
Narrative review of chronic active EBV infection—advances in clinical management
Introduction
Patients with persistent/recurrent illness of Epstein-Barr virus (EBV) infection were initially reported in the 1970s (1,2). These patients were extensively examined, and the concept of chronic active EBV infection (CAEBV) was introduced in the 1980s (3-7). Although EBV was previously shown to exhibit a tropism to a specific subset of lymphocytes, B cells, it was found to infect T and NK cells, resulting in the manifestation of symptoms, in patients with CAEBV in 1988 and 1989, respectively (8-11). B-cell neoplasms and T/NK-cell neoplasms are pathologically considered to differ. Therefore, “CAEBV of the T/NK-cell type” is a new nomenclature (12). CAEBV has historically included B-cell lymphoproliferative disease (LPD) as a small and milder subset in the literature; however, it has been redefined as T- and NK-cell LPD (T/NK-cell LPD) (13,14).
We herein reviewed advances in the clinical management of CAEBV in our institute and in the literature. This retrospective analysis was approved by the Research Ethics Committee of Osaka Women’s and Children’s Hospital. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/aol-20-34).
Methods
MEDLINE was searched using the term CAEBV between 1964 and June 2020. Since CAEBV is a rare disease and our department specializes in EBV, we also updated the retrospective analysis of patients with CAEBV treated in our institute before the end of 2019.
Diagnosis
CAEBV
Guidelines for diagnosing CAEBV were proposed in 2005 (15), and comprised the following: (I) persistent or recurrent infectious mononucleosis (IM)-like symptoms, (II) evidence of EBV activation, and (III) the exclusion of other known diseases. The most common symptoms of CAEBV are fever and elevated liver transaminase levels; however, other symptoms have also been reported (Table 1) (16). EBV activation is confirmed by abnormally elevated anti-EBV antibodies, with an EBV DNA load ≥317 copies/µg DNA (a rational number is now preferable to an irrational number 102.5 for definition) in peripheral blood (PB) mononuclear cells (15). The identification of EBV-infected T/NK cells in PB or affected tissues/organs, together with a medical history and clinical symptoms, is critical for diagnosing CAEBV. EBV-encoded small RNA (EBER) staining with in situ hybridization and a flow cytometric analysis was recently developed for rapid identification (17,18). A biopsy of any affected tissues/organs is not mandatory, but is required for a differential diagnosis in some cases (Table 2) (12,16,19). CAEBV, a T/NK-cell LPD, is sometimes considered to be similar to a lymphoma, but is a PB-diagnosable lymphoma or leukemia.
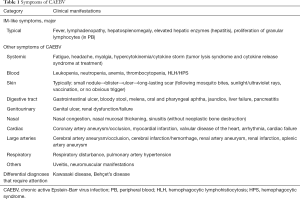
Full table
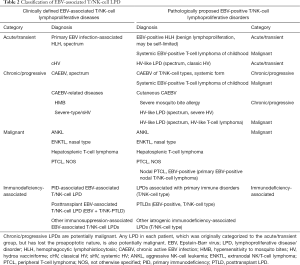
Full table
CAEBV shows a spectrum for its neoplastic nature, and, thus, is regarded as a potentially malignant disease. CAEBV is pathologically categorized as follows: polymorphic polyclonal/oligoclonal LPD, polymorphic monoclonal LPD, and monomorphic monoclonal LPD [similar to posttransplant LPD (PTLD)] (20,21). Based on this definition, truly malignant EBV-associated T/NK-cell LPD, such as aggressive NK-cell leukemia (ANKL) and extranodal NK/T-cell lymphoma (ENKTL), need to be excluded. However, the boundary between CAEBV and malignant T/NK-cell LPD is ambiguous (22-24). Some cases of ANKL and ENKTL developed from CAEBV (25,26). Furthermore, hemophagocytic lymphohistiocytosis (HLH) may emerge during the clinical course as a symptom of CAEBV.
Hypercytokinemia, including hemophagocytic syndrome (HPS) and HLH, is a life-threatening manifestation of CAEBV. Severe hypercytokinemia may rapidly develop, may be fatal in any patient with CAEBV, and is referred to as “CAEBV flare” (16,19). Hypercytokinemia/HLH in CAEBV is generally caused by EBV-infected T/NK cells themselves, indicating that affected cells retain the original nature of cytotoxic cells. Therefore, HLH itself does not indicate lymphoma/leukemia, including “systemic EBV-positive T-cell lymphoma of childhood”. The rapid initiation of treatment is strongly recommended for fulminant cases following the diagnosis of “EBV-associated T/NK-cell LPD (including CAEBV, lymphoma, and leukemia)” without further sub-categorization (13).
Mutations/variants in genes, which may be responsible for primary immunodeficiencies (PIDs), have been incidentally detected in patients with CAEBV (27). PIDs are generally suspected in patients with EBV-associated B-cell LPD. However, similar to PTLD, EBV-associated T/NK-cell LPD may also occur with immune dysregulation (28). CAEBV is diagnosed by the exclusion of other known diseases. Therefore, the relationship between the affected gene variants and the clinical history of patients in view of susceptibility to infections needs to be considered when attempting to reach a diagnosis of CAEBV.
CAEBV-related diseases
Systemic IM-like symptoms follow a topical skin reaction, which is induced by mosquito bites and sunlight in cases of hypersensitivity to mosquito bites (HMB) and severe-type/systemic hydroa vacciniforme (sHV), respectively (Table 2). HMB is characterized by a high load of EBV-infected NK cells in the skin and PB, and often has a similarly poor prognosis to CAEBV (29,30). Classical HV (cHV) is a self-limited disease in which EBV-infected gamma delta T cells are harbored in the skin and PB (31). In contrast, sHV has a poor prognosis similar to HMB and CAEBV. sHV is mainly caused by EBV-infected α/β T cells (32-34). EBV may infect two or more subsets of T/NK cells (35,36), and symptoms may manifest based on the nature of the major subset. Although sHV appears to progress from cHV in some cases, it is a distinct disease from cHV rather than a lineage switch in a single affected clone. EBV-infected NK cells have been detected in patients concomitantly manifesting HMB and sHV.
