
Older patients with mantle cell lymphoma: initial and subsequent therapies—a narrative review
Introduction
Mantle cell lymphoma (MCL) is a rare subtype of B cell lymphoma and accounts for 3–10% of the non-Hodgkin lymphoma (NHL) (1). It is characterized by translocation (11:14) which leads to overexpression of cyclin D1 both of which aid with identification of this disease at diagnosis. The median age at diagnosis is 68–71 years and the incidence increases with age (2). More than 80% of patients present with advanced-stage disease with involvement of multiple lymph nodes, bone marrow, gastrointestinal tract, and blood (3). MCL is not curable and initial therapy usually involves high-dose chemoimmunotherapy (CIT) followed by autologous stem cell transplant in younger patients. This approach has helped to improve progression-free survival (PFS) of the younger patients but there is still no firm data supporting that this modality improves the overall survival (OS) (4). The intensity and toxicities of the high-dose chemotherapy regimens are prohibitive for older/frail patients. Less intensive CIT regimens with or without maintenance strategies and the introduction of novel non-chemotherapy agents in the frontline setting have improved outcomes for older unfit patients. There is no clear age cut off for elderly patients diagnosed with MCL and fitness is sometimes the biggest driver of treatment decisions vs. chronological age. Several review studies in the past have described data of using CIT, followed by maintenance therapy in elderly patients (5,6). With the increasing understanding of the disease biology and introduction of newer agents, the treatment landscape of MCL is transforming. Recent studies have looked at introducing Bruton tyrosine kinase inhibitors (BTKis) in combination with CIT, chemotherapy free regimen in first-line setting (7,8). This review aims to give an overview of treating older patients with MCL in the era of newer target therapeutics. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aol.amegroups.com/article/view/10.21037/aol-22-8/rc).
Disease classification
The World Health Organization in 2016 classified MCL into classical MCL and leukemic nonnodal MCL based on their clinical presentation. Classical MCL is thought to derive from naïve pre-germinal B cells. Classical MCL is the most common variant that commonly presents with nodal involvement, has a heterogenous clinical course, unmutated immunoglobulin heavy-chain variable region (IGHV), and overexpression of SOX-11. Nonnodal leukemic MCL is thought to develop from the germinal center B-cells, that harbor somatic hypermutation of IGHV, lack of expression of SOX-11, have fewer genomic alterations. Patients with nonnodal MCL typically as the name indicates present with peripheral blood, spleen, and bone marrow involvement and have indolent course at presentation (9).
Methods
This narrative review was intended to include older patients with MCL. The analysis was performed in the Medline and Ovid database were searched for studies on older MCL patients with keywords listed in the Table 1 below to include a wide area to ensure all relevant publications were identified. There was no specified time frame. All international peer-reviewed papers in English language including retrospective, observational, prospective, randomized, real world studies were included. No exclusion criteria. All the authors were involved in the selection and reviewing of the relevant publications.
Table 1
| Items | Specification |
|---|---|
| Date of search | Search conducted between March 2022–June 2022 |
| Databases and other sources searched | Medline and Ovid |
| Search terms used | Mantle cell lymphoma, therapy, treatment, management, old, elder, geriatric |
| Timeframe | None specified |
| Inclusion and exclusion criteria | Included international peer-reviewed papers in the English language |
| Selection process | All the authors were involved in the selection and reviewing of the relevant publications |
Current treatment strategies
Indolent MCL
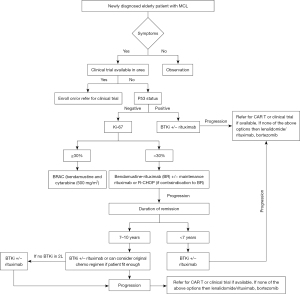
The majority of patients with MCL are symptomatic at presentation. However, 10–20% can be asymptomatic and include nonnodal leukemic phase patients and nodal MCL with no B symptoms, non-bulky disease with normal lactate dehydrogenase (LDH), low Ki-67% and good performance status (10). In selected asymptomatic patients, treatment could be deferred (11). A multicenter, phase II study conducted in Spain evaluated ibrutinib with rituximab (IR) in patients with untreated indolent MCL. These patients were characterized by no disease related symptoms, nonblastoid by morphology, Ki-67 <30%, and tumor size <3 cm. Fifty patients were enrolled and after 12 cycles of treatment, the overall response rate (ORR) was 84% including 80% with complete response (CR). At 2 years, 24 patients discontinued ibrutinib with undetectable minimal residual disease (MRD), four patients had disease progression (12). This study adds to the growing evidence of BTKi in front line MCL. Our treatment approach to elderly patients with MCL is summarized in Figure 1.
Treatment naïve symptomatic patients
Chemoimmunotherapy
(I) CHOP-based regimen
A randomized trial by European Mantle Cell Lymphoma Network randomly assigned 560 patients, 60 years or older with newly diagnosed stage II–IV MCL, who were ineligible for high-dose chemotherapy to receive 8 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) every 21 days or rituximab, fludarabine and cyclophosphamide (R-FC) every 28 days. There was a second randomization in patients who had response to either rituximab or interferon-alfa maintenance which was given until disease progression. The rate of progression was noted to be higher in the R-FC arm compared to the R-CHOP (14% vs. 5%) and the rate of infections causing death was also higher in the R-FC arm (13). After a median follow-up of 7.6 years, the OS was noted to be significantly higher in the R-CHOP (6.4 vs. 3.9 years). Patients who had responded to R-CHOP and randomly assigned to rituximab maintenance (MR) had median PFS of 5.4 years and OS of 9.8 years which were significantly higher compared to those randomized to interferon alfa at 1.9 and 7.1 years, respectively (14). This data suggested that R-CHOP with MR was an effective and tolerable option for elderly patients. Studies tried to improve on these outcomes by building on the R-CHOP backbone. LYM-3002 is a phase 3 trial that randomly assigned 487 adults who were not eligible for autologous transplant to receive 6–8 cycles of R-CHOP or VR-CAP (vincristine was replaced by bortezomib in R-CHOP regimen). More than half the patients were above 65 years, and 73% patients were over the age of 60. The median PFS was significantly higher with VR-CAP compared to R-CHOP (24.7 vs. 14.4 months). Higher rates of hematological toxicity were noted in the VR-CAP group, but the rates of peripheral neuropathy were similar (15). Long-term follow-up showed that after 82 months VR-CAP was associated with improved median OS (90.7 vs. 55.7 months). The role of maintenance rituximab was not analyzed in this study (16). Based on this study, VR-CAP was Food and Drug Administration (FDA)-approved to be used as a first-line treatment in transplant ineligible patients with MCL. The MCL R2 Elderly clinical trial tried to assess if the addition of lenalidomide to MR improved the PFS in elderly untreated patients with stage II–IV lymphoma, ineligible for transplant. A total of 624 untreated patients above the age of 60 years were randomized to the induction regimen of either 8 cycles of R-CHOP or 6 cycles of alternating R-CHOP every 21 days and R-HAD (rituximab, cytarabine, dexamethasone) every 28 days. Median age of the patients was 71 years, with 89% having stage IV disease. Out of 514 patients who achieved complete or partial response, 495 were randomized to maintenance rituximab every 2 months or R2 (lenalidomide 21/28 days plus rituximab) for 2 years. After median follow-up of 2.1 years from maintenance randomization, 2-year PFS was significantly prolonged at 76.6% in R2 arm compared to 60.8% in rituximab arm. OS was not different in both arms, but adverse events including grade 3 neutropenia, respiratory tract infection, skin cancer were noted in the R2 arm (17).
