
Risk stratification and management algorithms for patients with diffuse large B-cell lymphoma and CNS involvement
Introduction
The term secondary central nervous system (CNS) lymphoma (SCNSL) defines the involvement of the CNS at presentation or at failure in patients with systemic lymphoma (1). CNS dissemination can affect parenchymal organs of the CNS (i.e., brain, cerebellum and spinal cord), leptomeninges, cranial nerves, and, more rarely, the eyes; concomitant or sequential involvement of more CNS organs also occurs. There are limited data regarding the mechanisms underlining the trafficking of lymphoma cells to the CNS. Accordingly, three routes of invasion have been hypothesized: the hematogenous one, direct spread from adjacent bones and retrograde growth along neurovascular structures, which is paradigmatic of lymphomas abutting the skull base (2). CNS involvement in diffuse large B-cell lymphoma (DLBCL), if compared to highly-aggressive lymphoma, like Burkitt and lymphoblastic lymphomas, which are managed uniformly using CNS-directed strategies (3), is an uncommon event with reported incidence in the rituximab era oscillating between 2.3% and 10.2% (4-9). CNS relapses tend to occur earlier than systemic relapses (median 5 vs. 18 months), often during frontline chemoimmunotherapy or immediately after as part of first relapse, with a median survival after diagnosis of affected patients of only 2.2 months compared to 9 months for non-CNS relapse (10,11). Thus, it is clear that the early detection of CNS involvement as well as a timely and effective prophylaxis or treatment, are pivotal to decrease the incidence of SCNSL. Moreover, the increased risk of severe neurotoxicity and other relevant side effects associated with the different prophylaxis options enforce the identification of reliable prognostic markers and scores to identify patients at “high-risk” of CNS involvement as the best candidates for this strategy.
Although DLBCL is the most common lymphoid tumor, and CNS dissemination represents an important prognostic event, several questions are still open in this setting. Proposed risk-predicting variables and scores exhibit low diagnostic sensitivity, mostly due to evident selection biases in analyzed series. Among other caveats, analyzed series included variably managed study population, treated with and without rituximab (12,13), using varied CNS prophylaxis strategies (i.e., intrathecal, IT; intravenous, IV; or both routes), and following indications based on unclear “homemade” criteria. Most reported studies included small numbers of early-stage extranodal lymphomas, which resulted in unconfirmed definition of high-risk forms (14), and, more important, suitable internal control groups managed without prophylaxis and integration of clinical data with pathological or molecular parameters are usually lacking. The present review analyzes scores and factors proposed to identify DLBCL patients with “high-risk” of CNS involvement and algorithms for early detection, prevention and treatment of CNS dissemination.
DLBCL patients with increased risk of CNS dissemination
The first studies describing CNS dissemination focused mostly on pediatric patients and highly aggressive lymphomas (15). Once established the risk of CNS dissemination and the value of prophylaxis in highly aggressive lymphomas, investigators focused their efforts in DLBCL. Several retrospective analyses were performed to identify risk factors able to predict CNS involvement in DLBCL and other lymphoma entities. In the pre-rituximab era, most studies included all the categories of aggressive and highly aggressive lymphomas together, and several variables were proposed as predictors of CNS dissemination. However, some of these variables were not confirmed by different studies mostly due to evident selection biases. In the rituximab era, most consistent variables regarded those of the International Prognostic Index (IPI) and some forms of extranodal lymphomas (Figure S1). Some molecules with a putative “homing receptor” capability for the CNS were reported, but confirmatory studies of preliminary reports are lacking.
“High-risk” extranodal sites
A peculiar association between the risk of CNS dissemination and the involvement of some extranodal organs dates from the 90s to the early 2000s. Notably, the primary and secondary involvement of these extranodal sites should be distinguished (Figure S1). In the former form, lymphoma affects exclusively or prevalently an extranodal organ, which suggests an increased risk of CNS dissemination based on clinical experience. For instance, involvement of the testis, kidney and adrenal gland are well-documented extranodal lymphomas associated with a high-risk of CNS dissemination, and this is independent from stage of disease (16-18). On the other hand, dissemination of DLBCL to extranodal organs compels to ask whether the risk relates to biological characteristics of the lymphoma involving a specific site, or rather to the concomitant presence of high-risk factors, including elevated lactic acid dehydrogenase (LDH) serum concentrations, advanced stage and involvement of multiple extranodal sites. On the basis of involvement of extranodal organs, three high-risk groups of DLBCL can be distinguished: (I) lymphomas intrinsically prone to involve contemporaneously extranodal sites and CNS (intravascular large B-cell lymphoma, post-transplant lymphoproliferative disorders, HIV-associated lymphomas) (19-21); (II) DLBCL arising in direct proximity of the CNS (i.e., epidural space, orbit, nasal cavity, paranasal sinuses) that directly infiltrates the brain and meninges; and (III) DLBCL whose proclivity to invade the CNS is not justified by any anatomical contingency (i.e., adrenal glands, kidney, testis, breast) (22-25).
For what concern adrenal/kidney involvement (Table 1), most studies are focused on secondary disease describing a CNS relapse rate up to 35% (22,23). Given the rarity of primary adrenal/kidney DLBCL, the existing reports on stage I/II presented data rarely fully informative on patients’ clinical features (32,33). However, an increased CNS relapse risk has been reported also in this condition, especially in presence of many unfavorable features (bilateral disease, large lesions, increased LDH concentrations).
Table 1
| Anatomical site | Number of assessed patients (ref) | Cumulative risk of CNS relapse | Treatment (Induction + Prophylaxis) |
|---|---|---|---|
| Renal/adrenal gland | 55 (DLBCL) (23) | 35% | R-CHOP (46%)/CHOP-like (54%); IT (14%) |
| Testis | 371 (DLBCL) (24) | 34% | Anthracyclines-based chemo; IT (18% of pts) |
| 73 (DLBCL) (26) | 25% | R-CHOP + variable prophylaxis (6 HD-MTX; 2 HD-MTX + IT) | |
| Breast | 204 (DLBCL) (25) | 5% | Anthracycline-based chemo + IFRT; IT (4%) |
| 84 (51 high grade) (27) | 14% | Variable Treatment w/o prophylaxis | |
| 75 (DLBCL) (28) | 20% | Chemo with Rtx (in 69%) + IT (in 8%) | |
| Paranasal sinus | 44 (37 DLBCL) (17) | 11% | Anthracycline-based chemo; IT (89%) |
| 40 (DLBCL) (29) | 1.5% | R-CHOP + IT proph (in 30% of pt) | |
| Orbit | 143 (not specified) (30) | 5% | Not specified |
| Spine/epidural soft tissue | 48 (28 Intermediate; 12 High Grade) (31) | 8% | Anthracycline-based chemo; IT (19% of pts; none of those who relapsed) |
CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; IT, intrathecal drug delivery as CNS prophylaxis; HD-MTX, high-dose methotrexate; IFRT, involved-field radiotherapy; Rtx, rituximab.
Another extranodal organs widely studied are the testes. In the largest pre-rituximab series (24), limited-stage testicular DLBCL patients who received different treatments showed a 5-year overall survival of 60%, with a 3-year contralateral testis relapse of 15%, and a CNS relapse rate of 19% at 5 years and 34% at 10 years. This observation has been confirmed also in the rituximab era (16,26,34), pointing towards the need of a CNS-oriented management of those patients.
Direct invasion from neighboring structures was one of the first described mechanisms of CNS dissemination. In several pre-rituximab series of patients treated with CHOP and without prophylaxis, stage I–II DLBCL of the nasal cavity and paranasal sinuses had an increased risk of CNS dissemination, ranging from 10 to 30% of cases at relapse (17,35). This notion was questioned by a retrospective study in the rituximab era (29,36), where the addition of this antibody dropped down the CNS relapse rate to 1.9%. An increased risk of CNS involvement was described in patients with primary orbit DLBCL, particularly in the context of deep lesions, bone erosion, extension to other vault regions, or combinations of these features, and in secondary orbit DLBCL (30,37). Analogously, in a small retrospective analysis (36), the authors argued against the utility of CNS-directed prophylaxis for patients treated with rituximab containing regimens. However, to maximize the statistical power of the analysis, all the patients with extra-lymphatic craniofacial involvement were grouped into a single entity, regardless of the heterogeneity of the presentation and treatment. Recently, several large retrospective analysis on orbital DLBCL were published (38-40); unfortunately, most of these studies did not focus on the rate of CNS events, the induction treatment and prophylactic intervention, leaving several questions still unsolved (41). CNS-directed interventions could be considered in some patients with orbit DLBCL based on the presence of “high-risk” features (i.e., large masses, lesions close to the apex of the orbit), patient comorbidity and preference.