The diagnoses of HMB, sHV, and CAEBV are not mutually exclusive in a single patient with EBV-associated T/NK-cell LPD; therefore, these three are different aspects of one disease, rather than three overlapping diseases. Systemic IM-like symptoms may also be elicited by subcutaneous vaccination in patients with CAEBV. Treatment strategies have primarily been developed in the CAEBV approach, but may also be applied to HMB and SHV. Therefore, the following analyses include patients with CAEBV, HMB, and sHV.
Etiology and prognosis
Patients with CAEBV will not recover without radical treatment. A nationwide questionnaire survey, which was performed in the 1990s in Japan, revealed that 50% of patients with CAEBV died within 5 years, with the majority ultimately dying within 10–15 years (25). The outcome of adult-onset CAEBV is worse, with most patients dying within 5 years (37,38). CAEBV is a severe progressive disease with fatal organ failure (particularly hepatic and cardiac), hypercytokinemia/HLH (resulting in multiple organ failure), and true lymphoma/leukemia.
Although EBV is a B-cell tropic virus, it may infect T/NK cells at a low frequency (39,40). However, EBV-infected T/NK cells cannot be maintained and undergo apoptosis in vitro and in a healthy internal environment (41). Therefore, regarding carcinogenesis, EBV-infected T/NK cells in patients with CAEBV appear to have acquired mechanisms to evade apoptosis and self-expand (42). Okuno et al. reported an intragenic deletion in the EBV genome in BamH1 A rightward transcript (BART) microRNA clusters 1 and 2 in 35% (27/77) of patients with CAEBV, which promoted lymphomagenesis (36). Somatic mutations were also detected in a number of genes, such as DDX3X, BCOR/BCORL1, and TET2, in 20% (16/80) of patients with CAEBV (36). Some of these somatic mutations were frequently observed in healthy older individuals as clonal hematopoiesis of indeterminate potential (43), which may lead to myelodysplastic syndromes (MDS). Common somatic mutations may provide insights into the initiation and progression of CAEBV, as well as the poor prognosis of adult-onset CAEBV.
Treatment overview
Development of the three-step strategy
Immunotherapy and chemotherapy were attempted in the 1980s and 1990s to treat CAEBV and exerted some clinical effects, but failed to improve the final outcome (44). Regarding the use of anti-cancer drugs against CAEBV, some experts disagreed with its use claiming that CAEBV is just an infectious disease; however, based on our findings that CAEBV is a malignant (potentially neoplastic) disease (10,11), we introduced anti-cancer drugs as a treatment for CAEBV. A male pediatric patient with CAEBV was the first to successfully undergo bone marrow transplantation (BMT) from his HLA-matched elder brother in 1998 (45). Allogeneic hematopoietic stem cell transplantation (HSCT) became widely accepted in the late 2000s.
Since the initial success of HSCT, a treatment strategy consisting of three steps has been established (Figure 1) (19,46). The typical time schedule of the current treatment is shown in Figure 2. Our three-step strategy may also be applicable not only to children and adolescents, but also to adults with CAEBV (19). The 3-year overall survival (OS) rate was previously reported to be 76% (19). Planned HSCT is now performed for patients with stable disease or a less active disease status, i.e., without uncontrolled flare, and the 3-year OS rate is approximately 90%.

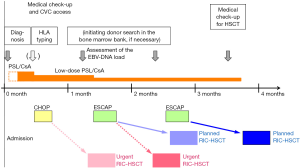
HSCT is currently the only cure for CAEBV patients, and complete donor-type chimerism is essential. We treated 92 patients with CAEBV before the end of 2019. Three out of 85 patients who underwent HSCT died soon after treatment (d1, d3, and d4 after HSCT). Among the remaining 82 patients, the cumulative incidence of mixed chimerism, insufficient autologous hematopoietic recovery (auto recovery), and engraftment failure was 2, 1, and 4, respectively. Although all patients with engraftment failure were successfully engrafted with 2nd HSCT, all three with mixed chimerism or auto recovery showed the early recurrence of disease (including one with no overt symptoms). Once complete donor-type chimerism was achieved, relapse was only observed in 3.8% of patients (3/79): one case of systemic relapse and two of local relapse as “hidden spaces” from immune surveillance, namely, the central nervous system (CNS) and skin (47). The numbers of chemotherapy courses received before HSCT were six, two, and two, respectively, EBV loads in PB at conditioning for HSCT were 500,000, 30,000, and 20,000 copies/mL (whole blood), ages at HSCT were 11, 38, and 16 years old, respectively, and the types of HSCT were BMT, umbilical cord blood transplantation (CBT), and CBT, respectively. As a result, no obvious risk factor for recurrence/relapse was identified other than mixed chimerism/auto recovery as described above.
Optimization of HSCT
In our institute, patients generally undergo HSCT after at least two courses of multidrug-combination chemotherapy. Myeloablative conditioning (MAC) was used in early series. The 1st case of successful reduced-intensity conditioning (RIC) followed by BMT (RIC-BMT) was a patient with well-controlled CAEBV in 2002. RIC was introduced with the aim of reducing late sequelae, and all patients have been treated with RIC since 2006. However, RIC also achieved better survival because of reductions in early toxicity: 3-year OS rates were 91–95% and 55–67% with RIC and MAC, respectively (19,46). The recovery rate of spontaneous menstruation was also higher (82% and 7% with RIC and MAC, respectively) (48). Although azoospermia and precocious menopause need to be considered, female patients and the partners of male patients have successfully become pregnant and given birth.