(II) Bendamustine and rituximab-based regimen
Bendamustine, a cytotoxic alkylating agent is effective as a monotherapy and in combination with rituximab for relapsed and refractory NHL with favorable safety profile (18,19). STiL was a prospective, open label study from Germany which assessed the efficacy of Bendamustine plus rituximab (BR) vs. R-CHOP as first-line treatment for indolent NHL and MCL. They enrolled 46 patients with stage II–IV MCL in BR arm and 48 in R-CHOP, median age was 70 years for MCL. After a median follow-up of 45 months, median PFS was significantly improved with BR, 35.4 months compared to 22.1 months in R-CHOP. BR arm experienced lower rates of hematological toxicity, infections, peripheral neuropathy, and alopecia (20). Similarly, an international phase III, BRIGHT study compared the efficacy of BR with R-CHOP or R-CVP in patients with MCL and NHL. Thirty-six patients were enrolled in BR arm and 38 in R-CHOP/R-CVP arm, median age was 60 years. BR showed improved CR rates 50% compared to 27% in the R-CHOP/R-CVP group (21). A follow-up report showed that the 5-year PFS, event-free survival, duration of response favored BR compared to R-CHOP/R-CVP. No OS benefit was noted. The incidence of second cancers were higher in the BR group. Forty-two patients in BR arm developed at least one secondary cancer including 22 patients who were diagnosed with secondary malignancy excluding NHL and nonmelanoma skin cancer (22). Based on these studies, BR has become the preferred regimen for transplant ineligible elderly patients with MCL. MR after BR induction has demonstrated differing outcomes in separate studies. A randomized study from Germany did not indicate a benefit to MR but this study is believed to be underpowered to detect a difference. A retrospective multicenter analysis by Karmali et al, showed that MR after frontline BR was associated with improved OS and PFS in elderly patients (23). Similarly, analysis of large cohort of patients treated in community setting showed that MR after BR for first-line treatment improved OS and real-world time to next treatment (24). MR currently is controversial in some respects but is supported by retrospective data suggesting clinical benefit.
Studies have explored augmenting the BR regimen with additional agents to improve the outcomes. High-dose cytarabine is an effective treatment of MCL in patients younger than 65 years, but is associated with high-grade hematological toxicity, febrile neutropenia, and renal toxicity (25). A phase 2 trial assessed the safety and efficacy of low dose cytarabine combined with BR as first-line treatment in patients 60–65 years and ineligible for stem cell transplant, or >65 years and fit as per comprehensive geriatric assessment. All patients received RBAC 500 (BR with cytarabine 500 mg/m2 on days 2–4) every 28 days for 6 cycles. Fifty-seven patients were enrolled, median age of 71 years, 52 (91%) achieved CR. After a median follow-up of 35 months, PFS was 76%. At 2 years, OS was 86%, duration of response was 90%. Forty-one patients required dose reduction, only 38 patients completed 6 cycles. Neutropenia and thrombocytopenia were the most frequent grade 3–4 hematological toxicities (26). High risk patients (blastoid variant, high Ki-76 proliferative index, TP53 mutation/TP53 deletions) were noted to have high risk of progression with 2-year PFS of 40%. A phase 2 study is assessing the addition of Venetoclax for 2 years after R-BAC to high-risk patients to improve the 2-year PFS is in process (27).
Bortezomib was added to BR along with dexamethasone (RiBVD) in a phase 2, prospective trial for first-line therapy of older MCL patients. Bortezomib was given subcutaneously on days 1, 4, 8, and 11 at dose of 1.3 mg/m2, dexamethasone 40 mg intravenously on day 2 along with BR for 6 cycles, at 28 days interval. The study enrolled 74 patients with median age of 73 years. The ORR was 84% and CR was 75.5% at the end of treatment with 2-year PFS of 70%. Molecular responses were evaluated by quantitative polymerase chain reaction targeted to patient specific, immunoglobulin heavy chain (IGH) V(D)J clono-specific rearrangements, to quantify tumor B cells. MRD analysis was performed on 54 patients who were eligible after completion of the treatment. The 4-year OS was noted to be significantly higher in patients who had undetectable MRD compared to patients who had detectable MRD (86.6% vs. 28.6%). Grade 3/4 toxicities included neuropathy in 15% and hematological toxicities (28). E1411 is a phase 2 study that evaluated the efficacy of adding Bortezomib to BR (BVR) compared to BR in untreated MCL. The induction therapy was followed by second randomization to rituximab alone or lenalidomide and rituximab combination for consolidation. The study enrolled 179 patients in BVR group and 180 in BR group with median age of 67 years. The PFS, ORR were similar in both arms. Grade >3 neutropenia and peripheral neuropathy was greater in BVR arm compared to BR (29). Follow-up is ongoing for the consolidation phase.
Lenalidomide was added to BR as for elderly patients with untreated stage II–IV MCL. Patients received Lenalidomide 10 mg from days 1–14 for first 6 cycles along with BR followed by single agent lenalidomide in days 1–21 from cycle 7–13. Fifty-one patients were enrolled, and after 6 cycles, CR was 64%. Median PFS was 42 months, and 3-year OS was noted to be 73%, however the regimen was associated with infection in 42% of patients and second primary cancer in 16%. One Hodgkin lymphoma, 1 renal cancer, 1 squamous lung cancer, 1 hepatocellular carcinoma, 1 prostate cancer and 2 noninvasive nonmelanoma skin cancer (30).