The picture is still more controversial in the primary DLBCL of the breast. In the largest pre-rituximab studies, CNS relapse rate ranged from 5% to 24% (25,27,28,42). The protective effect of rituximab on CNS dissemination and the role of earlier diagnosis in the last decade resulting in reduced proportion of patients diagnosed with large “high-risk” lesions remain two important open questions. Therefore, although some advocates CNS-oriented management of primary DLBCL of the breast tout court (43), CNS assessment and prophylaxis could be offered to patients with particular features like stage II, lesions >5 cm or bilateral involvement, based on most recent series (44-47).
Although infrequent, DLBCL localization to the epidural soft tissue was associated with subarachnoid spread in 10–16% of patients, irrespectively of disease stage (31,48). and is generally considered amenable of prophylaxis. Differently from the conditions previously discussed, other sites carry an increased risk of CNS relapse only when their involvement is in the context of a systemic disease. For instance, CNS recurrence occurs in less than 1% of patients with limited stage Waldeyer’s ring DLBCL, while this complication rises up to 17% in secondary Waldeyer’s ring involvement (49). Similarly, CNS dissemination is an unusual event in limited stage ovarian DLBCL, while published data reported this event in up to 46% of patients with secondary ovarian DLBCL (50). Conversely, in a recent retrospective analysis (51), seven (41%) of 17 patients with DLBCL involving the uterus, but not the ovaries, experienced CNS relapse. However, the limited sample size and the elevated number of patients within a high R-IPI score (13/17) seems to indicate an increased risk of CNS involvement in the context of a disseminated disease. Thus, confirmatory studies are needed in this setting. Lastly, CNS relapse was recently reported in a small case-series of cutaneous DLBCL, leg-type, but the frequency of this complication (4.4%), was similar to the general population of DLBCL and, therefore, no recommendation for prophylaxis can be done with current evidence (52).
Clinical variables and scores
Different variables and scores predicting CNS recurrence risk have been proposed on large retrospective series, most of them including IPI factors and other clinical features (Table 2). The first proposed risk model considered number of extranodal sites >1, albumin level <3.5 mg/dL, age >60 years, high LDH serum level, and retroperitoneal lymph-node involvement, which allowed to stratify patients as low risk (0 to 3 risk factors) or high risk (4 to 5 risk factors) (53). CNS relapse occurred in 25% of high-risk patients compared with a relapse rate of 6% in low-risk. A GELA study on 974 patients confirmed with the final multivariate model increased LDH and >1 extra-nodal sites as variables independently associated with higher risk (56). Authors also observed that IPI divided patients’ population into two risk groups for the incidence of CNS relapse: 0.6% in the low-low-intermediate risk group and 4.1% in the high-intermediate-high risk group. A survey of 1,693 patients treated between 1990 and 2000 in trials of the German High-Grade NHL Study Group (57) confirmed that elevated LDH serum levels (3-year CNS relapse rate: 3.7 vs. 1.3%) and initial involvement of >1 extra-nodal sites (5.8% vs. 1.4%) were associated with an increased risk of CNS involvement. However, these two factors identified only seven (37%) out of 19 patients with CNS recurrence, with a poor diagnostic sensitivity. In addition to that, an association between the involvement of testis and sino-orbital region was noted with a higher risk of subsequent CNS disease 10-fold higher, particularly in younger patients. Notably, these data were collected in the pre-rituximab era, a potentially relevant bias as this antibody has changed the natural history of DLBCL. However, as previously stated, despite rituximab improved systemic disease control and outcome, the impact on CNS relapse looks small, likely reflecting its poor CNS penetration (5,12).
Table 2
| Reference | N° (Cohort) | Histology | Treatment (prophylaxis) | Proposed Risk Variables | Cumulative CNS relapse risk |
|---|---|---|---|---|---|
| Hollender [2002] (53) | 1,220 | NHL (no BL/LBL) | CHOP or similar (variable, mostly IT, in 11% of pts) | Age >60 | Low risk: 5.6% |
| Albumin <3.5 mg/dL | High risk: 18.3% | ||||
| EN >1 | |||||
| High LDH | |||||
| Retroperitoneal LN | |||||
| Schmitz [2016] (9) | 1,735 | DLBCL | R-chemotherapy (not described) | Age >60 | Low risk: 0.8% |
| ECOG PS >1 | High risk: 10% | ||||
| EN >1 | |||||
| High LDH | |||||
| Stage >2 | |||||
| Kidney/Adrenal | |||||
| Schmitz [2016] (9) | 1,597 | DLBCL | R-CHOP (not described) | Age >60 | Low risk: 0.8% |
| ECOG PS >1 | High risk: 12% | ||||
| EN >1 | |||||
| High LDH | |||||
| Stage >2 | |||||
| Kidney/Adrenal | |||||
| Kanemasa [2016] (54) | 413 | DLBCL | R-CHOP or similar (IT in 62/112 high risk pts) | Albumin <3.5 mg/dL | Low risk: 3% |
| EN >1 | High risk: 26.4% | ||||
| Retroperitoneal LN | |||||
| Stage >2 | |||||
| Tomita [2017] (55) | 1,220 | DLBCL | R-CHOP (none) | Age >60 | Low risk: 1.3% |
| ECOG PS >1 | High risk: 12.9% | ||||
| EN >1 | |||||
| High LDH | |||||
| Stage >2 (breast and testis at high risk) |
DLBCL, diffuse large B-cell lymphoma; NHL, non-Hodgkin lymphomas; BL, Burkitt lymphoma; LBL, lymphoblastic lymphoma; IT, intrathecal drug delivery; EN, extranodal organs involved; LDH, lactic dehydrogenase serum level; LN, lymph nodes; ECOG PS, Eastern Cooperative Oncology Group performance status score.
In the rituximab era, advanced stage, increased LDH serum level, involvement of >1 extranodal site and high IPI score have been largely suggested as prognostic factors (4,5,8,34,58,59). More recently, the German High-Grade Lymphoma Study Group (DSHNHL) analyzed data from 2,164 DLBCL patients treated with R-CHOP-like therapy in prospective studies, and identified IPI parameters (stage III/IV, age>60, elevated serum LDH, multiple extra-nodal sites, ECOG performance status >1) and renal/adrenal involvement as independent predictors of CNS relapse (9). This model, called “CNS-IPI” stratified patients into three different groups: low risk (0–1), intermediate risk (2,3) or high risk (>3) with 2-year CNS relapse rates of 0.6%, 3.4% and 10.2%, respectively. This score was validated in an independent cohort of 1,597 patients from the British Columbia Cancer Agency (BCCA) with similar 2-year rates for CNS relapse of 0.8%, 3.9%, and 12% in the 3 risk groups. Despite being an easy and effective tool, the sensitivity of this model is still low with some DLBCL at higher risk of CNS involvement not considered amenable for prophylaxis. A retrospective analysis in 1,080 patients with DLBCL registered in the UK NCRI R-CHOP14vs21 trial showed no differences in CNS recurrence rates using the CNS-IPI (1.9% for the whole series vs. 2.8 for patients selected for prophylaxis), with no significant risk factor observed in multivariate analysis, probably due to the small number of events (13). Of note, 81% of CNS relapses occurred in patients with extra-nodal disease, but kidney and adrenal gland are the only ones incorporated into the CNS-IPI. Importantly, the association between some extranodal lymphomas and CNS infiltration is somehow independent of stage and IPI (24) The above-mentioned limitations of the CNS-IPI have been recently explored in a retrospective series of 126 patients with stage IE DLBCL: 15% of patients had a disease relapse, and one third of those relapses involved the CNS in patients with a low-risk CNS-IPI (60). The sensitivity of CNS–IPI could improve up to predict 90% of CNS relapses by the addition of some specific extranodal sites. However, these results should be addressed with caution since it implies a wider use of prophylaxis, with the possibility of increasing also the number of unnecessary and potentially toxic treatments (61). Furthermore, a model based on the standard R-IPI was proposed in a retrospective series of 1,221 DLBCL patients treated with R-CHOP without any CNS prophylaxis (55). Based on the results of this analysis, the authors proposed a new algorithm, considering at high risk patients with either a high R-IPI score or patients with testis or breast involvement independently from their R-IPI score. Indeed, it is well known that involvement of these extranodal organs is associated with CNS recurrence risk, however, most patients with primary testicular or breast lymphoma belong to the low- or low-intermediate risk category according to the standard IPI in most reported studies (24,62). Thus, although potentially appealing because patient population is free from CNS treatment, this study should be validated in a second large cohort (55), as occurred for the CNS-IPI. Moreover, other extranodal organs like kidney and adrenal gland might be included in the high-risk category regardless of the IPI, as it was done for testis and breast DLBCL.
