
Overview of the current treatment strategy in extranodal NK/T-cell lymphoma: from diagnosis to recurrence
Background
Extranodal natural killer (NK) T-cell lymphoma (ENKTL) is a singular non-Hodgkin’s lymphoma (NHL) that primary involves the nasal cavity and nasopharynx and can be affected by the Epstein-Barr virus (EBV) (1). Before the year 2000, most treatments were based on cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like chemotherapy or radiation alone (2). However, the outcomes from these treatments were dismal. In the era of (CHOP)-based chemotherapy, the prognosis was grim for most patients, with a 5-year overall survival (OS) of 30–40%. Even in the patients with limited-stage ENKTL, the 5-year progression-free survival (PFS) and OS of ENKTL was about 44% and 21% respectively. Moreover, the 5-year OS to the treatment of newly diagnosed advanced ENKTL was lower than 30%, and that of relapsed or refractory ENKTL was worse (3-5).
Recently, the treatment of ENKTL has undergone significant changes. Concurrent or sequential chemoradiotherapy has emerged as a vital treatment option for localized ENTKL, and nonanthracycline-based chemotherapy combined with L-asparaginase is drawing attention in advanced ENKTL treatment. Studies have shown that localized ENKTL can maintain long-term efficacy, with a 2-year OS of 88% and a 5-year OS of 65% (6,7). Also, patients with advanced-stage or relapsed/refractory ENKTL were found to obtain excellent extended survival results with a response rate of 70–80% and a 2-year PFS or OS of approximately 40% (8,9). Furthermore, the efficacy of the anti–programmed cell death protein 1 (PD-1) antibody (pembrolizumab, nivolumab, sintilimab) and anti-programmed death-ligand 1 (PD-L1) antibody (avelumab, CS1001) was reported for cases that failed previous systemic chemotherapy (10-12). However, experience concerning anti-PD-1 antibody and anti-PD-L1 antibody efficacy administered to patients with relapsed/refractory ENKTL is still lacking. Therefore, any significant improvements in clinical outcomes in daily practice in ENKTL patients have been achieved through use of a prospective registry (13).
Nevertheless, some patients with ENKTL still do not have satisfactory prognoses. Consequently, researchers have developed a prognostic index of natural killer lymphoma (PINK)/PINK-E (EBV) and central nervous system (CNS)-PINK. With objective and weighted scoring systems, we can segment the treatments applied to patients and predict their prognosis more accurately (14,15). Other studies have noted that the effect of immunotherapy is not the same in all ENKTL patients, and the difference in efficacy is based on the discrepancy in the tumor microenvironment, and applying immunotherapy that considers these tumor microenvironments has been recommended (16,17).
As described above, the treatment strategy and prognostic prediction model of ENKTL have continued to evolve, treatment experience has gradually been enriched, and related data have accumulated. To provide an account for these changes, we review the treatment options in the localized, advanced, and relapsed settings and discuss the factors to be considered in creating a plan for future patient care.
Why novel treatment strategies in ENKTL are drawing attention
Radiotherapy is an important treatment method in localized ENKTL, so the appropriate dose, clinical target volume (CTV), and radiotherapy delivery method have continued to develop (8). However, there is concern that radiotherapy alone is insufficient to reduce the rate of local failure, regional failure, and systemic disease recurrence (18,19). Several key approaches with combination radiation and chemotherapy (concurrent, sequential, and sandwich) for the locoregional disease have emerged.
Studies have found that CHOP combined with radiotherapy does not produce an effective survival outcome compared to radiotherapy alone (2,20,21) because of the high expression of P-glycoprotein, resulting in tumor multidrug resistance to anthracycline (22). Moreover, in vitro analysis to compare drug efficacy between L-asparaginase and anthracyclines demonstrated that L-asparaginase administration in NK-cell leukemia/lymphoma cell lines has moderate antitumor effect due to the decreased ability of asparagine synthesis in tumor cells; as a result, L-asparaginase has garnered attention as an essential therapeutic agent in ENKTL. Overall, radiotherapy and nonanthracycline with L-asparaginase have become the strategic treatments of localized and advanced ENKTL (23).