(III) Combination of BR with BTKis
A phase 3, double blind study investigated the safety and efficacy of adding Ibrutinib to the chemotherapy backbone of BR in patients above the age of 65 years as a first-line therapy. The study enrolled 523 patients with 262 patients to either BR + placebo vs. 261 patients to BR combined with Ibrutinib (8) [SHINE study (NCT01776840)]. All patients received 6 cycles of BR, and patients who achieved CR/partial response (PR) would receive MR for 2 years. All patients would receive either 560 mg of Ibrutinib orally or placebo along with BR and continue until disease progression or toxicity develops. At the end of median follow-up of 87.4 months, the PFS was significantly better in BR + Ibrutinib arm at 80.6 months compared to 50.2 months in BR + placebo arm. No difference was noted in the OS.
- Ongoing studies of BR with BTKi: ACE-LY-308 is a similar ongoing study to assess the efficacy of combining acalabrutinib to BR vs. BR + placebo for treatment naïve elderly MCL patients. Acalabrutinib is administered at a dose of 100 mg twice daily along with BR every 28 days in the experimental arm (NCT02972840). This study will allow patients who progression after BR to cross over to receive single agent acalabrutinib.
- Ongoing studies of BR with Venetoclax: a phase 2 single arm study, PrE0405, which is evaluating the addition of Venetoclax at 400 mg for 10 days along with BR every 28 days for 6 cycles in elderly patients with MCL as first-line therapy. MR will be at the discretion of the investigator and the primary outcome is CR rate assessed by the Lugano criteria, along with PFS and OS. The trial is currently recruiting (NCT03834688).
Chemotherapy-free initial therapy
The development of the novel agents has revolutionized the treatment of older patients with MCL and can be safely delivered in the outpatient setting. Several phase 1/2 studies have demonstrated the efficacy of the chemotherapy free regimens in first-line setting and larger studies are ongoing (31-34).
(I) Lenalidomide with rituximab in first-line therapy
An open label, single arm, multi-center phase 2 studied evaluated the efficacy of the lenalidomide combined with rituximab in untreated MCL patients. Lenalidomide was given at dose of 20 mg daily from days 1–21 every 28 days with dose escalation to 25 mg from second cycle if there were no dose limiting toxicities for 12 cycles. Rituximab was given weekly for 4 weeks and then every other cycle for a total of nine doses. MR was given every 8 weeks and lenalidomide was given at a dose of 15 mg daily on days 1–21 of every 28 cycles for 36 cycles or until disease progression or unacceptable toxicity. The study enrolled 38 patients with a median age of 65. There were no patients with blastoid or pleomorphic features. After a median follow-up of 30 months, among all patients who could be evaluated, the ORR was impressive at 92% and CR rate was 64%. The response to treatment improved over time with median time to partial response of 3 months and CR of 11 months. Grade 3 or 4 neutropenia was seen in 50% of patients, thrombocytopenia in 11% and anemia in 11%. Grade 3 or 4 rash was noted in 29%, tumor flare in 11%. Five patients developed noninvasive skin cancer and 2 patients had invasive cancer (35). At a median follow-up of 64 months, patients continued to show durable response with 5-year PFS of 64% and OS of 77% (36,37). Twelve of the 36 evaluable patients remain in remission at 7 years of follow-up on study.
A phase Ib study evaluated the safety of addition of Venetoclax to rituximab and lenalidomide (R2 + V) in adult patients with MCL as first-line treatment. The induction phase consisted of rituximab given weekly for cycle 1, followed by day 1 of every other even cycle, lenalidomide given at dose of 20 mg on days 1–21 of 28-day cycle and Venetoclax starting on day 8, escalated over 4 weeks to dose of 400 mg. After 12 months of induction, patients started maintenance phase, rituximab every 8 weeks for 36 months, lenalidomide 10 mg for 24 months and Venetoclax for 12 months. 28 patients were enrolled in the study, and in evaluable patients, PR was 96%, with CR rates of 89%. Grade 3 adverse events were reported in 93% patients, with >50% of patients experiencing grade 3 or 4 neutropenia, and thrombocytopenia (NCT03523975) (38).
A phase 2 study evaluating the addition of second generation BTKi acalabrutinib to lenalidomide and rituximab is untreated MCL is currently ongoing. The study includes 12 cycles of induction phase, followed by maintenance phase in responding patients (NCT03863184).
(II) BTKi with rituximab
A phase 2, single center trial assessed the efficacy and safety of chemotherapy-free regimen of IR in elderly patients above age 65 for first-line treatment of MCL. Patients with blastoid or pleomorphic history and Ki-67% of >50% were excluded from the study. Rituximab was given weekly for 4 weeks in cycle 1, followed by every month from cycles 3–9. From cycle 9, rituximab was given every 2 months for 2 years. Ibrutinib was given at 560 mg once along with rituximab and after 2 years was given alone until disease progression or unacceptable adverse events. Fifty patients were enrolled with a median age of 71 years. The ORR of the combination was 96%. After median follow-up of 45 months, 3-year PFS and OS was 87% and 94% respectively. It was noted that patients who achieved CR had significantly longer PFS and patients with high Ki-67% had a trend towards higher risk of disease progression or death. Twenty-one patients came off study because of intolerance, 10 due to atrial fibrillation, 3 due to bleeding and 8 due to other grade 3 toxicities. Given the adverse event profile, patients with cardiac comorbidities need to be selected carefully before initiating treatment with IR (7). A phase 2 study is evaluating acalabrutinib with rituximab is untreated elderly patients, is currently enrolling. We will need to see if the safety profile of acalabrutinib allows for a decrease in the risk of discontinuation from cardiac toxicities (NCT04765111).
As mentioned above a study conducted by Spanish Lymphoma Group treated asymptomatic indolent patients with MCL. Treatment consisted of rituximab given weekly for 4 doses followed day 1 of cycle 3, 5, 7 and 9. Ibrutinib was given at 560 mg daily and could be discontinued after 2 years if patients had sustained undetectable MRD. Fifty-five patients with median age of 65 years were included. Eighty-four percent of patients had ORR with 40% showing CR after 12 cycles of treatment. Eighty-seven percent of patients had achieved undetectable MRD and at 2 years 24 of 35 evaluable patients discontinued ibrutinib due to sustained negative MRD. The median OS was noted to be significantly lower in the TP53-mutated cases (12). This time limited treatment based on MRD results in indolent MCL needs to be studied in larger prospective trials. A randomized phase 2/3 study is ongoing in UK to compare IR against conventional CIT in elderly MCL patients [ENRICH trial (2015-000832-13)]. A phase 3 multicenter study is comparing Zanubrutinib combined with rituximab against BR as first line in MCL patients who are ineligible for autologous transplant and is actively recruiting (NCT04002297).