Histological and molecular variables
With more insight into tumor biology, a few biomarkers were also proposed as predictors of CNS relapse in DLBCL (Table 3). Overall, DLBCL with MYC translocation are more aggressive and less sensitive to conventional treatments, and this is particularly evident in DLBCLs with chromosomic translocations involving MYC and BCL-2, BCL-6 or both, usually called double-hit (DHL) or triple-hit (THL) lymphomas (13). Some authorities proposed that DHL are associated with a higher risk of CNS involvement than the other DLBCLs, with CNS infiltration at diagnosis in 4% of cases, which arises to 10% in series homogeneously assessed with CNS staging, and a 3-year CNS involvement rate of 13% (63,69). Although CNS-directed strategies are still recommended for DHL/THL patients, available evidence is not uniformly supportive. For instance, none of the patients with MYC rearrangements or DHL analyzed among the 1,080 patients with DLBCL registered in the UK NCRI R-CHOP14vs21 trial experienced CNS relapse (13). Importantly, despite acknowledging the controversial prognostic implication and management of MYC and BCL-2/BCL-6 rearrangement, current ESMO guidelines (70) recommend to perform this assessment using fluorescence in situ hybridization (FISH), whenever technically possible, in newly diagnosed and relapsed DLBCL. The cost-benefit of this strategy requires validation, albeit in part supported by a large retrospective analysis (71).
Table 3
| Reference | N° of patients | Histological/molecular variable | Cumulative risk of CNS relapse | Treatment: induction/consolidation/prophylaxis |
|---|---|---|---|---|
| Oki [2014] (63) | 129 | C-MYC+ BCL-2+ by FISH (DHL) (selection criterion) | 13% | R-CHOP, R-EPOCH or HCVAD)/ASCT (20%)/– |
| Savage [2016] (64) | 127 | c-myc+ bcl-2+ by IHC (DEL) (selection criterion) | 10% | R-CHOP/–/IT (if nasal sinus disease) |
| Klanova [2017] (65) | 933 | ABC plus high CNS-IPI (9.6% of cases) | 15% | R-CHOP or G-CHOP/–/IT MTX (if high risk) |
| Yamaguchi [2008] (66) | 120 | CD5+ (selection criterion) | 13% | R-CHOP/–/– |
| Xu-Monette [2015] (67) | 879 | CD5+ (5.5% of cases) | 8% | R-CHOP/–/– |
| Cox [2014] (68) | 151 | IgM Monoclonal (13% of cases) | 43% | R-CHOP/–/IT (if high risk) + EV-MTX (if CNS disease) |
CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; FISH, fluorescence
DLBCL expressing high levels of MYC and BCL-2, BCL-6 or both in immunohistochemistry are called double or triple “expresser” lymphomas (DEL or TEL). Cases of DEL and DHL are not consistent in reported series, probably indicating two different processes. In fact, DHL are more commonly, if not exclusively, germinal-center B-cell-like (GCB) genotype, whereas most of DEL are categorized as activated B-cell-like (ABC) genotype, and only a small proportion are also DHL. Studies focused on CNS risk in patients with DEL show contrasting results. A retrospective series of 428 patients treated with R-CHOP (64) showed that the 2-year CNS relapse risk was 9.7% in DEL vs. 2.2% in the other DLBCLs (P=0.001) and the rate comes up when coupled with high-risk CNS-IPI group (2-year risk: 22.7% versus 2.3%; P=0.02). Notwithstanding, it should also be noted that the overall frequency of CNS relapse was low (3.5%) and that a significant number of DEL patients displayed variables presumptively associated with a higher risk of CNS involvement (i.e., old age, advanced stage, elevated LDH levels, non-germinal center phenotype). Conversely, preliminary data of the GOYA trial carried out on 688 patients were not able to replicate the results of the previous study and no association between DEL and CNS risk was detected. Of note, in the GOYA trial, COO assessed by gene expression profiling (GEP; Nanostring Lymphoma Subtyping) was available in 933 pts (65.8%) and showed that ABC and unclassified subtypes had significantly higher CNS relapse risk vs. the GCB subtype (2-year rates: 6.9%, 4.8% vs. 1.5%, respectively). Interestingly, merging the COO analysis and the CNS-IPI score resulted in the selection of a subgroup of patients (75/933; 8%) with both ABC (or unclassifiable genotype) and a high CNS-IPI, characterized by a particularly high CNS relapse rate (15.2% at 2 year) (65). However, to implement this strategy in the current CNS prophylaxis algorithm, the exact sensitivity and specificity of the aforementioned approach is still needed both in this and in other independent cohorts (1).
Immunohistochemical expression of CD5 (66,67,72,73) and IgM secretion (68) have also been investigated and linked with lower outcome and higher CNS relapse risk (see Table 4 for more details).
Table 4
| Symptoms | Incidence |
|---|---|
| Cranial nerve palsy | 30% |
| Intracranial hypertension (nausea, vomiting) | 4–10% |
| Mental status changes | 20–30% |
| Gait/balance disturbance | 10% |
| Peripheral sensory/motor symptoms | 25% |
| Headaches | 20–50% |
| Visual symptoms (uveitis, floaters or campimeter deficits) | 5–10% |
| Seizures, brain stem or cerebellum symptoms | 5% |
| Focal CNS deficits | 50% |
| No symptoms | <5% |
CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma.
Another interesting field is represented by the molecular phenotype of extranodal lymphomas (74). Moving from the elevated frequency of MYD88 and CD79A mutations in primary DLBCL of the CNS, several groups have investigated the mutation rate of this molecular target in other extranodal lymphomas displaying similar immunohistochemical features. It is now well-known that also primary testicular DLBCL presents a high frequency of non-GCB phenotype based on the Hans algorithm, as well as elevated rates of MYD88 L265P mutation (75). Similar MYD88 mutation rates have been described in primary breast DLBCL (76,77), and, anecdotally in some cases of uterine DLBCL (76). However, the predictive role of CNS dissemination of MYD88 and CD79B mutations should be studied more in details as preliminary data seems to suggest that DLBCL relapsing in the CNS lack these oncogenic mutations (78).
How we manage DLBCL at high risk of CNS dissemination
Policy, in our Institution, is to candidate to CNS-oriented diagnostic procedures and prophylaxis DLBCL with either a high R-IPI score or with involvement of at least one high-risk extranodal site, independently from the IPI score (i.e., kidney, adrenal gland, testis, breast, epidural space, skull, spinal cord, orbit, and nasal/paranasal sinus) (8). Although still controversial, we consider DLBCL showing either MYC rearrangement alone or DHL/THL phenotype by FISH at high risk of CNS dissemination (Figure 1). Of note, asymptomatic cases of positive CSF by flow cytometry are considered pts with occult CNS disease, a condition associated with CNS relapse risk and mortality (79). Thus, despite the optimal treatment approach for such patients is still not known, policy in our Institution is to manage them as SCNSL.
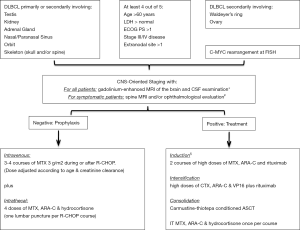
CNS prophylaxis
CNS prophylaxis should improve survival figures in patients with high-risk DLBCL. However, the optimal type of prophylaxis remains a matter of debate because this issue has been addressed in few single-arm prospective studies, and a single ongoing randomized trial exists (NCT02777736). In the last decades, whole-brain irradiation, the most commonly used prophylaxis strategy in highly-aggressive lymphomas was replaced by systemic and/or intrathecal chemotherapy, including methotrexate (MTX) and/or cytarabine (ARA-C) in particular. Actually, with all the limitation of a study not originally designed to test the efficacy of CNS prophylaxis, data reported by Bernstein et al. (10) failed to demonstrate benefit of CNS prophylaxis using intrathecal therapy or cranial irradiation after remission in patients with de novo, advanced-stage aggressive lymphomas and BM involvement at diagnosis. Indeed, in this setting both high-dose systemic chemotherapy and timely intrathecal chemotherapy are essential to decrease the incidence of CNS leukemia (80). Moreover, in the last decade, different retrospective and prospective analyses have questioned the efficacy of IT prophylaxis in DLBCL with controversial results. In some studies (36,81,82), IT alone did not avoid relapses in brain parenchyma or meninges in patients with high-risk DLBCL, with recurrence rates of up to 6%, suggesting that this modality is insufficient as an exclusive CNS prophylaxis. However, these and other studies (13) reported a lower number of leptomeningeal recurrences, which may suggest some benefit using this strategy. Moreover, it has to take into account that the number of leptomeningeal involvement rose up after the introduction of rituximab (12). Thus, although IT prophylaxis efficacy was observed only in small, retrospective series without a control arm as well as sometimes with co-administration of systemic MTX, discouraging its administration is still premature given the lack of a high-level evidence in contrast (59). Triple intrathecal therapy (TIT) based on the combination of MTX (12.5 mg), ARA-C (50 mg) and hydrocortisone (40 mg) is the most commonly used schedule for CNS prophylaxis in hematological malignances. Also liposomal cytarabine (LC) has been evaluated specifically in the lymphoma setting but it is not registered with this indication (83,84).