Treatment strategies for newly diagnosed patients with localized ENKTL
The current form of chemoradiotherapy, which we currently use for localized ENKTL, is based on previous failures. Its administration can be divided into 4 variations: simultaneous chemoradiotherapy, radiotherapy followed by chemotherapy, chemotherapy followed by radiotherapy, and sandwich chemoradiotherapy (Table 1).
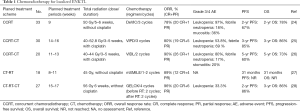
Full table
In one Japanese phase I/II study, 3 courses of 2/3 DeVIC (dexamethasone, etoposide, ifosfamide, and carboplatin) were administered for 5–6 weeks simultaneously. This concurrent chemoradiotherapy (CCRT) strategy yielded a 78% overall response rate (ORR), a 67% 2-year PFS, and 78% 2-year OS (24). Another study applied adjuvant CCRT with weekly cisplatin with radiation followed by VIPD (etoposide, ifosfamide, cisplatin, and dexamethasone) and yielded an ORR of 80%, a 3-year PFS of 85%, and a 3-year OS of 86%. This finding was similar to treatment results of DeVIC-based CCRT (25); however, this treatment scheme took more than 14–16 weeks to complete. Another phase II study conducted CCRT followed by chemotherapy with L-asparaginase (VIDL, etoposide, ifosfamide, dexamethasone, and L-asparaginase) (26). This approach shortened the treatment period slightly (11-13 weeks) and achieved similar therapeutic efficacy.
When it is too challenging to secure radiotherapy margin for patients with localized ENKTL, or it is impossible to wait for the time required to simulate radiotherapy, sequential chemotherapy followed by radiotherapy may be a viable option. Therapies that have proven effective in clinical researches include SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide), modified SMILE, and DICE-L (cisplatin, ifosfamide, etoposide, dexamethasone, and L-asparaginase) (27,29). Depending on the regimen, the potency is similar, but there is a difference in the incidence of adverse events (AEs), so it is necessary to consider the age, performance, and comorbidities of the individual.
Sandwich chemoradiotherapy comprises sequential chemoradiotherapy followed by additional chemotherapy. A prospective study was conducted to appraise the efficacy and toxicity of the treatment scheme consisting of GELOX (gemcitabine, oxaliplatin, and L-asparaginase) followed by radiotherapy, and then GELOX again (28). This yielded an excellent ORR (96%) and 2-year PFS (86%), with only a few grade 3 or 4 AEs occurring. The researchers recommended that sandwich chemoradiotherapy containing GELOX can be a practical and feasible treatment strategy for localized ENKTL.
The Asian Lymphoma Study Group analyzed an international cohort of patients with newly diagnosed stage I/II ENKTL to evaluate the efficacy and safety of CCRT and sequential chemotherapy followed by radiotherapy. The results indicated no difference in the response and survival results between the chemoradiotherapy types (30).
Based on the findings of previous trials, we can conclude that chemotherapy combined with radiation is essential. A recent prospective cohort study confirmed these observations. Fox et al. showed a definite survival benefit for chemoradiation (3-year OS, 70%) against chemotherapy alone (3-year OS, 12%) in patients with localized ENKTL (13). In addition to the application of a therapeutic concept for localized ENKTL, numerous clinical trials evaluating new chemoradiotherapy combinations are continuously being performed. The recent clinical trials for localized ENKTL are summarized in Table 2.