The 1st CBT for CAEBV to follow RIC was planned and successfully performed in 2003. Since then, we have been improving RIC (49). The latest combination of drugs for RIC was initiated in 2010 and worked well for BMT and peripheral blood stem cell transplantation (PBSCT). However, the rejection rate was higher than expected for CBT (49). We added one dose of melphalan (Mel) 70 mg/m2 for children and adolescents from 2012 (or systemic irradiation of 3 Gy for adults), which increased the engraftment rate from 57% to 100% (50).
Upfront HSCT without multidrug-combination chemotherapy also represents a treatment option. Although some patients may be cured by this approach, the following issues need to be considered (Table 3). (I) The disease activity of CAEBV widely varies. Conditioning before HSCT induces tumor lysis, resulting in conditioning-associated HPS in 33% of patients (49). Although most cases were self-limiting or controlled with etoposide (Etp), fatal conditioning-associated HPS was reported (51). The step-by-step cytoreduction of drug-susceptive EBV-infected T/NK cells is a safer approach. (II) In contrast to benign diseases, complete donor chimerism is required for CAEBV to prevent recurrence. However, even for BMT, RIC is sometimes insufficient, and the acquisition rate of complete donor chimerism is not adequate, similar to other diseases [40% in patients with familial HLH (FHL)] (52). (III) Chemotherapy may attenuate CAEBV before RIC-HSCT, similar to the management of advanced MDS before RIC-HSCT (53). (IV) CAEBV is a progressive disease. The earlier initiation of therapy results in better survival. The timing of chemotherapy and HSCT is restricted [case 2 in a previous study (19)] before bypassing “the point of no return” to a fatal clinical course.

Full table
Current three-step treatment strategy
Step 1 (cooling): immunochemotherapy
At the diagnosis of CAEBV, immunochemotherapy is initiated as step 1 (cooling) and consists of prednisolone (PSL), cyclosporine A (CsA), and Etp (Figure 1). PSL and CsA are used to suppress the abnormal self-activation of EBV-infected T/NK cells and hypercytokinemia, which may induce the activation of macrophages and histiocytes (potential HPS/HLH). A previous study suggested that Etp inhibited EBV nuclear antigen (EBNA) synthesis and EBV DNA synthesis in EBV-infected lymphocytes in vitro (54). In addition, high-dose Etp may induce apoptosis in the activated T cells of mice as a model of FHL (55). However, Etp mainly targets activated macrophages, and, thus, may be spared when HLH does not accompany EBV-associated T/NK-cell LPD.
Pulsed high-dose methylprednisolone (mPSL) needs to be considered for progressive hypercytokinemia. If a patient is accompanied by HLH, the HLH-94/HLH2004 protocol may be substituted for step 1, which was originally developed for FHL and consists of dexamethasone (Dex), CsA, and Etp (56,57). However, in contrast to FHL, CAEBV is less likely to involve CNS and Dex has more severe side effects than PSL; therefore, Dex is not mandatory for CAEBV. Further diagnostic examinations, medical check-ups, and other preparations for treatment (including HLA typing) are performed during 2 [1–3] weeks of immunochemotherapy (Figure 2). This step achieves the temporary control of disease activity, but does not contribute to the cytoreduction of EBV-infected T/NK cells in CAEBV (Figure 3) (58). Patients then move to the next step.
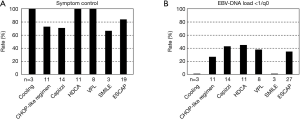
Step 2 (cytoreduction): multidrug-combination block chemotherapy
In step 2, the cytoreduction of EBV-infected T/NK cells is expected. Cytokine release syndrome and HPS/HLH may be induced with the tumor lysis of highly drug-susceptible EBV-infected T/NK cells at the time of chemotherapy and with the homeostatic proliferation of EBV-infected T/NK cells at recovery from myelosuppression (59). Therefore, low-dose PSL and CsA need to be continued during step 2, particularly when the burden of the residual disease is high, and pulsed high-dose mPSL and Etp also need to be loaded for hypercytokinemia and HPS/HLH, respectively (Figure 1). EBV-infected T/NK cells retain some characteristics of normal lymphocytes because they show homeostatic proliferation, but not infinite proliferation (in contrast to leukemic blasts). Although hypercytokinemia is sometimes severe during the administration of chemotherapy, it may be fatal during MAC for upfront HSCT in such patients. The cytokine storm (severe hypercytokinemia) was previously reported to be fatal, even after intensive chemotherapy (51). Therefore, the step-up strategy is preferable. The CHOP-like regimen needs to be considered as first-line chemotherapy, followed by more intensive chemotherapy and RIC-HSCT. Upfront HSCT is sometimes risky in this respect. The purposes and benefits of chemotherapy prior to HSCT are listed in Table 3.
The CHOP-like regimen is the most common 1st-line chemotherapy for lymphomas. THP-COP is the most frequently selected first-line chemotherapy in our institute (Figure 1) (49). CHOP and CHOEP are alternatives; however, pirarubicin is a widely used anthracycline in Japan because it is less cardiotoxic than doxorubicin (60,61). EBV-infected T/NK cells derived from most, if not all, patients are resistant to anthracycline due to their expression of p-glycoprotein (P-gp) (62). CsA down-regulates P-gp expression and restores T/NK-cell susceptibility to anthracycline (63). In addition, P-gp expressed in NK cells may not be the classical form, it may be a shorter form, which does not effectively export anthracycline (64). Therefore, the CHOP-like regimen may be more effective with CsA as described above or the COP regimen may be an alternative.