On the other hand, due to the high rate of parenchymal involvement, successful prophylactic strategies should ideally integrate agents that deeply penetrate all CNS compartments. The most widely studied drug is MTX that at doses ≥1 g/m2 appear to produce therapeutic levels in both CSF and parenchyma. The evidence in favor of high-dose MTX (HD-MTX) comes from prospective as well as retrospective studies both in pre- and post-rituximab era (8,85-89) (Table 5). Of note, some of these studies used dose-intensive regimens and/or high-dose ARA-C, which may also contribute to the risk reduction and only one has an internal control group who did not receive prophylactic treatment (8,85-89). Based on this evidence, despite the intrinsic limitations of collected data, our recommendation is to use 3–4 courses of MTX 3 g/m2 every two to three weeks at the end of R-CHOP treatment. In elderly patients and/or patients with dose-limiting comorbidity 2–3 courses of MTX 1.5 g/m2 (dose-adjusted according to creatinine clearance) is advisable. HD-MTX is contraindicated in patients with renal failure or effusions/generalized edema because this drug cumulates in third space and is slowly released, resulting in prolonged exposure and enhanced toxicity. Moreover, caution has to be used in case of hepatic failure. To deliver HD-MTX in day 15 of each course of R-CHOP to anticipate prophylaxis seems to be a soundly approach, but it should be used with caution as safety data are sparse, and MTX-related hepato- or nephrotoxicity may result in disappointing delay of R-CHOP.
Table 5
| Reference (author, year) | Type of study | Histology included (N° of patients) | Whole series | Treatment induction | Prophylaxis (N° of patients) | CNS relapse rate | Conclusions |
|---|---|---|---|---|---|---|---|
| Abramson [2010] (88) | R | HR-DLBCL [61] | 65 | R-CHOP [63] | MTX 3–3.5 g/m2 [65] | 3.0% | HD-MTX is associated with less CNS relapses |
| HR-PMBCL (4) | |||||||
| Guirguis [2012] (90) | R | DLBCL | 214 | R-CHOP | HD-MTX ± IT [27] | 3.7% | CNS prophylaxis not needed with R-CHOP, with the exception of testicular DLBCL |
| Kumar [2012] (59) | P | DLBCL | 989 | R-CHOP | IT MTX ± ARAC (72%) or HD-MTX [117] | 2.0% | Low CNS recurrence rate; no benefit with prophylaxis |
| Avilés [2013] (91) | R | DLBCL | 3,258 | CHOP ± R | None [2,253] | 5.9% | No benefit with prophylaxis |
| Varied [1,005] | 5.9% | ||||||
| Holte [2013] (86) | P | DLBCL [145] | 156 | R-CHOEP14 | MTX 3 g/m2 + ARAC [156] | 4.5% | CNS events lower than expected |
| FL 3A aaIPI >2 [11] | |||||||
| Cheah [2014] (89) | R | HR-DLBCL | 217 | Varied* | IT MTX [43] | 18.4% | IV MTX and/or ARAC reduced CNS relapses in comparison with IT alone |
| MTX 1–3 g/m2 [125] | 6.9% | ||||||
| HD-MTX + IT [43] | 2.3% | ||||||
| Ferreri [2015] (8) | R | HR-DLBCL | 107 | R-CHOP | None [67] | 14.0% | IV MTX reduces CNS relapses |
| MTX 3 g/m2 ± IT [40] | 0.0% |
CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma; R, retrospective; P, prospective; HR, high risk of CNS recurrence defined by homemade algorithm; FL, follicular lymphoma; aaIPI, age-adjusted International Prognostic Index; MTX, methotrexate; ARAC, cytarabine; IT, intrathecal drug delivery; IV, intravenous. *, Induction regimens included CHOP ± R, R ± CHOP-like, HyperCVAD or CODOX-M IVAC ± R.
HD-MTX-based prophylaxis is matter of debate even when dose-intensified regimens like DA-EPOCH-R are used (92,93). Of note, results of these studies need to be validated in larger cohorts and prospectively but have to be taken into account while using this infusional regimen.
Presentation and diagnosis of CNS involvement in DLBCL patients
DLBCL progression or relapse in the CNS occurs usually early, frequently within weeks to months after disease diagnosis (10,11). Accordingly, most investigators believe that a significant number of patients prone to develop SCNSL harbor lymphoma cells in the brain parenchyma or the CSF at the time of diagnosis. In an international, retrospective series of 291 SCNSL patients (11), CNS involvement occurred as part of first relapse in 87% of cases, 23% of these events occurred during first-line treatment, and CNS dissemination were detected at second or subsequent relapses only in 13% of patients. Moreover, authors highlighted that patients developing CNS disease during first-line therapy were younger (median age 61 vs. 65 years, P=0.015) and more likely to have concurrent systemic progression. A higher rate of leptomeningeal involvement was observed in patients with concomitant systemic involvement by DLBCL at the time of CNS event (n=113, 39%). In the larges reported series, relapse involves the brain parenchyma in 40–45% of cases, leptomeninges in 40% and both in 8% (94). There are several discrepancies among reported studies in the prevalence and timing of involved CNS sites at recurrence, which may be explained by partial effects of different types of prophylaxis used in analyzed series. For instance, a reduction of brain parenchymal lesions has been reported with the use of IV CNS prophylaxis (11).
Neurological symptoms are the first indication of CNS disease in many patients (Table 4). Usually, clinical presentation is influenced by the site of the CNS lesions that can be focal or multifocal. Histological or cytological diagnosis of CNS dissemination is always required with the exception of clear clinical and radiological presentations, especially if a previous or concomitant history of aggressive systemic lymphoma are coupled with poor clinical conditions, rapidly progressive disease and/or intracranial hypertension.
Treatment of patients with DLBCL and CNS involvement
The main goal of treatment in SCNSL is the equal control of systemic and CNS disease. Conventional drugs used for the treatment of systemic disease have a good anti-lymphoma activity but are not able to cross the blood-brain barrier (BBB), thus resulting inadequate for the treatment of SCNSL. Actually, first-line treatments are usually based on combinations of drugs like MTX and/or ARAC that administered at high doses exhibits a good CNS bioavailability. These could represent a problem in case of CNS relapse in a patient already treated with those drugs as prophylaxis, for an increased risk of CNS recurrence during the first-line, since few other drugs are able to cross the BBB. Of note, systemic glucocorticoids could help to treat rapidly progressive diseases, both by direct tumor cytolysis and reduction of perilesional edema.
Treatment of SCNSL is mainly translated by prospective and retrospective experience on patients with primary CNS lymphoma. In particular, the best upfront approach for lymphomas involving the CNS is represented by a HD-MTX-based combination. Recently, the largest retrospective analysis to date published on patients with CNS relapse in the brain parenchyma as initial event have been reported by the International PCNSL Collaborative Group (IPCG): systemic HD-MTX is independently associated with better outcome if CNS is the only site of relapse (95). The efficacy of MTX, is strictly linked to both administered dose and duration of tumour cells exposure, as it normally happens with the use of most antimetabolities. Studies on CSF demonstrate that after systemic administration of MTX, cytotoxic concentrations are reached in 44% of patients treated at 2.5 g/m2, while the percentage comes up 66–81% when the drug concentration is 5 g/m2 (96-98). Thus, the recommended dose of MTX to effectively treat CNS lymphomas is ≥3 g/m2, which is a good compromise between efficacy and tolerability (99).
Due to the fast but very short (0.5 to 5 months) improvement registered in patients treated by systemic and intrathecal/intraventricular chemotherapy with, in some cases, further whole-brain irradiation (WBRT), those strategy are now used as complementary part of wider approach with the aim to reach the chemotherapy sanctuaries (see below).
The highest level of evidence in the treatment of SCNSL is represented by three multicenter single-arm phase II trials (Table 6). The background of these trials is based on old retrospective studies suggesting that only patients who received ASCT after remission of CNS disease could have some opportunities to be cured (103,104). Accordingly, experimental treatments addressed in the above-mentioned trial intuitively incorporated BBB-crossing drugs in the induction phase, followed by consolidation with myeloablative chemotherapy supported by ASCT. In particular, data on conditioning regimens obtained in primary CNS lymphomas have been translated into SCNSL patients (105) with BCNU, busulfan and thiotepa showing superior results in comparison to BEAM (etoposide, conventional-dose ARAC and melphalan) regimen. Indeed, these drugs have a well-documented CNS penetration while the ability of BEAM component to cross the BBB is questionable. Actually, busulfan, thiotepa and carmustine reported an excellent CNS penetration rate, with CSF levels in excess of 80% serum levels for busulfan and thiotepa, and of 50–80% for carmustine (106). On the other hand, BEAM drugs exhibited a significant lower CNS distribution: etoposide is 5%, AraC is 6–22%, and melphalan is 10% (106). Moreover, anecdotal cases of primary CNS lymphomas relapsed after BEAM reported a long-lasting remission after a salvage thiotepa-busulfan conditioning chemotherapy and ASCT (107).