A single arm, multicenter, phase 1/2 prospective trial (Oasis), studied Venetoclax in combination with fixed doses of ibrutinib and obinutuzumab in a cohort of 15 untreated patients with MCL with median age of 65 years. After a median follow-up of 14 months, patients had high response rates with 1-year PFS of 93.3% and CR rate of 86.6% after cycle 6. More than 50% of the patients experienced grade 3/4 adverse events (34). Similarly, a multicenter, open label study phase 1b study evaluated the efficacy and safety of acalabrutinib, Venetoclax, and rituximab as first-line therapy in 21 patients with MCL. After 6 cycles, ORR was 100% including CR in 90% of patients, with median duration of response of 19 months. The 1-year PFS rates were 89% and OS rates 95%, with infections, neutropenia, hemorrhage, cardiac events being the common adverse events (39). Table 2 summarizes the major frontline studies that have included patients above the age of 60 years.
Table 2
| Regimen | Patients (above age 65 years), n | Median age, years | ORR | CR | PFS | OS | Reference |
|---|---|---|---|---|---|---|---|
| R-CHOP | 280 | 70 | 86% | 34% | mPFS: 5.4 y | mOS: 6.4 y | (14) |
| VR-CAP | 243 | 65 | 92% | 53% | mPFS: 25 m | mOS: 90.7 m | (15) |
| BR | 46 | 70 | 93% | 40% | mPFS: 35 m | – | (20) |
| BR | 36 | 60 | 94% | 50% | 5-y PFS: 40% | 5-y OS: 59% | (21) |
| R-BAC | 57 | 71 | 91% | 91% | 3-y PFS: 76% | 2-y OS: 86% | (26) |
| RiBVD | 74 | 73 | 84% | 75.5% | 4-y PFS: 58% | 4-y OS: 71% | (28) |
| BR + Bortezomib | 179 | 67 | 89% | 66% | mPFS: 5.3 y | – | (29) |
| BR + Lenalidomide | 51 | 71 | 80% | 64% | mPFS: 42 m | 3-y OS: 73% | (30) |
| BR + Ibrutinib | 261 | 71 | 90% | 66% | mPFS: 6.7 y | 7-y OS: 55% | (8) |
| R2 | 38 | 65 | 92% | 64% | 5-y PFS: 64% | 5-y OS: 77% | (36) |
| IR | 50 | 71 | 96% | 68% | 3-y PFS: 87% | 3-y OS: 94% | (7) |
BR, bendamustine, rituximab; CR, complete response; IR, ibrutinib, rituximab; ORR, overall response rate; PFS progression-free survival; mPFS, median PFS; OS, overall survival; mOS, median OS; R2, rituximab, lenalidomide; R-BAC, rituximab, bendamustine, and cytarabine; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; VR-CAP, bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone; RiBVD, bortezomib, dexamethasone, bendamustine, rituximab; m, months; y, years.
Relapsed-refractory MCL
BTKis
BTK is a non-receptor kinase that plays a key role in the activation of the B cell receptor signaling which is important for the proliferation and survival of the cell (40). There are three BTKi approved for the treatment of relapsed or refractory (R/R) MCL (5). In the R/R setting BTKi have improved outcomes, and unique toxicity profile compared to conventional CIT in older adults (41-43).
Ibrutinib is a covalent, irreversible BTKi, that has interaction with other TEC kinases besides BTK leading to toxicities like atrial fibrillation, rash, bleeding (44). A phase 2 international studied oral ibrutinib at dose of 560 mg until disease progression or intolerance in patients with R/R MCL. A total of 111 patients were enrolled with median age of 68 years and had received median of 3 prior therapies. Sixty-eight percent of the patients had ORR with CR in 21% (45). An update of the study showed that Ibrutinib has durable response. The 2-year PFS and OS were 31% and 47% respectively, 22% of the patients were treated for >2 years. The grade 3 or higher adverse effects were pneumonia, urinary tract infections, cellulitis and >2% of patients experienced grade 3 or higher bleeding events (42). A phase 3 randomized study compared the efficacy of ibrutinib with temsirolimus in R/R MCL. Ibrutinib had significantly better median PFS of 14.6 months compared to temsirolimus at 6.2 months and was also noted to be better tolerated (46). A follow-up study after a median follow-up of 38.7 months, ibrutinib showed a trend towards improved OS (47). Three-point-five years follow-up of pooled analysis revealed durable response with median PFS of 12.5 months and median OS of 26.7 months. Patients who received ibrutinib in second line had better PFS and OS compared to those who received in later lines. Serious adverse events included pneumonia and atrial fibrillation (48).
Ibrutinib was studied in combination with rituximab in relapsed MCL setting in a single center, phase 2 study. Fifty patients with median age of 67 years were enrolled. Eighty-eight percent of the patients achieved ORR with 44% having CR Jeny(49). After a median follow-up on 47 months, median PFS was 43 months and OS was note reached. Fifty-eight percent of the patients achieved CR and 12 patients continued on the study. Common grade 3 adverse events on long-term follow-up included infection, atrial fibrillation. Patients with high-risk disease, i.e., high Mantle Cell Lymphoma Prognostic Index (MIPI) score, high Ki-67%, blastoid morphology had poor survival (50). PHILEMON was a multicenter phase 2 study that evaluated the addition of lenalidomide to ibrutinib and rituximab in R/R MCL. Fifty patients were enrolled, median age of 69.5 years and received a median of 2 prior treatments. At a median follow-up of 17.8 months, 76% had ORR, including 56% who had CR. Grade 3 or higher toxicities included neutropenia in 38%, infection in 22% and cutaneous toxicity in 14% (51).
A phase 2 study studied addition of Venetoclax to ibrutinib in R/R MCL which was continued until disease progression or intolerance. Twenty-three patients with median age of 68 years were enrolled. Patients had high-risk disease features including alterations of TP53 (50%) and high-risk prognostic score by MIPI (75%). At 16 weeks, CR assessed by positron -emission tomography was 62% and ORR of 71%. Grade 3 or higher adverse events were noted in 71% patients and included diarrhea, neutropenia, thrombocytopenia, anemia (52). An ongoing phase 3 study SYMPATICO is evaluating the efficacy and safety of ibrutinib with Venetoclax vs. ibrutinib. ORR was 81% with 62% showing CR after a median follow-up of 31 months. Most adverse events were low grade, common grade 3 adverse effect included infections, diarrhea, neutropenia, atrial fibrillation, and hemorrhage (53). The Oasis study included an arm for R/R MCL patients and demonstrated a CR of 67% in this group (34).