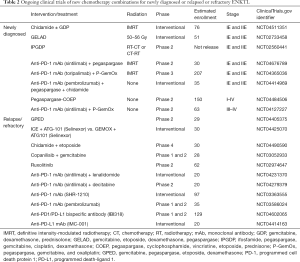
Full table
Treatment strategies for patients with advanced ENKTL (Table 3)

Full table
Recently, CHOP or CHOP-like chemotherapy has been discontinued. The chemotherapy regimens most frequently used in clinic are SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide), VIDL (etoposide, ifosfamide, dexamethasone, and L-asparaginase), MIDLE (methotrexate, ifosfamide, dexamethasone, L-asparaginase, and etoposide), and DDGP (dexamethasone, cisplatin, gemcitabine, and peg-asparaginase) (13). In a phase I and II study of a SMILE regimen for newly diagnosed advanced or R/R ENKTL (31-33,37), the ORR after 2 SMILE cycles were approximately 80%, and the 1-year OS and PFS were 55% and 53%, respectively. Although there were no subsequent treatment-related deaths, grade 3 or 4 neutropenia (92%) and infection (16%) were common. Unexpectedly, the allergic reaction caused by L-asparaginase was manageable, and the chemotherapy did not stop due to hypersensitivity. Thus, researchers recommended the need for rigorous monitoring of myelosuppression and infection during and after SMILE.
In a French phase 2 study, a cohort of 19 patients who received AspaMetDex (L-asparaginase, methotrexate, and dexamethasone) had an ORR of 78%, and a 2-year PFS and OS of both about 40%. Furthermore, grade 3 or 4 neutropenia developed after chemotherapy in 42% of patients (35).
Gemcitabine has shown substantial efficacy according to a retrospective study (38); therefore, the gemcitabine and L-asparaginase-based chemotherapy regimen, DDGP has been further developed. In a retrospective study to evaluate the efficacy and safety of DDGP, 28 patients with stage III or IV ENKTL who received DDGP showed an ORR of 89.3%, a 2-year PFS of 68.4%, and a 2-year OS of 84.5% (35). A randomized trial for newly diagnosed advanced ENKTL was performed to compare the efficacy and survival of DDGP versus those of the SMILE regimen (36). The ORR (90% vs. 60%), 3-year PFS rate (56.6% vs. 41.8%), and 5-year OS rate (74.3% vs. 51.7%) in the DDGP group were superior to those of the SMILE group, while, grade 3 or 4 leukopenia, neutropenia, and mucositis were significantly higher in the SMILE group. Moreover, treatment-related mortality was about 17.5% in the SMILE group and 10% in the DDGP group. Researchers thus emphasized the prolonged survival, better tolerability, and superior safety of the DDGP regimen in newly diagnosed stage III or IV ENKTL patients. Kwong et al. also pointed out the same shortcomings of SMILE therapy, including a high incidence of grade 3 or 4 hematologic AEs (67%) (32). Among 87 patients who were given SMILE, death from severe infections only occurred in 5 patients (6%). However, the sample size was not sufficiently large to determine which chemotherapy regimen is better. Therefore, although the priority of regimens for advanced-stage or R/R ENKTL remains controversial, the common opinion based on current data suggest that the treatment scheme of advanced-stage ENKTL should consist of non-MDR-dependent drugs with L-asparaginase.
Treatment strategies for patients with R/R ENKTL
Patients with advanced and R/R ENKTL have poor outcomes. Therefore, many clinicians have performed high-dose chemotherapy with hematopoietic stem cell transplantation. In a retrospective study in 2008, Lee et al. reported the outcome of autologous stem cell transplantation (auto-SCT) in 59 patients with ENKTL. With a median follow-up of 116.5 months, the median survival was not reached for the auto-SCT group, but it was 43.5 months for the patients who did not receive auto-SCT. Also, the authors emphasized that the disease status of the auto-SCT patients was more significantly influenced by disease-specific survival (39). Another retrospective study that estimated the efficacy of auto-SCT noted that achieving complete response (CR) before auto-SCT was the only significant prognostic factor (40). Kim et al. assessed the efficacy and safety of induction treatment with SMILE and consolidation with upfront auto-SCT. Among 27 patients with advanced-stage ENKTL, only 11 patients underwent auto-SCT, and there was no transplantation-related mortality (TRM) (33). However, the role of auto-SCT for patients with advanced ENKTL has not yet been established clearly, due to the fact that ENKTL frequently recurs during or after induction treatment.