Second-line chemotherapy often contains cytosine arabinoside (CA) or L-asparaginase (65), and ESCAP has been the preferred choice at our institute since 2007. The effects of each chemotherapy are shown in Figure 3 (58). Based on the EBV load as a molecular marker of measurable residual disease (MRD), 4 out of 92 patients achieved molecular complete remission (CR). Two patients received an additional single course of chemotherapy to maintain continuous CR without HSCT (patients #2 and #N03 in our previous studies) (19,66). The two other patients successfully underwent HSCT with their parents’ and physician’s choice (patients #362 and #668 in our previous study) (19).
Step 3 (reconstruction): allogeneic HSCT
The preparation of HSCT consists of three parts. The first part involves the pre-preconditioning (pre-RIC) of low-dose Etp and CA (LDEC). LDEC was historically innovated for better control of the leukemic-cell burden before HSCT (67,68), and for the better engraftment of upfront HSCT in benign diseases. However, regarding CAEBV, LDEC for 1–2 weeks has also provided a safe bridge to HSCT by controlling the tumor burden and disease activity.
The early stages of RIC consist of low-dose rabbit anti-thymocyte globulin (ATG; Thymoglobulin®, Sanofi, France; 1.25 mg/kg/d × 2 days) and preceding Mel. The aim of ATG is not to prevent graft-versus-host disease (GVHD), but to reduce recipient T-cell immunity in order to enforce donor-cell engraftment. ATG also reduces EBV-infected T/NK-cell numbers for better disease control (42). Preceding Mel 70 mg/m2 (resulting in 210 mg/m2 in total RIC) is administered for better engraftment in children and adolescents undergoing CBT after only 2 or 3 courses of chemotherapy. It is replaced by the systemic irradiation of 3 Gy with gonadal blockade in adults (50).
The main stage of RIC consists of fludarabine, Mel, and Etp. Mel ≤240 mg/m2 is expected to preserve fertility in women (69). Etp was originally introduced to suppress antigen-presenting cells, thereby reducing GVHD and HLH after HSCT, and this concept was partially proven by a retrospective analysis (70). During RIC in patients with CAEBV, Etp provides a safety net for conditioning-associated HLH. Therefore, although Etp 100 mg/m2/d is scheduled on days –3 and –2, it may be flexibly administered whenever HLH occurs (49).
Dose reductions in RIC according to a formula for organ dysfunction are reasonable. However, our RIC regimen has been fine-tuned. Therefore, excessive reductions may result in a higher rate of rejection or mixed chimerism.
Other considerations for clinical management
Virological CR
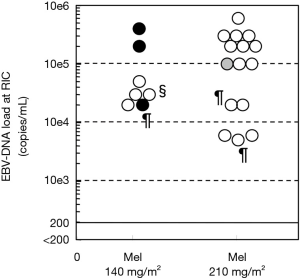
The main effector against CAEBV after HSCT is alloimmunity, not anti-EBV cytotoxic T lymphocytes (CTLs) (42). The EBV load (the MRD level) at RIC has a negligible impact on the success of CBT (Figure 4) (50). As described above, once CR is achieved after HSCT, the disease relapse rate is less than 5%. Furthermore, HSCT may be avoided when the EBV load is below the lower detection limit. Therefore, virological CR is beneficial, but not mandatory before HSCT.
Disease activity
Disease activity is important. Patients with mild symptoms may undergo successful HSCT. However, the prognosis of patients with severe disease activity even after chemotherapy is poor (OS rate <20%) (19,71). Among 12 patients with uncontrolled active disease, 4 died before HSCT, 3 died very early (d1, d3, and d4 after HSCT), and 3 died after emergent HSCT (19).
Caution is required when interpreting the findings of retrospective studies. In a previous study, 3-year OS rates in the upfront HSCT group (n=12), chemotherapy-HSCT group (n=47), and chemotherapy only group (n=20) were 82%, 65%, and 0%, respectively (14). However, a mild disease status may be included in the upfront HSCT group, a more active status in the chemotherapy-HSCT group, and progression before HSCT in the chemotherapy only group.
Advanced cases
CAEBV has a spectrum of disease severity, and there are two types of ANKL: de novo ANKL and ANKL transformed from CAEBV (26). ANKL may provide a more detailed understanding of advanced CAEBV. The OS rate of ANKL is <10% because it is mostly chemoresistant and mainly occurs in the elderly (72). However, recent findings indicate that OS is better at approximately 50% in CR patients (73). CAEBV is also generally a chemoresistant disease; however, a transient improvement is often observed during myelosuppression after chemotherapy. The initiation of RIC under myelosuppression and before organ dysfunction progresses to irreversible organ failure may overcome CAEBV with an advanced status.
Perspectives
New drugs
The 3-step strategy has become the standard platform for the treatment of CAEBV. However, further advances are needed for a better prognosis and fewer late sequelae with new drugs and methods in the perspective of: (I) more sophisticated alternative approaches, (II) the better management of advanced disease, and (III) a radical cure other than HSCT. JAK 1/2 inhibitors, such as ruxolitinib, may provide an additive effect with PSL/CsA/Etp on CAEBV (74,75); however, it may not be sufficient as a single agent for EBV-associated hypercytokinemia/HLH (76). In contrast, emapalumab, a monoclonal antibody against interferon-gamma, achieved improvements in 65% of patients with HLH, including CR in 26%; however, single cytokine blockade does not appear to be sufficient for a multiple cytokine disease (77).
Ganciclovir is an antiviral agent that is effective against EBV-lytic infection, but not CAEBV. It is activated via phosphorylation with EBV protein kinase, which is not expressed during latent infection, including EBV-infected T/NK cells in CAEBV. Proteosome inhibitors, such as bortezomib, induce EBV protein kinase expression to change latent into lytic infection (78), and the combination of bortezomib and ganciclovir may exert cytoreductive effects on EBV-infected T/NK cells (79). Histone deacetylase inhibitors, such as romidepsin, also induce EBV-lytic infection (80). However, close monitoring is warranted because these lytic infection-inducible drugs may cause severe EBV reactivation (81,82).