Acalabrutinib is a second generation, potent, covalent BTKi that is more selective to BTK and thus has less off target activity (54). In chronic lymphocytic leukemia (CLL) studies, acalabrutinib had lower risk of atrial fibrillation, bleeding and cardiovascular events compared to ibrutinib (55). A phase 2 study of patients with R/R MCL with median age of 68 years who had received a median of 2 therapies were enrolled. Acalabrutinib was given 100 mg twice a day until disease progression or intolerable toxicity. Eighty-one percent of patients achieved ORR including 49% who had CR (43). After a median follow-up of 38.1 months, median PFS was 22 months and median OS was reached at 59.1 months. The most common side effects included headache, diarrhea, fatigue, cough, myalgia, and nausea. Atrial fibrillation, was seen in 2.4%, and hemorrhage in 37% and infections in 67.7% (56).
Zanubrutinib is a third covalent BTKi that has more selective off target inhibition compared to ibrutinib. A phase II, single arm study in China treated patients with R/R MCL with 160 mg twice daily of Zanubrutinib. Patients were excluded if they had prior exposure to BTKi. Eighty-six patients were included in the study and 25.6% were above the age of 65 years. Patients had median of 2 lines of prior therapy and after a median follow-up of 18.4 months, 84% patients achieved ORR and 68.6% had CR. Median duration of response was 19.5 months and median PFS was 22.1 months. The older patients (>65 years) were noted to have inferior outcomes and required two or more interruptions (41). A phase 1/2 multicenter study of single agent zanubrutinib enrolled patients with R/R B-cell malignancies. An analysis of 32 patients with R/R MCL patients was done. Twenty-four of the enrolled patients were above the age of 65. After a median follow-up 18.8 months, ORR was seen in 85% including 25% achieving a CR. A proportion of 59.4% patients experienced at least one adverse event higher than grade 3, anemia, pneumonia and myalgia were most common adverse event greater than grade 3 (57). A pooled analysis evaluated all 112 patients treated at recommended phase 2 dose. The patients had a median age of 61.5 years with a median PFS and OS of 25.8 and 38.2 months respectively. The common adverse effects included hypertension in 11.6%, major hemorrhage in 5.4% and atrial fibrillation/flutter in 1.8%. US FDA approved Zanubrutinib in 2018 for MCL patients who had received at least one prior therapy (58).
Pirtobrutinib is a non-covalent, highly selective BTKi that has the ability to inhibit both the wild type and C481 mutated BTK. BRUIN is a phase 1/2 study of oral pirtobrutinib in patients with advanced B cell malignancies which included 61 patients with MCL with median age of 69 years and median 3 prior lines of therapy including 90% treated with prior BTKi. Patients who were previously treated with a covalent BTKi had ORR of 51% and CR of 25%, while BTKi naïve patients had an ORR and CR of 82% and 18% respectively. Fatigue, diarrhea, and contusion were the most common adverse events and grade >3 neutropenia was seen in 10%. Responses were seen even in patients who previously had undergone transplantation or treated with chimeric antigens receptor (CAR) T cell therapy (47).
Venetoclax
Venetoclax is an oral, selective BCL2 inhibitor, that has activity in B cell malignancies. A phase 1 trial involving 28 relapsed MCL patients treated with median of 3 prior lines, demonstrated an ORR of 75% and CR in 21%, median PFS of 14 months in a BTKi naïve patient population. After a median follow-up of 38.5 months, the median duration of response was 15.7 months for MCL (59). Outcomes are not as promising for those previously exposed to a BTKi. A retrospective study from Europe on 20 R/R MCL patients, with median age of 69 years, who were treated with Venetoclax monotherapy after being treated with BTKi showed an ORR of 53%, median PFS of 3.2 months and median OS of 9.4 months. No cases of clinical tumor lysis were noted, and the drug was well-tolerated with grade 3 pneumonia, grade 4 sepsis and grade 2 fatigue, neutropenia and diarrhea being the common adverse events (60). Mechanisms of resistance were studied in 24 patients with R/R MCL who were treated with Venetoclax (12 as single agent, eight with obinutuzumab, three with BTKi with/without obinutuzumab and one with chemotherapy). Sixty-seven percent of the patients had progressed on BTKi and 92% were exposed to prior BTKi. Fifty percent had ORR including 21% with CR, and 29% with PR. ORR was not significantly different between single agent and combination. Median PFS was 8 months and median OS of 13.5 months, post Venetoclax survival was 7.3 months. The resistance to Venetoclax in this MCL population was mostly related to alterations other than BCL2 mutations (61).
Bortezomib
Bortezomib is a selective reversible proteosome inhibitor that has shown clinical activity in R/R B cell NHL as a single agent in a phase 2 study (62). PINNACLE trial evaluated 155 patients with R/R MCL, median number of prior therapies was one, and achieved an ORR of 33% including CR of 8%. The median duration of response was only 9.2 months with median time to progression being only 6.7 months (63). A phase 2 study combining bortezomib to Bendamustine and rituximab in patients with R/R B cell NHL and included 7 patients with MCL with a median of four prior lines of treatment was conducted. Addition of bortezomib to chemotherapy improved the ORR to 83% with 2-year PFS of 47%. Common adverse events included thrombocytopenia, neutropenia, nausea, neuropathy, fatigue, fever, and eight patients experienced serious adverse events (64).
Lenalidomide
Lenalidomide is an immunomodulatory agent that was approved as monotherapy in R/R MCL based on phase 2 studies with ORR ranging from 28–53% and median duration of response of 16.6 months (65-67). Efficacy of combination of rituximab with lenalidomide in R/R MCL was assessed in a phase 1/2 study. Fifty-seven percent patients had ORR and 35% had CR. The median PFS, duration of response, OS were 11.1 months, 18.9 months, and 24.3 months respectively. The combination was well tolerated (31). Lenalidomide based therapy has shown modest clinical activity in patients who had progressed on BTKi based on observational MCL -004 study. This study assessed 58 patients, with median age of 71 years who were treated with various lenalidomide based therapies, after being exposed to a BTKi. Patients had an ORR of 29% and median duration of response of 20 weeks (68). A phase 2 study evaluated lenalidomide and BR followed by consolidation with lenalidomide, and rituximab followed by lenalidomide maintenance. Forty-two patients with median age of 70 years were included and 55% achieved CR after the consolidation phase with median PFS of 20 months. Hematological toxicities were the most common adverse events during the induction and consolidation with grade 3/4 neutropenia noted in 72% even in the maintenance phase (69).