There is also no consensus opinion regarding performing allogeneic stem cell transplantation (allo-SCT) for advanced or relapsed ENKTL patients. In Japan, the retrospective data of 28 NK cell neoplasm patients who underwent allo-SCT were reviewed. With a median follow-up of 34 months, the 2-year PFS and OS were 34% and 40%, respectively. However, the incidence of acute and chronic graft-versus-host disease (GVHD) was estimated at 43% (12/28) and 28% (8/28), respectively, with the TRM being 28% (8/28) (41). In another retrospective study that reviewed 12 patients with ENKTL who received allo-SCT, the 3-year event-free survival (EFS) and OS were 53% and 55%, respectively. All-grade acute GVHD was observed in 50% (6/12) of the patients, and atypical infections, such as cytomegalovirus, adenovirus, and BK virus, were also recorded in 50% (6/12) of the patients (42). Given these results, it is still questionable whether the benefits of allo-SCT outweigh the costs. Thus, the role of transplantation in ENKTL treatment remains ambiguous due to the limited data. However, auto-SCT or allo-SCT can be considered when patients with advanced or relapsed ENKTL achieve remission by salvage treatment strategy.
One study found that patients who relapsed after being treated with L-asparaginase-containing regimens received only a few months of survival (43). Gene expression profiling data has established that the expression of JAK/STAT pathway genes is higher in ENKTL cells than in normal NK cells (44). Song et al. conducted a study to determine the relationship between JAK/STAT mutation and PD-L1 expression (45). Oncogenic activation of the STAT3 pathway showed a high prevalence of about 21% in ENKTL cases. Moreover, activated STAT3 was found to affect PD-L1 expression by strongly binding to the promoter site of the PD-L1 gene. The use of anti-PD-1 antibodies (pembrolizumab, nivolumab, and sintilimab) or anti–PD-L1 antibodies (avelumab and CS1001) can thus interrupt PD-L1 and PD-1 interaction enabling host immune surveillance (Table 4).
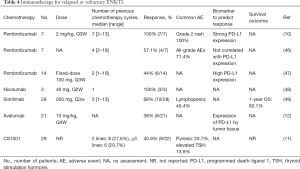
Full table
Kwong et al. retrospectively analyzed the outcomes of anti-PD-1 antibody administration in R/R EKNTL lymphomas after L-asparaginase combination chemotherapy. All 7 patients (100%) included in the study obtained an objective response (10). However, another retrospective study reported that pembrolizumab could only yield an ORR of 44% (6/14) (47). For nivolumab, Chan et al. indicated that, similar to pembrolizumab, the smallest available dose is also useful for R/R ENKTL; although this outcome was reported in only 3 patients, nivolumab showed less toxicity and 100% response in relapsed ENKTL cases (48). A multicenter, single-arm, phase II study examined the efficacy of another anti-PD-1 antibody, sintilimab, in R/R ENKTL patients: 28 patients were treated with about 3 cycles (range, 1–13) of sintilimab. The ORR, including the number of patients with CR and partial response (PR), was 68% (19/28) (49).
Kim et al. assessed the efficacy and safety of treatment with anti-PD-L1 antibody (avelumab) in R/R ENKTL. A total of 21 patients were administrated 10 mg/kg of avelumab every 4 weeks. The ORR was 38%, with CR achieved in 24%. Based on the results showing a significant response to immunotherapy in patients with high expression of PD-L1, the expression of PD-L1 in tumor tissue has been considered critical for predicting response (12). Furthermore, the efficacy and safety of CS1001, another PD-L1 monoclonal immunoglobulin reported by a phase II clinical trial, has yielded an appreciable response rate and durable response duration without severe toxicities (11,50).