Cellular therapy and immunotherapy
Immunological approaches are now being revisited 30 years after early treatment with interleukin-2 (44). Wang et al. reported that 3 out of 5 children with EBV-positive T-cell LPD achieved clinical remission following the infusion of HLA-haploidentical lymphocytes without HSCT (83). Although EBV-specific CTL therapy is currently being developed (84,85), the induction of CTLs targeting EBNA1 and latent membrane proteins (LMPs), which are proteins expressed in type-2 latencies, such as CAEBV, is limited (86). PD-1 and PD-L1 inhibitors, including nivolumab and pembrolizumab, exerted promising effects in a small case series (87,88). They were safely and effectively administered, but with careful monitoring for cytokine release syndrome in the short term and disease recurrence in the long term.
To conclude this manuscript as a narrative review, CAEBV is a rare disease, and clinical data are limited; therefore, future research is awaited.
Conclusions
CAEBV is a diverse disease that may rapidly progress. The three-step treatment strategy has provided a platform for the management of CAEBV. Some of the novel modalities described above may contribute to further improvements in the prognosis of these patients, particularly those with advanced CAEBV.
Acknowledgments
The authors thank Prof. Keisei Kawa and Prof. Alan B. Rickinson for leading us in the EBV world in Japan and the UK, respectively. The authors thank Dr. Takayuki Okamura and Dr. Emiko Sato-Miyashita for their contributions to early studies. The authors thank Prof. Hiroshi Kimura and Prof. Ken-ichi Imadome for their cooperation with pathology. The authors thank all patients and staff included in this review.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Christopher P. Fox, Claire Shannon-Lowe) for the series “Lymphoma and Viruses” published in Annals of Lymphoma. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/aol-20-34
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-34). The series “Lymphoma and Viruses” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Horwitz CA, Henle W, Henle G, et al. Clinical evaluation of patients with infectious mononucleosis and development of antibodies to the R component of the Epstein-Barr virus-induced early antigen complex. Am J Med 1975;58:330-8. [Crossref] [PubMed]
- Virelizier JL, Lenoir G, Grisceili C. Persistent Epstein-Barr virus infection in a child with hypergammaglobulinaemia and immunoblastic proliferation associated with a selective defect in immune interferon secretion. Lancet 1978;2:231-4. [Crossref] [PubMed]
- Jones JF, Ray CG, Minnich LL, et al. Evidence for active Epstein-Barr virus infection in patients with persistent, unexplained illnesses: elevated anti-early antigen antibodies. Ann Intern Med 1985;102:1-7. [Crossref] [PubMed]
- Straus SE, Tosato G, Armstrong G, et al. Persisting illness and fatigue in adults with evidence of Epstein-Barr virus infection. Ann Intern Med 1985;102:7-16. [Crossref] [PubMed]
- Tobi M, Straus SE. Chronic Epstein-Barr virus disease: a workshop held by the National Institute of Allergy and Infectious Diseases. Ann Intern Med 1985;103:951-3. [Crossref] [PubMed]
- Rickinson AB. Chronic, symptomatic Epstein-Barr virus infections. Immunol Today 1986;7:13-4. [Crossref] [PubMed]
- Okano M, Sakiyama Y, Matsumoto S, et al. Unusual lymphoproliferation associated with chronic active Epstein-Barr virus infection. AIDS Res 1986;2:S121-3. [PubMed]
- Jones JF, Shurin S, Abramowsky C, et al. T-cell lymphomas containing Epstein-Barr viral DNA in patients with chronic Epstein-Barr virus infections. N Engl J Med 1988;318:733-41. [Crossref] [PubMed]
- Kikuta H, Taguchi Y, Tomizawa K, et al. Epstein-Barr virus genome-positive T lymphocytes in a boy with chronic active EBV infection associated with Kawasaki-like disease. Nature 1988;333:455-7. [Crossref] [PubMed]
- Ishihara S, Tawa A, Yumura-Yagi K, et al. Clonal T-cell lymphoproliferation containing Epstein-Barr (EB) virus DNA in a patient with chronic active EB virus infection. Jpn J Cancer Res 1989;80:99-101. [Crossref] [PubMed]
- Kawa-Ha K, Ishihara S, Ninomiya T, et al. CD3-negative lymphoproliferative disease of granular lymphocytes containing Epstein-Barr viral DNA. J Clin Invest 1989;84:51-5. [Crossref] [PubMed]
- Quintanilla-Martinez L, Ko YH, Kimura H, et al. EBV-positive T-cell and NK-cell lymphoproliferative diseases of childhood. In: Swerdlow SH, Campo E, Harris NL, et al. editors. WHO classification of tumors of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press, 2017:355-63.