Table 6
| Reference (author, year) | N° of patients | Median age [range] | Treatment induction → consolidation (% completed) | Intrathecal therapy | Pre-ASCT ORR (CRR) | PFS | OS | TRM |
|---|---|---|---|---|---|---|---|---|
| Korfel [2013] (100) | DLBCL [27] | 58 [29–65] | HD-MTX/IFO → HD-ARAC/TT → ASCT (80%) | Liposomal cytarabine 50 mg | 67% (23%) | 2-year: 49% | 2-year: 52%; |
3% |
| PTCL [3] | ||||||||
| Ferreri [2015] (101) | DLBCL [32] | 59 [36–70] | Rituximab-MTX-ARAC → Rituximab-HDS → ASCT (53%) | Liposomal cytarabine 50 mg | 63% (61%) | 4-year: 50% | 5-year: 41%; |
10% |
| FL [3] | ||||||||
| MCL [3] | ||||||||
| Doorduijn [2016] (102) | DLBCL [35] | 57 [23–65] | Rituximab-DHAP-HDMTX → ASCT (42%) | Rituximab | 53% (22%) | 2-year: 14% | 2-year: 22% | 8% |
| FL g 3 [1] |
SCNSL, secondary central nervous system lymphoma; DLBCL, diffuse large B-cell lymphoma; PTCL, peripheral T-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; HD-MTX, high-dose methotrexate; IFO, ifosfamide; ARAC, cytarabine; TT, thiotepa; ASCT, autologous stem cell transplantation; HDS, high-dose sequential chemotherapy; ORR, overall response rate (complete and partial response); CRR, complete remission rate; PFS, progression-free survival (called also “event-free survival” in the original articles); OS, overall survival; TRM, treatment-related mortality. *, Outcome in transplanted patients.
The first reported study addressing the treatment of SCNSL was a German phase II trial performed on 30 immunocompetent adult patients not older than 65 years with CNS relapse of systemic aggressive lymphoma (100). The treatment schedule consisted of three courses of induction therapy containing HD-MTX and ifosfamide and HD-ARAC and thiotepa plus intrathecal liposomal cytarabine followed by carmustine, thiotepa and etoposide-conditioned ASCT in responders. After 3 cycles of induction the complete remission rate was 23%, with ASCT being performed in 80% of the patients, and a 2-year OS of 52%. The second one was an Italian study, called SCNSL1 (101), on 38 patients aged 18–70 years with SCNSL, associated with concurrent systemic disease in two-thirds of cases. Induction regimen consisted of rituximab, HD-MTX, and ARAC was followed by intensification with R-HDS (sequential delivery of high doses of cyclophosphamide, cytarabine and VP16) and consolidated with carmustine-thiotepa-conditioned ASCT. Intrathecal liposomal cytarabine was used in the induction phase. Importantly, 61% of registered patients received ASCT in complete remission. In this trial, which has the longest follow-up period duration (median 48 months), the 5-year OS was 41% for the whole series and 68% for patients who received transplantation. Of note, tolerability was acceptable in patients older than 65 and/or with ECOG PS of 3, who were nor registered in the other trials, and systemic (extra-CNS) and/or meningeal disease did not affect outcome. The third study was conducted by the HOVON group on 36 SCNSL patients aged ≤65 years (102). Treatment consisted of 2 cycles of R-DHAP alternating with HD-MTX and was combined with intrathecal rituximab. Responding patients received a third R-DHAP-MTX cycle followed by busulfan-cyclophosphamide-conditioned ASCT. Efficacy of this treatment was inferior to those reported in the other two trials, with a complete remission rate of 22%, with half of transplanted patients experiencing progressive disease at a median time shorter than 4 months, and a 1-year PFS of only 19%.
At the best of our knowledge, there is a single ongoing prospective trial focused on patients with SCNS-DLBCL. This is an international phase II trial called MARIETTA, which is addressing an induction with 3 courses of MATRix (MTX, ARAC, rituximab, thiotepa) combination, an effective standard regimen for primary CNS lymphomas, followed by 3 courses of R-ICE (rituximab, ifosfamide, carboplatin, etoposide) combination, a largely used salvage regimen in relapsed/refractory DLBCL (NCT02329080). Ideally, included drugs are capable to cross BBB and not cross-resistant with antimetabolites, and R-ICE combination should provide adequate treatment of CNS disease and improve systemic disease control, which is a relevant issue considering that half of DLBCL patients with CNS involvement die of systemic progressive disease. Moreover, with respect to SCNSL1 trial (101), the MARIETTA regimen includes the last version thiotepa-BCNU conditioning regimen, with higher doses of thiotepa, which has been largely tested in German centers with the same tolerability and higher efficacy (108). Similarly to the SCNSL1, the MARIETTA regimen offers the possibility to start with R-CHOP regimen in the case of extensive and life-threatening extra-CNS disease and to deliver WBRT in patients with residual CNS disease after ASCT.
The role of intrathecal chemotherapy
Intrathecal chemotherapy, twice a week, administered by lumbar puncture or by intraventricular route using an Ommaya reservoir is incorporated in the treatment of SCNSL. Several drugs can be delivered by intrathecal route, but MTX, ARAC and steroids are the most commonly used ones to treat lymphomatous meningosis, with or without focal neurological deficits. Unluckily, this strategy, even if always associated with a reduction in the number of tumor cells in the CSF, led to a symptomatic improvement in less than 20% of cases. Moreover, the risk of acute and delayed, severe side effects reduce the worldwide application of intrathecal chemotherapy in SCNSL patients. Indeed, at least two-three doses per week of conventional drugs are needed to reach adequate CSF concentrations when administrated by lumbar puncture, thus increasing the risk of infective complications and limiting patient’s quality of life. In models of Cynomolgus monkey pharmacokinetic analysis of rituximab suggests a biphasic clearance of the drug from the CSF, with a terminal half-life of 4.96 h, with no significant acute or late toxicity detected after intrathecal delivery. Responses are reported as case reports and confirmed in a small phase I trial (109). Usually, doses up to 25 mg, administered twice a week by Ommaya’s reservoir, are well tolerated, while side effects like nausea, vomiting, arterial hypertension, diplopia, and tachypnea are reported when used at 50 mg. Nearly half of patients with primary CNS lymphoma achieve an objective response, but typically a fast cytological failure or cerebral progression occur (109). Thus, intraventricular rituximab can be securely delivered in patients with CNS lymphoma, even if combinations with other strategies are needed to obtain long-lasting results.
The role of radiation therapy
In the 90s, induction treatment before total-body irradiation- or alkylating-based conditioning and transplantation was represented by WBRT in combination with intrathecal chemotherapy in 70% of SCNSL patients (103). In particular, a median OS of 10 months, and a 2-year EFS of 40% were reached when primary WBRT was followed by ASCT. The major concern with this strategy regards the occurrence of severe, sometimes lethal, neurotoxicity in one third of patients (103). In the last decades, primary WBRT was abandoned in patients with highly-aggressive NHL or DLBCL, being replaced by modern chemotherapy as induction therapy before ASCT. Moreover, in order to drop the risk of delayed sequelae such as second cancers, endocrinopathy and cognitive defects, radiotherapy for CNS disease and prophylaxis is less used especially in children, without registering an excessive CNS relapse rate (110). On the other hand, in patients with SCNS-DLBCL, radiotherapy is now part of the induction therapy, followed or not by ASCT, or as consolidation after primary chemotherapy. A transitory clinical benefit with WBRT is reported when used as palliative strategy in over 65% of patients with focal neurological deficits, or with intraparenchymal lesions in the brain, cranial nerves or spinal cord.
The role of allogeneic transplantation
Anecdotal case-series studies on different hematological malignancies and active CNS disease assessed the role of allogeneic transplantation in this setting. In particular, activity on CNS disease mediated by a graft versus leukemia/lymphoma within the brain have been reported (111,112). Unfortunately, it was recorded an elevated treatment-related mortality with a possible influence on the real survival benefit. Moreover, based on the results reported in the setting of highly-aggressive lymphomas, in which CNS recurrence rate was lower with allogeneic transplantation respect to ASCT, both in children and in adults, this strategy should be take into account especially for patients with chemosensitive disease; nevertheless, the true impact might be influenced by the interpretation bias related to the effect of immunosuppressive therapy used for prevention or treatment of graft-versus-host disease.
Conclusion and future perspectives
Effective prevention and treatment of CNS dissemination will be a milestone in the progress of the management of patients with DLBCL. CNS prophylaxis, in particular with HD-MTX, seems to prevent successful this disappointing event. However, this strategy is associated with side effects that preclude its use in a wide DLBCL population. Selection of high-risk patients using conventional clinical parameters, especially IPI variables, is often unsuccessful given the low sensitivity of the resulting scores. Perhaps, based on these considerations, we have to come up with new ideas able to help us to better stratify patients’ risk. In particular, thanks to the better knowledge achieved, we must start exploring the integration between conventional clinical parameters and pathological and molecular factors. Ideally, any progress achieved with the combined use of these factors should give information on sensitivity and specificity, and possibly propose the use of molecular techniques that could be easily translate into routine practice. Moreover, independent confirmatory studies should be conducted to support the results obtained. The aim of future studies should be focused on the identification of molecules with a good predictive value that are involved in lymphocyte activation, adhesion and trafficking towards the CNS. Some of these molecules have already been reported, but important investments and collaborative efforts are required in order to perform morphological and molecular studies able to identify many others. Indeed, identifying reliable predictors of CNS dissemination and defining effective prophylaxis will result in a substantial reduction in the number of patients with SCNSL. In the meantime, new combinations and strategies to treat SCNSL should be investigated in prospective trials. Among others, the use of new agents active against DLBCL, immunomodulation and BBB permeabilization are exciting therapeutic approaches to be addressed in this setting.