Although the efficacy of immune checkpoint inhibitors (ICIs) on ENKTL has been reported on several occasions, the correlation of PD-L1 expression to therapeutic efficacy remains controversial. Therefore, many researchers have speculated using the relationship between tumors and their surrounding tumor immune microenvironment (TIME) as a valuable tool in predictions. Somasundaram et al. developed 4 categories of TIME for ENKTL by applying immunohistochemistry (IHC) and gene expression. They defined the depletion of effector T cells around the tumor as immune silencing (IS) and predicted a decrease in the effectiveness of immunotherapy in patients with the IS type (51). In a multi-omics study, Xiong et al. identified the molecular subtypes of ENKTL based on the RNA helicase family, tumor suppressors, the JAK-STAT pathway, epigenetic modifiers, and the RAS-MAPK pathway (17). They recommended choosing a PD-1 blocker while also considering NK cell-associated immunity and the PD-L1 marker. Lim et al. conducted a genetic analysis study on tumor tissues from 19 patients with R/R ENKTL. Using genetic profiling, they found that PD-L1 structural rearrangements are more useful than PD-L1 as a biomarker to predict the response to ICI (52).
Based on these findings, we believe that treatment strategies should factor in genetic alterations, as well as the molecular and cellular interactions related to EBV-associated lymphomagenesis, before the initiation of immunotherapy. However, the limitation of these technical methods is that these testing techniques are not used widely and are not accessible in general clinics.
Although a diversity of research streams have enhanced our understanding of the disease aspects and therapeutic strategies of ENKTL over the past decade, treatment limitations still exist in advanced and R/R ENKTL cases. Fortunately, various clinical trials currently are being conducted with the aim of evaluating the efficacy and safety of chemotherapies or ICIs for advanced or R/R ENKTL patients (Table 2).
Criteria for CNS prophylaxis intervention in ENKTL patients
According to the previous studies, 0% to 6% of patients with ENKTL have experienced CNS recurrence (53,54). Although the incidence of CNS disease in ENKTL is low, the means for prognosis is inferior, and more attention is needed to improve this.
Kim et al. conducted developed a model to predict CNS events in 253 ENKTL patients (14). Using to Cox regression analyses, they established a CNS prognostic index of natural killer lymphoma (CNS-PINK) composed of 2 risk groups: 0 or 1 point was considered low risk while 2 points was considered high risk (PINK group III/IV or extranodal involvement; Table 5). It was found that the incidence of CNS recurrence increased to 22.8% in the CNS-PINK high-risk group. Additionally, among patients categorized into the high-risk group, patients who were administered S-ID-MTX presented a lower rate of CNS recurrence than patients who were not. However, there was no difference in CNS recurrence according to S-ID-MTX in patients classified in the low-risk group. Based on these findings, the authors recommended considering active CNS prophylaxis of patients with a high-risk CNS-PINK (15).
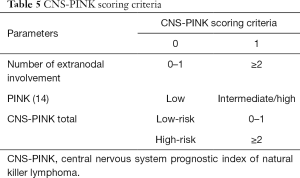
Full table
Conclusions