- Kawa K, Okamura T, Yasui M, et al. Allogeneic hematopoietic stem cell transplantation for Epstein-Barr virus-associated T/NK-cell lymphoproliferative disease. Crit Rev Oncol Hematol 2002;44:251-7. [Crossref] [PubMed]
- Yonese I, Sakashita C, Imadome KI, et al. Nationwide survey of systemic chronic active EBV infection in Japan in accordance with the new WHO classification. Blood Adv 2020;4:2918-26. [Crossref] [PubMed]
- Okano M, Kawa K, Kimura H, et al. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol 2005;80:64-9. [Crossref] [PubMed]
- Sawada A, Inoue M. Pathology and treatment of chronic active Epstein-Barr virus infection. Journal of Hematopoietic Cell Transplantation 2014;3:1-11. [Crossref]
- Kimura H, Miyake K, Yamauchi Y, et al. Identification of Epstein-Barr virus (EBV)-infected lymphocyte subtypes by flow cytometric in situ hybridization in EBV-associated lymphoproliferative diseases. J Infect Dis 2009;200:1078-87. [Crossref] [PubMed]
- Fournier B, Boutboul D, Bruneau J, et al. Rapid identification and characterization of infected cells in blood during chronic active Epstein-Barr virus infection. J Exp Med 2020;217:e20192262 [Crossref] [PubMed]
- Sawada A, Inoue M, Kawa K. How we treat chronic active Epstein-Barr virus infection. Int J Hematol 2017;105:406-18. [Crossref] [PubMed]
- Suzuki K, Ohshima K, Karube K, et al. Clinicopathological states of Epstein-Barr virus-associated T/NK-cell lymphoproliferative disorders (severe chronic active EBV infection) of children and young adults. Int J Oncol 2004;24:1165-74. [Crossref] [PubMed]
- Ohshima K, Kimura H, Yoshino T, et al. Proposed categorization of pathological states of EBV-associated T/natural killer-cell lymphoproliferative disorder (LPD) in children and young adults: overlap with chronic active EBV infection and infantile fulminant EBV T-LPD. Pathol Int 2008;58:209-17. [Crossref] [PubMed]
- Ohtsuka R, Abe Y, Sada E, et al. Adult patient with Epstein-Barr virus (EBV)-associated lymphoproliferative disorder: chronic active EBV infection or de novo extranodal natural killer (NK)/T-cell lymphoma, nasal type? Intern Med 2009;48:471-4. [Crossref] [PubMed]
- Takahashi E, Ohshima K, Kimura H, et al. Clinicopathological analysis of the age-related differences in patients with Epstein-Barr virus (EBV)-associated extranasal natural killer (NK)/T-cell lymphoma with reference to the relationship with aggressive NK cell leukaemia and chronic active EBV infection-associated lymphoproliferative disorders. Histopathology 2011;59:660-71. [Crossref] [PubMed]
- Isobe Y, Aritaka N, Setoguchi Y, et al. T/NK cell type chronic active Epstein-Barr virus disease in adults: an underlying condition for Epstein-Barr virus-associated T/NK-cell lymphoma. J Clin Pathol 2012;65:278-82. [Crossref] [PubMed]
- Kawa K, Ishihara S, Okamura T, et al. Chronic active Epstein-Barr virus infection and lymphoproliferative diseases. Gann Monograph on Cancer Research 1998;45:139-47.
- Ishida F. Aggressive NK-cell leukemia. Front Pediatr 2018;6:292. [Crossref] [PubMed]
- Tanita K, Hoshino A, Imadome KI, et al. Epstein-Barr virus-associated γδ T-cell lymphoproliferative disorder associated with hypomorphic IL2RG mutation. Front Pediatr 2019;7:15. [Crossref] [PubMed]
- Ishimura M, Eguchi K, Shiraishi A, et al. Systemic Epstein-Barr virus-positive T/NK lymphoproliferative diseases with SH2D1A/XIAP hypomorphic gene variants. Front Pediatr 2019;7:183. [Crossref] [PubMed]
- Ishihara S, Ohshima K, Tokura Y, et al. Hypersensitivity to mosquito bites conceals clonal lymphoproliferation of Epstein-Barr viral DNA-positive natural killer cells. Jpn J Cancer Res 1997;88:82-7. [Crossref] [PubMed]
- Ishihara S, Okada S, Wakiguchi H, et al. Clonal lymphoproliferation following chronic active Epstein-Barr virus infection and hypersensitivity to mosquito bites. Am J Hematol 1997;54:276-81. [Crossref] [PubMed]
- Hirai Y, Yamamoto T, Kimura H, et al. Hydroa vacciniforme is associated with increased numbers of Epstein-Barr virus-infected γδT cells. J Invest Dermatol 2012;132:1401-8. [Crossref] [PubMed]
- Miyake T, Yamamoto T, Hirai Y, et al. Survival rates and prognostic factors of Epstein-Barr virus-associated hydroa vacciniforme and hypersensitivity to mosquito bites. Br J Dermatol 2015;172:56-63. [Crossref] [PubMed]
- Iwatsuki K, Miyake T, Hirai Y, et al. Hydroa vacciniforme: a distinctive form of Epstein-Barr virus-associated T-cell lymphoproliferative disorders. Eur J Dermatol 2019;29:21-8. [PubMed]
- Miyake T, Iwatsuki K, Hirai Y, et al. The aim of the measurement of Epstein-Barr virus DNA in hydroa vacciniforme and hypersensitivity to mosquito bites. J Med Virol 2020; Epub ahead of print. [Crossref] [PubMed]
- Ohga S, Ishimura M, Yoshimoto G, et al. Clonal origin of Epstein-Barr virus (EBV)-infected T/NK-cell subpopulations in EBV-positive T/NK-cell lymphoproliferative disorders of childhood. J Clin Virol 2011;51:31-7. [Crossref] [PubMed]
- Okuno Y, Murata T, Sato Y, et al. Defective Epstein-Barr virus in chronic active infection and haematological malignancy. Nat Microbiol 2019;4:404-13. [Crossref] [PubMed]
- Arai A, Imadome KI, Watanabe Y, et al. Clinical features of adult-onset chronic active Epstein-Barr virus infection: a retrospective analysis. Int J Hematol 2011;93:602-9. [Crossref] [PubMed]
- Kawamoto K, Miyoshi H, Suzuki T, et al. A distinct subtype of Epstein-Barr virus-positive T/NK-cell lymphoproliferative disorder: adult patients with chronic active Epstein-Barr virus infection-like features. Haematologica 2018;103:1018-28. [Crossref] [PubMed]
- Kasahara Y, Yachie A, Takei K, et al. Differential cellular targets of Epstein-Barr virus (EBV) infection between acute EBV-associated hemophagocytic lymphohistiocytosis and chronic active EBV infection. Blood. 2001;98:1882-8. [Crossref] [PubMed]