Chimeric antigen receptor
KTE-X19 is an autologous anti-CD19 CAR T-cell therapy approved in the US for the treatment of R/R MCL based on the ZUMA 2 trial. This phase 2 study was conducted across 20 sites in US and Europe in patients who had MCL that was either relapsed or refractory and had received up to five prior therapies. The median age of enrolled patients was 65 years, all of them had disease that was relapsed or refractory to BTKi and included high risk disease, 31% with blastoid, 82% with Ki-67 index >30%, intermediate or high risk MIPI in 56%. Sixty-eight patients with R/R MCL underwent leukapheresis and conditioning chemotherapy followed by infusion of KTE-X19. After a median follow-up of 28.8 months, the ORR was 91% with a CR of 68%. It was noted that patients with progression of disease within 24 months of initial diagnosis and treatment had lower PFS compared to patients without progression during this defined time period. Durable responses were noted, as 49% of patients who had obtained CR remained in CR, median duration of response was 25 months, median PFS was 25 months and OS was not reached. All patients had at least one adverse event and 99% of the patients had grade 3 or higher adverse events. The most common grade 3 or higher adverse events included cytopenias and infections. Grade 3 or higher cytokine release syndrome (CRS) and neurotoxicity seen in 15% and 31% respectively (70,71). A real-world retrospective analysis of 95 patient was reported from the US Lymphoma CAR T Consortium. Median age of the patients was 67 years and 73% of patients did not meet eligibility of ZUMA 2. At a median follow-up of 3 months, ORR was 86% with CR of 64%. Grade 3 or higher CRS and neurotoxicity were noted in 8% and 33% respectively but use of steroids and tocilizumab were more frequent (72,73) as compared to the clinical trial. Brexucabtagene autoleucel (BA) is currently approved to be given after CIT and BTKi.
Lisocabtagene maraleucel is a CD19 directed CAR with 4-1BB costimulatory domain and has demonstrated good preliminary efficacy and safety in R/R MCL in the ongoing Transcend NHL-001 phase 1 (74).
Emerging treatment options
Bispecific antibodies (BsAb)
BsAb are agents that can bind to an epitope on T cells and an epitope on the target tumor cell of interest, leading to activation of the immune response of the host. Agents that are being investigated include epcoritamab, mosunetuzumab with polatuzumab, odronextamab and glofitamab. Glofitamab is a T cell engaging BsAb with 2:1 configuration with bivalency for CD20 and has showed good efficacy and safety in combination with obinutuzumab pretreatment. The agent has shown high ORR of 81% and CR of 66.7% in patients with R/R MCL who had received prior BTKi therapy (73).
Zilovertamab (ZV) is an antibody- drug conjugate against anti-receptor tyrosine kinase like orphan receptor 1 (ROR1). The agent links monomethyl-auristatin E (MMAE) to an antibody again ROR1 which is present on the surface of MCL cells. In a phase 1 study, including 17 patients with MCL, an ORR of 53% and CR of 12% was achieved (75).
Conclusions
MCL is a heterogenous disease of mostly elderly patients that is currently without a standard option in the frontline setting. MCL remains incurable, and the main therapeutic goal is to deliver effective therapies without acute and long-term toxicities thus preserving quality of life. In an unfit, elderly patients CIT with or without maintenance therapies are regimens most frequently used in frontline treatment. BTKi with rituximab as upfront therapy has shown good response in selected patients. Despite the advances in therapeutic approach in front-line setting, relapses invariably occur. Currently, the treatment remains without a standard of care in the first line setting standard of care and clinical trials should be considered. Treatment with BTKi is a most common approach in second-line but with a shift toward chemo-free approaches in up front treatment newer options are needed in the R/R setting. Given that patients who progress on BTKi have poor prognosis. Recent advances in alternative treatments including the non-covalent BTKi (pirtobrutinib), cellular therapy, BsAb and ROR1 directed therapies are promising and offer hope to patients who are afflicted with this disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jonathon B. Cohen and Craig Portell) for the series “Management of Elderly Patients with HL and NHL” published in Annals of Lymphoma. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aol.amegroups.com/article/view/10.21037/aol-22-8/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aol.amegroups.com/article/view/10.21037/aol-22-8/coif). The series “Management of Elderly Patients with HL and NHL” was commissioned by the editorial office without any funding or sponsorship. TJP received consulting fees from Abbvie, ADCT, BMS, Beigene, Epizyme, Eli Lily Genentech, Incyte, Genmab, Gilead, Xencor, TG therapeutics. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Fu S, Wang M, Lairson DR, et al. Trends and variations in mantle cell lymphoma incidence from 1995 to 2013: A comparative study between Texas and National SEER areas. Oncotarget 2017;8:112516-29. [Crossref] [PubMed]
- Ferrer A, Salaverria I, Bosch F, et al. Leukemic involvement is a common feature in mantle cell lymphoma. Cancer 2007;109:2473-80. [Crossref] [PubMed]
- Eskelund CW, Kolstad A, Jerkeman M, et al. 15-year follow-up of the Second Nordic Mantle Cell Lymphoma trial (MCL2): prolonged remissions without survival plateau. Br J Haematol 2016;175:410-8. [Crossref] [PubMed]
- Romancik JT, Cohen JB. Management of Older Adults with Mantle Cell Lymphoma. Drugs Aging 2020;37:469-81. [Crossref] [PubMed]
- Pease DF, Morrison VA. Treatment of mantle cell lymphoma in older adults. J Geriatr Oncol 2018;9:308-14. [Crossref] [PubMed]
- Jain P, Zhao S, Lee HJ, et al. Ibrutinib With Rituximab in First-Line Treatment of Older Patients With Mantle Cell Lymphoma. J Clin Oncol 2022;40:202-12. [Crossref] [PubMed]
- Wang ML, Jurczak W, Jerkeman M, et al. Ibrutinib plus Bendamustine and Rituximab in Untreated Mantle-Cell Lymphoma. N Engl J Med 2022;386:2482-94. [Crossref] [PubMed]