Chemotherapy combined with radiation should be recommended as standard care for localized ENKTL patients regardless of the sequence. Different chemotherapy regimens for localized ENKTL show no significant differences in efficacy, but the specifics of the patient’s condition should be included into the treatment plan to account for potential AEs. For advanced-stage ENKTL, systemic chemotherapy, including L-asparaginase, is regarded as the front-line treatment. Immunotherapy is also effective in advanced or R/R ENKTL, but there are no significant biomarkers to predict treatment efficacy. Thus, further research concerning the EBV-related tumor microenvironment is needed to identify new predictive biomarkers of immunotherapy, while studies for developing prognostic tools for ENKTL should be continuously conducted.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Christopher P. Fox, Claire Shannon-Lowe) for the series “Lymphoma and Viruses” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-35). The series “Lymphoma and Viruses” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia 2005;19:2186-94. [Crossref] [PubMed]
- Kim WS, Song SY, Ahn YC, et al. CHOP followed by involved field radiation: is it optimal for localized nasal natural killer/T-cell lymphoma? Ann Oncol 2001;12:349-52. [Crossref] [PubMed]
- Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin's lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin's Lymphoma Classification Project. Ann Oncol 1998;9:717-20. [Crossref] [PubMed]
- Bu S, Yuan F, Wei X, et al. L-asparaginase-based regimen as a first-line treatment for newly diagnosed nasal type extranodal natural killer cell/T-cell lymphoma. Exp Ther Med 2016;11:2437-45. [Crossref] [PubMed]
- Suzuki R, Suzumiya J, Yamaguchi M, et al. Prognostic factors for mature natural killer (NK) cell neoplasms: aggressive NK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann Oncol 2010;21:1032-40. [Crossref] [PubMed]
- Kim BS, Kim TY, Kim CW, et al. Therapeutic outcome of extranodal NK/T-cell lymphoma initially treated with chemotherapy--result of chemotherapy in NK/T-cell lymphoma. Acta Oncol 2003;42:779-83. [Crossref] [PubMed]
- Tse E, Kwong YL. How I treat NK/T-cell lymphomas. Blood 2013;121:4997-5005. [Crossref] [PubMed]
- Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood 2018;131:2528-40. [Crossref] [PubMed]
- Xiong J, Zhao WL. Advances in multiple omics of natural-killer/T cell lymphoma. J Hematol Oncol 2018;11:134. [Crossref] [PubMed]
- Kwong YL, Chan TSY, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 2017;129:2437-42. [Crossref] [PubMed]
- Huang HQ, Tao R, Zou L, et al. Preliminary Results from a Multicenter, Single-Arm, Phase 2 Study of CS1001, an Anti-Programmed Death-Ligand 1 (PD-L1) Human Monoclonal Antibody (mAb), in Patients (pts) with Relapsed or Refractory Extranodal Natural Killer/T Cell Lymphoma (rr-ENKTL). Blood 2019;134:2833. [Crossref]
- Kim SJ, Lim JQ, Laurensia Y, et al. Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: an open-label phase 2 study. Blood 2020;136:2754-63. [Crossref] [PubMed]
- Fox CP, Civallero M, Ko YH, et al. Survival outcomes of patients with extranodal natural-killer T-cell lymphoma: a prospective cohort study from the international T-cell Project. Lancet Haematol 2020;7:e284-94. [Crossref] [PubMed]
- Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol 2016;17:389-400. [Crossref] [PubMed]
- Kim H, Jeong H, Yamaguchi M, et al. Prediction and prevention of central nervous system relapse in patients with extranodal natural killer/T-cell lymphoma. Blood 2020;136:2548-56. [Crossref] [PubMed]
- Cho J, Kim SJ, Park WY, et al. Immune subtyping of extranodal NK/T-cell lymphoma: a new biomarker and an immune shift during disease progression. Mod Pathol 2020;33:603-15. [Crossref] [PubMed]
- Xiong J, Cui BW, Wang N, et al. Genomic and Transcriptomic Characterization of Natural Killer T Cell Lymphoma. Cancer Cell 2020;37:403-19.e6. [Crossref] [PubMed]