Acknowledgments
We are indebted to oncologists, hematologists, neuroradiologists, neurologists, neurosurgeons, radiation oncologists, pathologists, physiotherapists and nurses of the San Raffaele Scientific Institute in Milan, Italy, for the dedication and expertise always shown in the care of our patients and the sustained scientific collaboration.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Astrid Pavlovsky) for the series “Diffuse Large B-Cell Lymphoma” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol.2019.06.01). The series “Diffuse Large B-Cell Lymphoma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferreri AJM. Secondary CNS lymphoma: the poisoned needle in the haystack. Ann Oncol 2017;28:2335-7. [Crossref] [PubMed]
- Ferreri AJM, Assanelli A, Crocchiolo R, et al. Central nervous system dissemination in immunocompetent patients with aggressive lymphomas: incidence, risk factors and therapeutic options. Hematol Oncol 2009;27:61-70. [Crossref] [PubMed]
- Ferreri AJM, Bruno Ventre M, Donadoni G, et al. Safety and activity of a new intensive short-term chemoimmunotherapy in HIV-positive patients with Burkitt lymphoma. Br J Haematol 2012;159:252-5. [Crossref] [PubMed]
- Feugier P, Virion JM, Tilly H, et al. Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large-B-cell lymphoma: influence of rituximab. Ann Oncol 2004;15:129-33. [Crossref] [PubMed]
- Boehme V, Schmitz N, Zeynalova S, et al. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood 2009;113:3896-902. [Crossref] [PubMed]
- Villa D, Connors JM, Shenkier TN, et al. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol 2010;21:1046-52. [Crossref] [PubMed]
- Deng L, Song Y, Zhu J, et al. Secondary central nervous system involvement in 599 patients with diffuse large B-cell lymphoma: are there any changes in the rituximab era? Int J Hematol 2013;98:664-71. [Crossref] [PubMed]
- Ferreri AJM, Bruno-Ventre M, Donadoni G, et al. Risk-tailored CNS prophylaxis in a mono-institutional series of 200 patients with diffuse large B-cell lymphoma treated in the rituximab era. Br J Haematol 2015;168:654-62. [Crossref] [PubMed]
- Schmitz N, Zeynalova S, Nickelsen M, et al. CNS International Prognostic Index: A Risk Model for CNS Relapse in Patients With Diffuse Large B-Cell Lymphoma Treated With R-CHOP. J Clin Oncol 2016;34:3150-6. [Crossref] [PubMed]
- Bernstein SH, Unger JM, Leblanc M, et al. Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516 -- the Southwest Oncology Group. J Clin Oncol 2009;27:114-9. [Crossref] [PubMed]
- El-Galaly TC, Cheah CY, Bendtsen MD, et al. Treatment strategies, outcomes and prognostic factors in 291 patients with secondary CNS involvement by diffuse large B-cell lymphoma. Eur J Cancer 2018;93:57-68. [Crossref] [PubMed]
- Ghose A, Elias HK, Guha G, et al. Influence of Rituximab on Central Nervous System Relapse in Diffuse Large B-Cell Lymphoma and Role of Prophylaxis--A Systematic Review of Prospective Studies. Clin Lymphoma Myeloma Leuk 2015;15:451-7. [Crossref] [PubMed]
- Gleeson M, Counsell N, Cunningham D, et al. Central nervous system relapse of diffuse large B-cell lymphoma in the rituximab era: results of the UK NCRI R-CHOP-14 versus 21 trial. Ann Oncol 2017;28:2511-6. [Crossref] [PubMed]
- Ferreri AJM. Risk of CNS dissemination in extranodal lymphomas. Lancet Oncol 2014;15:e159-69. [Crossref] [PubMed]
- van Besien K, Ha CS, Murphy S, et al. Risk factors, treatment, and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood 1998;91:1178-84. [PubMed]
- Vitolo U, Chiappella A, Ferreri AJM, et al. First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international phase II trial. J Clin Oncol 2011;29:2766-72. [Crossref] [PubMed]
- Laskin JJ, Savage KJ, Voss N, et al. Primary paranasal sinus lymphoma: natural history and improved outcome with central nervous system chemoprophylaxis. Leuk Lymphoma 2005;46:1721-7. [Crossref] [PubMed]
- Ferry JA, Fung CY, Zukerberg L, et al. Lymphoma of the ocular adnexa: A study of 353 cases. Am J Surg Pathol 2007;31:170-84. [Crossref] [PubMed]
- Ponzoni M, Ferreri AJM, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol 2007;25:3168-73. [Crossref] [PubMed]
- Buell JF, Gross TG, Hanaway MJ, et al. Posttransplant lymphoproliferative disorder: significance of central nervous system involvement. Transplant Proc 2005;37:954-5. [Crossref] [PubMed]
- Desai J, Mitnick RJ, Henry DH, et al. Patterns of central nervous system recurrence in patients with systemic human immunodeficiency virus-associated non-hodgkin lymphoma. Cancer 1999;86:1840-7. [Crossref] [PubMed]
- Kim YR, Kim JS, Min YH, et al. Prognostic factors in primary diffuse large B-cell lymphoma of adrenal gland treated with rituximab-CHOP chemotherapy from the Consortium for Improving Survival of Lymphoma (CISL). J Hematol Oncol 2012;5:49. [Crossref] [PubMed]
- Villa D, Connors JM, Sehn LH, et al. Diffuse large B-cell lymphoma with involvement of the kidney: outcome and risk of central nervous system relapse. Haematologica 2011;96:1002-7. [Crossref] [PubMed]
- Zucca E, Conconi A, Mughal TI, et al. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol 2003;21:20-7. [Crossref] [PubMed]
- Ryan G, Martinelli G, Kuper-Hommel M, et al. Primary diffuse large B-cell lymphoma of the breast: prognostic factors and outcomes of a study by the International Extranodal Lymphoma Study Group. Ann Oncol 2008;19:233-41. [Crossref] [PubMed]
- Kridel R, Telio D, Villa D, et al. Diffuse large B-cell lymphoma with testicular involvement: outcome and risk of CNS relapse in the rituximab era. Br J Haematol 2017;176:210-21. [Crossref] [PubMed]
- Jeanneret-Sozzi W, Taghian A, Epelbaum R, et al. Primary breast lymphoma: patient profile, outcome and prognostic factors. A multicentre Rare Cancer Network study. BMC Cancer 2008;8:86. [Crossref] [PubMed]
- Salzberg MP, Maragulia JC, Press OW, et al. Primary Breast Diffuse Large B Cell Lymphoma: A Distinct Clinical Entity. Blood 2012;120:1618.