- Nadeu F, Martin-Garcia D, Clot G, et al. Genomic and epigenomic insights into the origin, pathogenesis, and clinical behavior of mantle cell lymphoma subtypes. Blood 2020;136:1419-32. [Crossref] [PubMed]
- Ye H, Desai A, Zeng D, et al. Smoldering mantle cell lymphoma. J Exp Clin Cancer Res 2017;36:185. [Crossref] [PubMed]
- Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol 2009;27:1209-13. [Crossref] [PubMed]
- Giné E, de la Cruz F, Jiménez Ubieto A, et al. Ibrutinib in Combination With Rituximab for Indolent Clinical Forms of Mantle Cell Lymphoma (IMCL-2015): A Multicenter, Open-Label, Single-Arm, Phase II Trial. J Clin Oncol 2022;40:1196-205. [Crossref] [PubMed]
- Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med 2012;367:520-31. [Crossref] [PubMed]
- Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of Older Patients With Mantle Cell Lymphoma (MCL): Long-Term Follow-Up of the Randomized European MCL Elderly Trial. J Clin Oncol 2020;38:248-56. [Crossref] [PubMed]
- Robak T, Huang H, Jin J, et al. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. N Engl J Med 2015;372:944-53. [Crossref] [PubMed]
- Robak T, Jin J, Pylypenko H, et al. Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: final overall survival results of a randomised, open-label, phase 3 study. Lancet Oncol 2018;19:1449-58. [Crossref] [PubMed]
- Ribrag V, Safar V, Kluin-Nelemans H, et al. Rituximab-Lenalidomide(R2) Maintenance Is Superior to Rituximab Maintenance after First Line Immunochemotherapy in Mantle Cell Lymphoma: Results of the MCL R2 Elderly Clinical Trial. Blood 2021;138:379. [Crossref]
- Kahl BS, Bartlett NL, Leonard JP, et al. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a Multicenter Study. Cancer 2010;116:106-14. [PubMed]
- Robinson KS, Williams ME, van der Jagt RH, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin's lymphoma. J Clin Oncol 2008;26:4473-9. [Crossref] [PubMed]
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203-10. [Crossref] [PubMed]
- Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 2014;123:2944-52. [Crossref] [PubMed]
- Flinn IW, van der Jagt R, Kahl B, et al. First-Line Treatment of Patients With Indolent Non-Hodgkin Lymphoma or Mantle-Cell Lymphoma With Bendamustine Plus Rituximab Versus R-CHOP or R-CVP: Results of the BRIGHT 5-Year Follow-Up Study. J Clin Oncol 2019;37:984-91. [Crossref] [PubMed]
- Karmali R, Switchenko JM, Goyal S, et al. Multi-center analysis of practice patterns and outcomes of younger and older patients with mantle cell lymphoma in the rituximab era. Am J Hematol 2021;96:1374-84. [Crossref] [PubMed]
- Martin P, Cohen JB, Wang M, et al. Treatment Outcomes and Roles of Transplantation and Maintenance Rituximab in Patients With Previously Untreated Mantle Cell Lymphoma: Results From Large Real-World Cohorts. J Clin Oncol 2022; Epub ahead of print. [Crossref] [PubMed]
- Hermine O, Hoster E, Walewski J, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet 2016;388:565-75. [Crossref] [PubMed]
- Visco C, Chiappella A, Nassi L, et al. Rituximab, bendamustine, and low-dose cytarabine as induction therapy in elderly patients with mantle cell lymphoma: a multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet Haematol 2017;4:e15-23. [Crossref] [PubMed]
- Visco C, Tabanelli V, Peracchio C, et al. Rituximab, Bendamustine and Cytarabine Followed By Venetoclax (V-RBAC) in High-Risk Elderly Patients with Mantle Cell Lymphoma. Blood. 2021;138:2427. [Crossref]
- Gressin R, Daguindau N, Tempescul A, et al. A phase 2 study of rituximab, bendamustine, bortezomib and dexamethasone for first-line treatment of older patients with mantle cell lymphoma. Haematologica 2019;104:138-46. [Crossref] [PubMed]
- Smith MR, Jegede O, Martin P, et al. ECOG-ACRIN E1411 randomized phase 2 trial of bendamustine-rituximab (BR)-based induction followed by rituximab (R) ± lenalidomide (L) consolidation for Mantle cell lymphoma: Effect of adding bortezomib to front-line BR induction on PFS. J Clin Oncol 2021;39:abstr 7503.
- Albertsson-Lindblad A, Kolstad A, Laurell A, et al. Lenalidomide-bendamustine-rituximab in patients older than 65 years with untreated mantle cell lymphoma. Blood 2016;128:1814-20. [Crossref] [PubMed]
- Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol 2012;13:716-23. [Crossref] [PubMed]
- Gine E. Efficacy and Safety of Ibrutinib in Combination with Rituximab As Frontline Treatment for Indolent Clinical Forms of Mantle Cell Lymphoma (MCL): Preliminary Results of Geltamo IMCL-2015 Phase II Trial. Blood. 2019;134:752. [Crossref]
- Jain P, Lee HJ, Steiner RE, et al. Frontline Treatment with Ibrutinib with Rituximab (IR) Combination Is Highly Effective in Elderly (≥65 years) Patients with Mantle Cell Lymphoma (MCL) - Results from a Phase II Trial. Blood 2019;134:3988. [Crossref]
- Le Gouill S, Morschhauser F, Chiron D, et al. Ibrutinib, obinutuzumab, and venetoclax in relapsed and untreated patients with mantle cell lymphoma: a phase 1/2 trial. Blood 2021;137:877-87. [Crossref] [PubMed]
- Ruan J, Martin P, Shah B, et al. Lenalidomide plus Rituximab as Initial Treatment for Mantle-Cell Lymphoma. N Engl J Med 2015;373:1835-44. [Crossref] [PubMed]
- Ruan J, Martin P, Christos P, et al. Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood 2018;132:2016-25. [Crossref] [PubMed]
- Yamshon S, Martin P, Shah B, et al. Initial Treatment with Lenalidomide Plus Rituximab for Mantle Cell Lymphoma (MCL): 7-Year Analysis from a Multi-Center Phase II Study. Blood 2020;136:45-6. [Crossref]
- Phillips TJ, Danilov AV, Bond DA, et al. The combination of venetoclax, lenalidomide, and rituximab in patients with newly diagnosed mantle cell lymphoma induces high response rates and MRD undetectability. J Clin Oncol 2021;39:7505. [Crossref]
- Wang M, Robak T, Maddocks KJ, et al. Safety and Efficacy of Acalabrutinib Plus Venetoclax and Rituximab in Patients with Treatment-Naïve (TN) Mantle Cell Lymphoma (MCL). Blood 2021;138:2416. [Crossref]
- Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int Rev Immunol 2012;31:119-32. [Crossref] [PubMed]
- Song Y, Zhou K, Zou D, et al. Treatment of Patients with Relapsed or Refractory Mantle-Cell Lymphoma with Zanubrutinib, a Selective Inhibitor of Bruton's Tyrosine Kinase. Clin Cancer Res 2020;26:4216-24. [Crossref] [PubMed]
- Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood 2015;126:739-45. [Crossref] [PubMed]
- Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet 2018;391:659-67. [Crossref] [PubMed]
- McMullen JR, Boey EJ, Ooi JY, et al. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood 2014;124:3829-30. [Crossref] [PubMed]
- Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369:507-16. [Crossref] [PubMed]
- Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet 2016;387:770-8. [Crossref] [PubMed]
- Rule S, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus: 3-year follow-up of patients with previously treated mantle cell lymphoma from the phase 3, international, randomized, open-label RAY study. Leukemia 2018;32:1799-803. [Crossref] [PubMed]
- Rule S, Dreyling M, Goy A, et al. Ibrutinib for the treatment of relapsed/refractory mantle cell lymphoma: extended 3.5-year follow up from a pooled analysis. Haematologica 2019;104:e211-4. [Crossref] [PubMed]
- Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol 2016;17:48-56. [Crossref] [PubMed]
- Jain P, Romaguera J, Srour SA, et al. Four-year follow-up of a single arm, phase II clinical trial of ibrutinib with rituximab (IR) in patients with relapsed/refractory mantle cell lymphoma (MCL). Br J Haematol 2018;182:404-11. [Crossref] [PubMed]
- Jerkeman M, Eskelund CW, Hutchings M, et al. Ibrutinib, lenalidomide, and rituximab in relapsed or refractory mantle cell lymphoma (PHILEMON): a multicentre, open-label, single-arm, phase 2 trial. Lancet Haematol 2018;5:e109-16. [Crossref] [PubMed]
- Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus Venetoclax for the Treatment of Mantle-Cell Lymphoma. N Engl J Med 2018;378:1211-23. [Crossref] [PubMed]
- Wang M, Ramchandren R, Chen R, et al. Concurrent ibrutinib plus venetoclax in relapsed/refractory mantle cell lymphoma: the safety run-in of the phase 3 SYMPATICO study. J Hematol Oncol 2021;14:179. [Crossref] [PubMed]
- Witzig TE, Inwards D. Acalabrutinib for mantle cell lymphoma. Blood 2019;133:2570-4. [Crossref] [PubMed]
- Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J Clin Oncol 2021;39:3441-52. [Crossref] [PubMed]
- Wang M, Rule S, Zinzani PL, et al. Acalabrutinib monotherapy in patients with relapsed/refractory mantle cell lymphoma: final results from a phase 2 study. Hematol Oncol 2021; [Crossref]
- Tam CS, Opat S, Simpson D, et al. Zanubrutinib for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv 2021;5:2577-85. [Crossref] [PubMed]
- Zhou K, Zou D, Zhou J, et al. Zanubrutinib monotherapy in relapsed/refractory mantle cell lymphoma: a pooled analysis of two clinical trials. J Hematol Oncol 2021;14:167. [Crossref] [PubMed]
- Davids MS, Roberts AW, Kenkre VP, et al. Long-term Follow-up of Patients with Relapsed or Refractory Non-Hodgkin Lymphoma Treated with Venetoclax in a Phase I, First-in-Human Study. Clin Cancer Res 2021;27:4690-5. [Crossref] [PubMed]
- Eyre TA, Walter HS, Iyengar S, et al. Efficacy of venetoclax monotherapy in patients with relapsed, refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor therapy. Haematologica 2019;104:e68-71. [Crossref] [PubMed]
- Zhao S, Kanagal-Shamanna R, Navsaria L, et al. Efficacy of venetoclax in high risk relapsed mantle cell lymphoma (MCL) - outcomes and mutation profile from venetoclax resistant MCL patients. Am J Hematol 2020;95:623-9. [Crossref] [PubMed]
- Goy A, Younes A, McLaughlin P, et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. J Clin Oncol 2005;23:667-75. [Crossref] [PubMed]
- Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol 2006;24:4867-74. [Crossref] [PubMed]
- Friedberg JW, Vose JM, Kelly JL, et al. The combination of bendamustine, bortezomib, and rituximab for patients with relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma. Blood 2011;117:2807-12. [Crossref] [PubMed]
- Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol 2013;31:3688-95. [Crossref] [PubMed]
- Zinzani PL, Vose JM, Czuczman MS, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann Oncol 2013;24:2892-7. [Crossref] [PubMed]
- Trněný M, Lamy T, Walewski J, et al. Lenalidomide versus investigator's choice in relapsed or refractory mantle cell lymphoma (MCL-002; SPRINT): a phase 2, randomised, multicentre trial. Lancet Oncol 2016;17:319-31. [Crossref] [PubMed]
- Wang M, Schuster SJ, Phillips T, et al. Observational study of lenalidomide in patients with mantle cell lymphoma who relapsed/progressed after or were refractory/intolerant to ibrutinib (MCL-004). J Hematol Oncol 2017;10:171. [Crossref] [PubMed]
- Zaja F, Ferrero S, Stelitano C, et al. Second-line rituximab, lenalidomide, and bendamustine in mantle cell lymphoma: a phase II clinical trial of the Fondazione Italiana Linfomi. Haematologica 2017;102:e203-6. [Crossref] [PubMed]
- Wang M, Munoz J, Goy A, et al. Outcomes with KTE-X19 in patients (pts) with relapsed/refractory (R/R) mantle cell lymphoma (MCL) in ZUMA-2 who had progression of disease within 24 months of diagnosis (POD24). J Clin Oncol 2021;39:abstr 7547.
- Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med 2020;382:1331-42. [Crossref] [PubMed]
- Wang Y, Jain P, Locke F, et al. Brexucabtagene Autoleucel for Relapsed/Refractory Mantle Cell Lymphoma: Real World Experience from the US Lymphoma CAR T Consortium. Blood. 2021;138:744. [Crossref]
- Phillips T, Dickinson M, Morschhauser F, et al. Glofitamab Step-up Dosing Induces High Response Rates in Patients (pts) with Relapsed or Refractory (R/R) Mantle Cell Lymphoma (MCL), Most of Whom Had Failed Prior Bruton's Tyrosine Kinase Inhibitor (BTKi) Therapy. Blood 2021;138:130. [Crossref]
- Palomba M, Gordon L, Siddiqi T, et al. Safety and Preliminary Efficacy in Patients with Relapsed/Refractory Mantle Cell Lymphoma Receiving Lisocabtagene Maraleucel in Transcend NHL 001. Blood 2020;136:10-1. [Crossref]
- Wang ML, Barrientos JC, Furman RR, et al. Zilovertamab Vedotin Targeting of ROR1 as Therapy for Lymphoid Cancers. NEJM Evidence 2022;1:EVIDoa2100001.
Cite this article as: Vuyyala S, Phillips TJ. Older patients with mantle cell lymphoma: initial and subsequent therapies—a narrative review. Ann Lymphoma 2022;6:12.