- Kim GE, Cho JH, Yang WI, et al. Angiocentric lymphoma of the head and neck: patterns of systemic failure after radiation treatment. J Clin Oncol 2000;18:54-63. [Crossref] [PubMed]
- Cheung MMC, Chan JKC, Lau WH, et al. Early stage nasal NK/T-cell lymphoma: clinical outcome, prognostic factors, and the effect of treatment modality. Int J Radiat Oncol Biol Phys 2002;54:182-90. [Crossref] [PubMed]
- Lee SH, Ahn YC, Kim WS, et al. The effect of pre-irradiation dose intense CHOP on anthracyline resistance in localized nasal NK/T-cell lymphoma. Haematologica 2006;91:427-8. [PubMed]
- Jiang L, Li SJ, Jiang YM, et al. The significance of combining radiotherapy with chemotherapy for early stage extranodal natural killer/T-cell lymphoma, nasal type: a systematic review and meta-analysis. Leuk Lymphoma 2014;55:1038-48. [Crossref] [PubMed]
- Wang B, Li XQ, Ma X, et al. Immunohistochemical expression and clinical significance of P-glycoprotein in previously untreated extranodal NK/T-cell lymphoma, nasal type. Am J Hematol 2008;83:795-9. [Crossref] [PubMed]
- Aller SG, Yu J, Ward A, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009;323:1718-22. [Crossref] [PubMed]
- Yamaguchi M, Tobinai K, Oguchi M, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol 2009;27:5594-600. [Crossref] [PubMed]
- Kim SJ, Kim K, Kim BS, et al. Phase II Trial of Concurrent Radiation and Weekly Cisplatin Followed by VIPD Chemotherapy in Newly Diagnosed, Stage IE to IIE, Nasal, Extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma Study. J Clin Oncol 2009;27:6027-32. [Crossref] [PubMed]
- Kim SJ, Yang DH, Kim JS, et al. Concurrent chemoradiotherapy followed by L-asparaginase-containing chemotherapy, VIDL, for localized nasal extranodal NK/T cell lymphoma: CISL08-01 phase II study. Ann Hematol 2014;93:1895-901. [Crossref] [PubMed]
- Ghione P, Qi S, Imber BS, et al. Modified SMILE (mSMILE) and intensity-modulated radiotherapy (IMRT) for extranodal NK-T lymphoma nasal type in a single-center population. Leuk Lymphoma 2020;61:3331-41. [Crossref] [PubMed]
- Wang L, Wang ZH, Chen XQ, et al. First-line combination of gemcitabine, oxaliplatin, and L-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer 2013;119:348-55. [Crossref] [PubMed]
- Kim SJ, Yoon SE, Kim WS. Treatment of localized extranodal NK/T cell lymphoma, nasal type: a systematic review. J Hematol Oncol 2018;11:140. [Crossref] [PubMed]
- Kwong YL, Kim SJ, Tse E, et al. Sequential chemotherapy/radiotherapy was comparable with concurrent chemoradiotherapy for stage I/II NK/T-cell lymphoma. Ann Oncol 2018;29:256-63. [Crossref] [PubMed]
- Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol 2011;29:4410-6. [Crossref] [PubMed]
- Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood 2012;120:2973-80. [Crossref] [PubMed]
- Kim SJ, Park S, Kang ES, et al. Induction treatment with SMILE and consolidation with autologous stem cell transplantation for newly diagnosed stage IV extranodal natural killer/T-cell lymphoma patients. Ann Hematol 2015;94:71-8. [Crossref] [PubMed]
- Jaccard A, Gachard N, Marin B, et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood 2011;117:1834-9. [Crossref] [PubMed]
- Zhang L, Li S, Jia S, et al. The DDGP (cisplatin, dexamethasone, gemcitabine, and pegaspargase) regimen for treatment of extranodal natural killer (NK)/T-cell lymphoma, nasal type. Oncotarget 2016;7:58396-404. [Crossref] [PubMed]
- Wang X, Zhang L, Liu X, et al. Efficacy and Survival in Newly Diagnosed Advanced Extranodal Natural Killer/T-Cell Lymphoma: A Randomized, Controlled, Multicenter and Open-Labled Study with Ddgp Regimen Versus SMILE Regimen. Blood 2019;134:463. [Crossref]