- Lee GW, Go SI, Kim SH, et al. Clinical outcome and prognosis of patients with primary sinonasal tract diffuse large B-cell lymphoma treated with rituximab-cyclophosphamide, doxorubicin, vincristine and prednisone chemotherapy: a study by the Consortium for Improving Survival of Lymphoma. Leuk Lymphoma 2015;56:1020-6. [Crossref] [PubMed]
- Jenkins C, Rose GE, Bunce C, et al. Clinical features associated with survival of patients with lymphoma of the ocular adnexa. Eye (Lond) 2003;17:809-20. [Crossref] [PubMed]
- Monnard V, Sun A, Epelbaum R, et al. Primary spinal epidural lymphoma: patients’ profile, outcome, and prognostic factors: a multicenter Rare Cancer Network study. Int J Radiat Oncol Biol Phys 2006;65:817-23. [Crossref] [PubMed]
- Mozos A, Ye H, Chuang WY, et al. Most primary adrenal lymphomas are diffuse large B-cell lymphomas with non-germinal center B-cell phenotype, BCL6 gene rearrangement and poor prognosis. Mod Pathol 2009;22:1210-7. [Crossref] [PubMed]
- Vélayoudom FL, Cardot-Bauters C, Decouvelaere AV, et al. Non Hodgkin’s lymphoma involving the adrenal glands and the central nervous system (CNS): a particular evolution after chemotherapy. Ann Endocrinol (Paris) 2005;66:527-31. [Crossref] [PubMed]
- Zhang J, Chen B, Xu X. Impact of rituximab on incidence of and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: a systematic review and meta-analysis. Leuk Lymphoma 2014;55:509-14. [Crossref] [PubMed]
- Oprea C, Cainap C, Azoulay R, et al. Primary diffuse large B-cell non-Hodgkin lymphoma of the paranasal sinuses: a report of 14 cases. Br J Haematol 2005;131:468-71. [Crossref] [PubMed]
- Murawski N, Held G, Ziepert M, et al. The role of radiotherapy and intrathecal CNS prophylaxis in extralymphatic craniofacial aggressive B-cell lymphomas. Blood 2014;124:720-8. [Crossref] [PubMed]
- Cheung CW, Burton C, Smith P, et al. Central nervous system chemoprophylaxis in non-Hodgkin lymphoma: current practice in the UK. Br J Haematol 2005;131:193-200. [Crossref] [PubMed]
- Olsen TG, Holm F, Mikkelsen LH, et al. Orbital Lymphoma - An International Multicenter Retrospective Study. Am J Ophthalmol 2019;199:44-57. [Crossref] [PubMed]
- Munch-Petersen HD, Rasmussen PK, Coupland SE, et al. Ocular Adnexal Diffuse Large B-cell Lymphoma: A Multicenter International Study. JAMA Ophthalmol 2015;133:165-73. [Crossref] [PubMed]
- Ahmed AH, Foster CS, Shields CL. Association of Disease Location and Treatment With Survival in Diffuse Large B-Cell Lymphoma of the Eye and Ocular Adnexal Region. JAMA Ophthalmol 2017;135:1062-8. [Crossref] [PubMed]
- Ferreri AJM. The Orbit and the Brain: Cheek by Jowl or Very Far Away? JAMA Ophthalmol 2015;133:726-7. [Crossref] [PubMed]
- Yhim HY, Kang HJ, Choi YH, et al. Clinical outcomes and prognostic factors in patients with breast diffuse large B cell lymphoma; Consortium for Improving Survival of Lymphoma (CISL) study. BMC Cancer 2010;10:321. [Crossref] [PubMed]
- Cheah CY, Campbell BA, Seymour JF. Primary breast lymphoma. Cancer Treat Rev 2014;40:900-8. [Crossref] [PubMed]
- Guo HY, Zhao XM, Li J, et al. Primary non-Hodgkin’s lymphoma of the breast: eight-year follow-up experience. Int J Hematol 2008;87:491-7. [Crossref] [PubMed]
- Fukuhara S, Watanabe T, Munakata W, et al. Bulky disease has an impact on outcomes in primary diffuse large B-cell lymphoma of the breast: a retrospective analysis at a single institution. Eur J Haematol 2011;87:434-40. [Crossref] [PubMed]
- Hosein PJ, Maragulia JC, Salzberg MP, et al. A multicentre study of primary breast diffuse large B-cell lymphoma in the rituximab era. Br J Haematol 2014;165:358-63. [Crossref] [PubMed]
- Ludmir EB, Milgrom SA, Pinnix CC, et al. Primary breast diffuse large B-cell lymphoma: treatment strategies and patterns of failure. Leuk Lymphoma 2018;59:2896-903. [Crossref] [PubMed]
- Salvati M, Cervoni L, Artico M, et al. Primary spinal epidural non-Hodgkin’s lymphomas: a clinical study. Surg Neurol 1996;46:339-43. [Crossref] [PubMed]
- Mian M, Ferreri AJM, Rossi A, et al. Role of radiotherapy in patients with early-stage diffuse large B-cell lymphoma of Waldeyer’s ring in remission after anthracycline-containing chemotherapy. Leuk Lymphoma 2013;54:62-8. [Crossref] [PubMed]
- Yun J, Kim SJ, Won JH, et al. Clinical features and prognostic relevance of ovarian involvement in non-Hodgkin’s lymphoma: A Consortium for Improving Survival of Lymphoma (CISL) report. Leuk Res 2010;34:1175-9. [Crossref] [PubMed]
- El-Galaly TC, Cheah CY, Hutchings M, et al. Uterine, but not ovarian, female reproductive organ involvement at presentation by diffuse large B-cell lymphoma is associated with poor outcomes and a high frequency of secondary CNS involvement. Br J Haematol 2016;175:876-83. [Crossref] [PubMed]
- Gardette E, Maraval A, Brunet-Possenti F, et al. Central nervous system involvement of primary cutaneous diffuse large B-cell lymphoma, leg type: 13 cases. J Eur Acad Dermatol Venereol 2017;31:e498-501. [Crossref] [PubMed]
- Hollender A, Kvaloy S, Nome O, et al. Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: a risk model. Ann Oncol 2002;13:1099-107. [Crossref] [PubMed]
- Kanemasa Y, Shimoyama T, Sasaki Y, et al. Central nervous system relapse in patients with diffuse large B cell lymphoma: analysis of the risk factors and proposal of a new prognostic model. Ann Hematol 2016;95:1661-9. [Crossref] [PubMed]
- Tomita N, Yokoyama M, Yamamoto W, et al. The standard international prognostic index for predicting the risk of CNS involvement in DLBCL without specific prophylaxis. Leuk Lymphoma 2018;59:97-104. [Crossref] [PubMed]
- Haioun C, Besson C, Lepage E, et al. Incidence and risk factors of central nervous system relapse in histologically aggressive non-Hodgkin’s lymphoma uniformly treated and receiving intrathecal central nervous system prophylaxis: a GELA study on 974 patients. Groupe d’Etudes des Lymphomes de l’Adulte. Ann Oncol 2000;11:685-90. [Crossref] [PubMed]
- Boehme V, Zeynalova S, Kloess M, et al. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma--a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Ann Oncol 2007;18:149-57. [Crossref] [PubMed]
- Yamamoto W, Tomita N, Watanabe R, et al. Central nervous system involvement in diffuse large B-cell lymphoma. Eur J Haematol 2010;85:6-10. [PubMed]
- Kumar A, Vanderplas A, LaCasce AS, et al. Lack of benefit of central nervous system prophylaxis for diffuse large B-cell lymphoma in the rituximab era: findings from a large national database. Cancer 2012;118:2944-51. [Crossref] [PubMed]
- Nijland M, Boslooper K, van Imhoff G, et al. Relapse in stage I(E) diffuse large B-cell lymphoma. Hematol Oncol. 2018;36:416-21. [Crossref] [PubMed]
- Ferreri AJ, Calimeri T, Cecchetti C, et al. Prophylaxis with high-dose methotrexate significantly reduces CNS dissemination in patients with diffuse large B-cell lymphoma (DLBCL) and high-risk CNS-IPI score. Hematol Oncol 2017;35:198. [Crossref]
- Avilés A, Delgado S, Nambo MJ, et al. Primary breast lymphoma: results of a controlled clinical trial. Oncology 2005;69:256-60. [Crossref] [PubMed]
- Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol 2014;166:891-901. [Crossref] [PubMed]
- Savage KJ, Slack GW, Mottok A, et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood 2016;127:2182-8. [Crossref] [PubMed]
- Klanova M, Sehn LH, Bence-Bruckler I, et al. Cell of Origin Combined with Cns International Prognostic Index Improves Identification of Dlbcl Patients with High Cns Relapse Risk After Initial Immunochemotherapy. Hematol Oncol 2017;35:43-4. [Crossref]
- Yamaguchi M, Nakamura N, Suzuki R, et al. De novo CD5+ diffuse large B-cell lymphoma: results of a detailed clinicopathological review in 120 patients. Haematologica 2008;93:1195-202. [Crossref] [PubMed]
- Xu-Monette ZY, Tu M, Jabbar KJ, et al. Clinical and biological significance of de novo CD5+ diffuse large B-cell lymphoma in Western countries. Oncotarget 2015;6:5615-33. [PubMed]
- Cox MC, Di Napoli A, Scarpino S, et al. Clinicopathologic characterization of diffuse-large-B-cell lymphoma with an associated serum monoclonal IgM component. PLoS One 2014;9:e93903 [Crossref] [PubMed]
- Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood 2014;124:2354-61. [Crossref] [PubMed]
- Tilly H, Vitolo U, Walewski J, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii78-82. [Crossref] [PubMed]
- Scott DW, King RL, Staiger AM, et al. High grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood 2018;131:2060-4. [Crossref] [PubMed]
- Miyazaki K, Yamaguchi M, Suzuki R, et al. CD5-positive diffuse large B-cell lymphoma: a retrospective study in 337 patients treated by chemotherapy with or without rituximab. Ann Oncol 2011;22:1601-7. [Crossref] [PubMed]