- Trempat P, Tabiasco J, Andre P, et al. Evidence for early infection of nonneoplastic natural killer cells by Epstein-Barr virus. J Virol 2002;76:11139-42. [Crossref] [PubMed]
- Isobe Y, Sugimoto K, Yang L, et al. Epstein-Barr virus infection of human natural killer cell lines and peripheral blood natural killer cells. Cancer Res 2004;64:2167-74. [Crossref] [PubMed]
- Sawada A, Inoue M. Hematopoietic stem cell transplantation for the treatment of Epstein-Barr virus-associated T- or NK-cell lymphoproliferative diseases and associated disorders. Front Pediatr 2018;6:334. [Crossref] [PubMed]
- Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015;126:9-16. [Crossref] [PubMed]
- Kawa-Ha K, Franco E, Doi S, et al. Successful treatment of chronic active Epstein-Barr virus infection with recombinant interleukin-2. Lancet 1987;1:154. [Crossref] [PubMed]
- Okamura T, Hatsukawa Y, Arai H, et al. Blood stem-cell transplantation for chronic active Epstein-Barr virus with lymphoproliferation. Lancet 2000;356:223-4. [Crossref] [PubMed]
- Kawa K, Sawada A, Sato M, et al. Excellent outcome of allogeneic hematopoietic SCT with reduced-intensity conditioning for the treatment of chronic active EBV infection. Bone Marrow Transplant 2011;46:77-83. [Crossref] [PubMed]
- Watanabe S, Okada M, Tokugawa T, et al. Recurrence of chronic active Epstein-Barr virus infection presenting with myelopathy after umbilical cord blood transplantation. Rinsho Shinkeigaku 2014;54:809-13. [Crossref] [PubMed]
- Shimizu M, Sawada A, Yamada K, et al. Encouraging results of preserving ovarian function after allo-HSCT with RIC. Bone Marrow Transplant 2012;47:141-2. [Crossref] [PubMed]
- Sawada A, Inoue M, Koyama-Sato M, et al. Umbilical cord blood as an alternative source of reduced-intensity hematopoietic stem cell transplantation for chronic Epstein-Barr virus-associated T or natural killer cell lymphoproliferative diseases. Biol Blood Marrow Transplant 2014;20:214-21. [Crossref] [PubMed]
- Mayumi A, Sawada A, Sato M, et al. Impact of melphalan dose during reduced-intensity conditioning on engraftment of cord blood transplantation for chronic Epstein-Barr virus-associated T or NK cell lymphoproliferative diseases. Pediatr Blood Cancer 2020;67:e28536 [Crossref] [PubMed]
- Suma S, Kurita N, Baba N, et al. Fatal exacerbations of chronic active Epstein-Barr virus infection subsequent to cytotoxic chemotherapy. Rinsho Ketsueki 2019;60:286-90. [PubMed]
- Marsh RA, Vaughn G, Kim MO, et al. Reduced-intensity conditioning significantly improves survival of patients with hemophagocytic lymphohistiocytosis undergoing allogeneic hematopoietic cell transplantation. Blood 2010;116:5824-31. [Crossref] [PubMed]
- Warlick ED, Cioc A, Defor T, et al. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant 2009;15:30-8. [Crossref] [PubMed]
- Kikuta H, Sakiyama Y. Etoposide (VP-16) inhibits Epstein-Barr virus determined nuclear antigen (EBNA) synthesis. Br J Haematol 1995;90:971-3. [Crossref] [PubMed]
- Johnson TS, Terrell CE, Millen SH, et al. Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis. J Immunol 2014;192:84-91. [Crossref] [PubMed]
- Trottestam H, Horne A, Aricò M, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood 2011;118:4577-84. [Crossref] [PubMed]
- Bergsten E, Horne A, Aricó M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood 2017;130:2728-38. [Crossref] [PubMed]
- Koyama M, Higuchi B, Kim JY, et al. Effectiveness of multi-drug chemotherapy on chronic active Epstein-Barr virus infection. Hematology Oncology 2004;49:415-6.
- Schluns KS, Kieper WC, Jameson SC, et al. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol 2000;1:426-32. [Crossref] [PubMed]
- Zhai L, Guo C, Cao Y, et al. Long-term results of pirarubicin versus doxorubicin in combination chemotherapy for aggressive non-Hodgkin's lymphoma: single center, 15-year experience. Int J Hematol 2010;91:78-86. [Crossref] [PubMed]
- Zheng S, Zhou S, Qiao G, et al. Pirarubicin-based chemotherapy displayed better clinical outcomes and lower toxicity than did doxorubicin-based chemotherapy in the treatment of non-metastatic extremity osteosarcoma. Am J Cancer Res 2014;5:411-22. [PubMed]
- Nam YS, Im KI, Kim N, et al. Down-regulation of intracellular reactive oxygen species attenuates P-glycoprotein-associated chemoresistance in Epstein-Barr virus-positive NK/T-cell lymphoma. Am J Transl Res 2019;11:1359-73. [PubMed]
- Yoshimori M, Takada H, Imadome K, et al. P-glycoprotein is expressed and causes resistance to chemotherapy in EBV-positive T-cell lymphoproliferative diseases. Cancer Med 2015;4:1494-504. [Crossref] [PubMed]
- Trambas C, Wang Z, Cianfriglia M, et al. Evidence that natural killer cells express mini P-glycoproteins but not classic 170 kDa P-glycoprotein. Br J Haematol 2001;114:177-84. [Crossref] [PubMed]
- Nagafuji K, Fujisaki T, Arima F, et al. L-asparaginase induced durable remission of relapsed nasal NK/T-cell lymphoma after autologous peripheral blood stem cell transplantation. Int J Hematol 2001;74:447-50. [Crossref] [PubMed]
- Koyama M, Takeshita Y, Sakata A, et al. Cytotoxic chemotherapy successfully induces durable complete remission in 2 patients with mosquito allergy resulting from Epstein-Barr virus-associated T-/natural killer cell lymphoproliferative disease. Int J Hematol 2005;82:437-40. [Crossref] [PubMed]
- Tsurumi H, Miura T, Yamada T, et al. Continuous infusion therapy with low dose cytosine arabinoside and etoposide in acute myelogenous leukemia patients hardly tolerable for intensive combination chemotherapy. Rinsho Ketsueki 1995;36:657-64. [PubMed]
- Ishihara T, Yasui M, Nakayama K, et al. Efficacy of continuous chemotherapy consisting of low dose etoposide and cytarabine in children. Japanese Journal of Pediatric Hematology 2008;22:347-53.