- Yamaguchi M, Suzuki R, Kwong YL, et al. Phase I study of dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide (SMILE) chemotherapy for advanced-stage, relapsed or refractory extranodal natural killer (NK)/T-cell lymphoma and leukemia. Cancer Sci 2008;99:1016-20. [Crossref] [PubMed]
- Ahn HK, Kim SJ, Hwang DW, et al. Gemcitabine alone and/or containing chemotherapy is efficient in refractory or relapsed NK/T-cell lymphoma. Invest New Drugs 2013;31:469-72. [Crossref] [PubMed]
- Lee J, Au WY, Park MJ, et al. Autologous hematopoietic stem cell transplantation in extranodal natural killer/T cell lymphoma: a multinational, multicenter, matched controlled study. Biol Blood Marrow Transplant 2008;14:1356-64. [Crossref] [PubMed]
- Kwong YL. High-dose chemotherapy and hematopoietic SCT in the management of natural killer-cell malignancies. Bone Marrow Transplant 2009;44:709-14. [Crossref] [PubMed]
- Gyurkocza B, Rezvani A, Storb RF. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol 2010;3:285-99. [Crossref] [PubMed]
- Ennishi D, Maeda Y, Fujii N, et al. Allogeneic hematopoietic stem cell transplantation for advanced extranodal natural killer/T-cell lymphoma, nasal type. Leuk Lymphoma 2011;52:1255-61. [Crossref] [PubMed]
- Lim SH, Hong JY, Lim ST, et al. Beyond first-line non-anthracycline-based chemotherapy for extranodal NK/T-cell lymphoma: clinical outcome and current perspectives on salvage therapy for patients after first relapse and progression of disease. Ann Oncol 2017;28:2199-205. [Crossref] [PubMed]
- Nairismägi M, Gerritsen ME, Li ZM, et al. Oncogenic activation of JAK3-STAT signaling confers clinical sensitivity to PRN371, a novel selective and potent JAK3 inhibitor, in natural killer/T-cell lymphoma. Leukemia 2018;32:1147-56. [Crossref] [PubMed]
- Song TL, Nairismägi ML, Laurensia Y, et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood 2018;132:1146-58. [Crossref] [PubMed]
- Li X, Cheng Y, Zhang M, et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol 2018;11:15. [Crossref] [PubMed]
- Kim SJ, Hyeon J, Cho I, et al. Comparison of Efficacy of Pembrolizumab between Epstein-Barr Virus-Positive and -Negative Relapsed or Refractory Non-Hodgkin Lymphomas. Cancer Res Treat 2019;51:611-22. [Crossref] [PubMed]
- Chan TSY, Li J, Loong F, et al. PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing L-asparaginase: efficacy and safety. Ann Hematol 2018;97:193-6. [Crossref] [PubMed]
- Tao R, Fan L, Song Y, et al. Sintilimab for relapsed/refractory (r/r) extranodal NK/T-cell lymphoma (ENKTL): A multicenter, single-arm, phase 2 trial (ORIENT-4). J Clin Oncol 2019;37:7504. [Crossref]
- Zhang J, Li Z, Tang L, et al. Abstract 3260: The preclinical characterization of CS1001, an anti-PD-L1 IgG4 monoclonal antibody and its activity beyond T cell regulation. Cancer Res 2020;80:3260.
- Somasundaram N, Lim JQ, Ong CK, et al. Pathogenesis and biomarkers of natural killer T cell lymphoma (NKTL). J Hematol Oncol 2019;12:28. [Crossref] [PubMed]
- Lim JQ, Huang D, Tang T, et al. Whole-genome sequencing identifies responders to Pembrolizumab in relapse/refractory natural-killer/T cell lymphoma. Leukemia 2020;34:3413-9. [Crossref] [PubMed]
- Cheung MM, Chan JK, Lau WH, et al. Primary non-Hodgkin's lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol 1998;16:70-7. [Crossref] [PubMed]
- Kim GE, Koom WS, Yang WI, et al. Clinical relevance of three subtypes of primary sinonasal lymphoma characterized by immunophenotypic analysis. Head Neck 2004;26:584-93. [Crossref] [PubMed]
Cite this article as: Yoon SE, Kim SJ, Kim WS. Overview of the current treatment strategy in extranodal NK/T-cell lymphoma: from diagnosis to recurrence. Ann Lymphoma 2021;5:17.