- Singhal S, Powles R, Treleaven J, et al. Melphalan alone prior to allogeneic bone marrow transplantation from HLA-identical sibling donors for hematologic malignancies: alloengraftment with potential preservation of fertility in women. Bone Marrow Transplant 1996;18:1049-55. [PubMed]
- Kobayashi R, Tanaka J, Hashino S, et al. Etoposide-containing conditioning regimen reduces the occurrence of hemophagocytic lymphohistiocytosis after SCT. Bone Marrow Transplant 2014;49:254-7. [Crossref] [PubMed]
- Arai A, Sakashita C, Hirose C, et al. Hematopoietic stem cell transplantation for adults with EBV-positive T- or NK-cell lymphoproliferative disorders: efficacy and predictive markers. Bone Marrow Transplant 2016;51:879-82. [Crossref] [PubMed]
- Oshimi K, Kawa K, Nakamura S, et al. NK-cell neoplasms in Japan. Hematology 2005;10:237-45. [Crossref] [PubMed]
- Hamadani M, Kanate AS, DiGilio A, et al. Allogeneic hematopoietic cell transplantation for aggressive NK cell leukemia. a center for international blood and marrow transplant research analysis. Biol Blood Marrow Transplant 2017;23:853-6. [Crossref] [PubMed]
- Onozawa E, Shibayama H, Takada H, et al. STAT3 is constitutively activated in chronic active Epstein-Barr virus infection and can be a therapeutic target. Oncotarget 2018;9:31077-89. [Crossref] [PubMed]
- Jin Z, Wang Y, Wang J, et al. Long-term survival benefit of ruxolitinib in a patient with relapsed refractory chronic active Epstein-Barr virus. Ann Hematol 2019;98:2003-4. [Crossref] [PubMed]
- Wei A, Ma H, Li Z, et al. Short-term effectiveness of ruxolitinib in the treatment of recurrent or refractory hemophagocytic lymphohistiocytosis in children. Int J Hematol 2020;112:568-76. [Crossref] [PubMed]
- Vallurupalli M, Berliner N. Emapalumab for the treatment of relapsed/refractory hemophagocytic lymphohistiocytosis. Blood 2019;134:1783-6. [Crossref] [PubMed]
- Fu DX, Tanhehco YC, Chen J, et al. Virus-associated tumor imaging by induction of viral gene expression. Clin Cancer Res 2007;13:1453-8. [Crossref] [PubMed]
- Bollard CM, Cohen JI. How I treat T cell chronic active Epstein barr virus disease. Blood 2018;131:2899-905. [Crossref] [PubMed]
- Hui KF, Cheung AK, Choi CK, et al. Inhibition of class I histone deacetylases by romidepsin potently induces Epstein-Barr virus lytic cycle and mediates enhanced cell death with ganciclovir. Int J Cancer 2016;138:125-36. [Crossref] [PubMed]
- Kim SJ, Kim JH, Ki CS, et al. Epstein-Barr virus reactivation in extranodal natural killer/T-cell lymphoma patients: a previously unrecognized serious adverse event in a pilot study with romidepsin. Ann Oncol 2016;27:508-13. [Crossref] [PubMed]
- Kim SJ, Kim WS. Reply to the letter to the editor 'Epstein-Barr virus reactivation in extranodal natural killer/T-cell lymphoma patients: a previously unrecognized serious adverse event in a pilot study with romidepsin, histone deacetylase (HDAC) inhibitors when combined with a proteasome inhibitor are safe and effective in patients with extranodal natural killer/T-cell lymphoma' by Tan et al. Ann Oncol 2016;27:2133-4. [Crossref] [PubMed]
- Wang Q, Liu H, Zhang X, et al. High doses of mother's lymphocyte infusion to treat EBV-positive T-cell lymphoproliferative disorders in childhood. Blood 2010;116:5941-7. [Crossref] [PubMed]
- Savoldo B, Huls MH, Liu Z, et al. Autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells for the treatment of persistent active EBV infection. Blood 2002;100:4059-66. [Crossref] [PubMed]
- Bollard CM, Gottschalk S, Torrano V, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol 2014;32:798-808. [Crossref] [PubMed]
- Long HM, Chagoury OL, Leese AM, et al. MHC II tetramers visualize human CD4+ T cell responses to Epstein-Barr virus infection and demonstrate atypical kinetics of the nuclear antigen EBNA1response. J Exp Med 2013;210:933-49. [Crossref] [PubMed]
- Kwong YL, Chan TSY, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 2017;129:2437-42. [Crossref] [PubMed]
- Liu P, Pan X, Chen C, et al. Nivolumab treatment of relapsed/refractory Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in adults. Blood 2020;135:826-33. [Crossref] [PubMed]
Cite this article as: Sawada A, Inoue M. Narrative review of chronic active EBV infection—advances in clinical management. Ann Lymphoma 2021;5:7.