- Chuang WY, Chang H, Shih LY, et al. CD5 positivity is an independent adverse prognostic factor in elderly patients with diffuse large B cell lymphoma. Virchows Arch 2015;467:571-82. [Crossref] [PubMed]
- Ollila TA, Olszewski AJ. Extranodal Diffuse Large B Cell Lymphoma: Molecular Features, Prognosis, and Risk of Central Nervous System Recurrence. Curr Treat Options Oncol 2018;19:38. [Crossref] [PubMed]
- Kraan W, Horlings HM, van Keimpema M, et al. High prevalence of oncogenic MYD88 and CD79B mutations in diffuse large B-cell lymphomas presenting at immune-privileged sites. Blood Cancer J 2013;3:e139 [Crossref] [PubMed]
- Cao XX, Li J, Cai H, et al. Patients with primary breast and primary female genital tract diffuse large B cell lymphoma have a high frequency of MYD88 and CD79B mutations. Ann Hematol 2017;96:1867-71. [Crossref] [PubMed]
- Taniguchi K, Takata K, Chuang S, et al. Frequent myd88 L265p and cd79b Mutations in Primary Breast Diffuse Large B-cell Lymphoma. Am J Surg Pathol 2016;40:324-34. [Crossref] [PubMed]
- Kersten MJ, Kraan W, Doorduijn J, et al. Diffuse large B cell lymphomas relapsing in the CNS lack oncogenic MYD88 and CD79B mutations. Blood Cancer J 2014;4:e266 [Crossref] [PubMed]
- Benevolo G, Stacchini A, Spina M, et al. Final results of a multicenter trial addressing role of CSF flow cytometric analysis in NHL patients at high risk for CNS dissemination. Blood 2012;120:3222-8. [Crossref] [PubMed]
- Cortes J, O’Brien SM, Pierce S, et al. The value of high-dose systemic chemotherapy and intrathecal therapy for central nervous system prophylaxis in different risk groups of adult acute lymphoblastic leukemia. Blood 1995;86:2091-7. [PubMed]
- Aviles A, Neri N, Nambo MJ. The role of genotype in 104 cases of diffuse large B-cell lymphoma primary of breast. Am J Clin Oncol 2012;35:126-9. [Crossref] [PubMed]
- Wilson WH, Bromberg JEC, Stetler-Stevenson M, et al. Detection and outcome of occult leptomeningeal disease in diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica 2014;99:1228-35. [Crossref] [PubMed]
- Corazzelli G, Frigeri F, Russo F, et al. RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and «unclassifiable» highly aggressive B-cell lymphoma. Br J Haematol 2012;156:234-44. [Crossref] [PubMed]
- Calimeri T, Cecchetti C, Vignati A, et al. High-dose methotrexate (HD-MTX) as CNS prophylaxis significantly improves outcome in patients with high-risk diffuse large B-cell lymphoma (DLBCL). Ann Oncol [published 11 October 2016]. Available online: https://academic.oup.com/annonc/article/27/suppl_6/908O/2799645
- Tilly H, Lepage E, Coiffier B, et al. Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood 2003;102:4284-9. [Crossref] [PubMed]
- Holte H, Leppä S, Björkholm M, et al. Dose-densified chemoimmunotherapy followed by systemic central nervous system prophylaxis for younger high-risk diffuse large B-cell/follicular grade 3 lymphoma patients: results of a phase II Nordic Lymphoma Group study. Ann Oncol 2013;24:1385-92. [Crossref] [PubMed]
- Phillips EH, Kirkwood AA, Lawrie A, et al. Low Rates of CNS Relapse in High Risk DLBCL Patients Treated with R-CODOX-M and R-IVAC: Results from a Phase 2 UK NCRI/Bloodwise Trial. Blood 2016;128:1855.
- Abramson JS, Hellmann M, Barnes JA, et al. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer 2010;116:4283-90. [Crossref] [PubMed]
- Cheah CY, Herbert KE, O’Rourke K, et al. A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. Br J Cancer 2014;111:1072-9. [Crossref] [PubMed]
- Guirguis HR, Cheung MC, Mahrous M, et al. Impact of central nervous system (CNS) prophylaxis on the incidence and risk factors for CNS relapse in patients with diffuse large B-cell lymphoma treated in the rituximab era: a single centre experience and review of the literature. Br J Haematol 2012;159:39-49. [Crossref] [PubMed]
- Avilés A, Jesús Nambo M, Neri N. Central nervous system prophylaxis in patients with aggressive diffuse large B cell lymphoma: an analysis of 3,258 patients in a single center. Med Oncol 2013;30:520. [Crossref] [PubMed]
- Malecek MK, Rozell S, Chu BA, et al. Risk Factors for CNS Relapse Among Patients with DLBCL Treated with EPOCH-R. Blood 2015;126:1500.
- Decker D. Survival and Risk of Central Nervous System Recurrence in Burkitt or High-Grade B-Cell Lymphoma in the DA-EPOCH-R Era. In ASH; 2018. Available online: https://ash.confex.com/ash/2018/webprogram/Paper115676.html
- Bromberg JEC, Doorduijn JK, Illerhaus G, et al. Central nervous system recurrence of systemic lymphoma in the era of stem cell transplantation - an international primary central nervous system lymphoma study group project. Haematologica. 2013;98:808-13. [Crossref] [PubMed]
- Patrij K, Reiser M, Wätzel L, et al. Isolated central nervous system relapse of systemic lymphoma (SCNSL): clinical features and outcome of a retrospective analysis. Ger Med Sci 2011;9:Doc11. [PubMed]
- Thyss A, Milano G, Deville A, et al. Effect of dose and repeat intravenous 24 hr infusions of methotrexate on cerebrospinal fluid availability in children with hematological malignancies. Eur J Cancer Clin Oncol 1987;23:843-7. [Crossref] [PubMed]
- Milano G, Thyss A, Debeauvais FS, et al. CSF drug levels for children with acute lymphoblastic leukemia treated by 5 g/m2 methotrexate: A study from the EORTC childrens’ leukemia cooperative group. Eur J Cancer 1990;26:492-5. [Crossref] [PubMed]
- Millot F, Rubie H, Mazingue F, et al. Cerebrospinal fluid drug levels of leukemic children receiving intravenous 5 g/m2 methotrexate. Leuk Lymphoma 1994;14:141-4. [Crossref] [PubMed]
- Ferreri AJM. How I treat primary CNS lymphoma. Blood 2011;118:510-22. [Crossref] [PubMed]
- Korfel A, Elter T, Thiel E, et al. Phase II study of central nervous system (CNS)-directed chemotherapy including high-dose chemotherapy with autologous stem cell transplantation for CNS relapse of aggressive lymphomas. Haematologica 2013;98:364-70. [Crossref] [PubMed]
- Ferreri AJM, Donadoni G, Cabras MG, et al. High Doses of Antimetabolites Followed by High-Dose Sequential Chemoimmunotherapy and Autologous Stem-Cell Transplantation in Patients With Systemic B-Cell Lymphoma and Secondary CNS Involvement: Final Results of a Multicenter Phase II Trial. J Clin Oncol 2015;33:3903-10. [Crossref] [PubMed]
- Doorduijn JK, van Imhoff GW, van der Holt B, et al. Treatment of secondary central nervous system lymphoma with intrathecal rituximab, high-dose methotrexate, and R-DHAP followed by autologous stem cell transplantation: results of the HOVON 80 phase 2 study. Hematol Oncol 2017;35:497-503. [Crossref] [PubMed]
- Williams CD, Pearce R, Taghipour G, et al. Autologous bone marrow transplantation for patients with non-Hodgkin’s lymphoma and CNS involvement: those transplanted with active CNS disease have a poor outcome--a report by the European Bone Marrow Transplant Lymphoma Registry. J Clin Oncol 1994;12:2415-22. [Crossref] [PubMed]
- Alvarnas JC, Negrin RS, Horning SJ, et al. High-dose therapy with hematopoietic cell transplantation for patients with central nervous system involvement by non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant 2000;6:352-8. [Crossref] [PubMed]
- Ferreri AJM, Illerhaus G. The role of autologous stem cell transplantation in primary CNS lymphoma. Blood 2016;127:1642-9. [Crossref] [PubMed]
- Wiebe VJ, Smith BK, Degregorio M, et al. Pharmacology of agents used in bone marrow transplant conditioning regimens. Critical reviews in oncology/hematology 1992;13:241-70. [Crossref] [PubMed]
- Abrey LE, Moskowitz CH, Mason WP, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol 2003;21:4151-6. [Crossref] [PubMed]
- Kasenda B, Schorb E, Fritsch K, et al. Prognosis after high-dose chemotherapy followed by autologous stem-cell transplantation as first-line treatment in primary CNS lymphoma—a long-term follow-up study. Ann Oncol 2012;23:2670-5. [Crossref] [PubMed]
- Rubenstein JL, Fridlyand J, Abrey L, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 2007;25:1350-6. [Crossref] [PubMed]
- Bluhm EC, Ronckers C, Hayashi RJ, et al. Cause-specific mortality and second cancer incidence after non-Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood 2008;111:4014-21. [Crossref] [PubMed]
- Varadi G, Or R, Kapelushnik J, et al. Graft-versus-lymphoma effect after allogeneic peripheral blood stem cell transplantation for primary central nervous system lymphoma. Leuk Lymphoma 1999;34:185-90. [Crossref] [PubMed]
- Lotze C, Schüler F, Krüger WH, et al. Combined immunoradiotherapy induces long-term remission of CNS relapse of peripheral, diffuse, large-cell lymphoma after allogeneic stem cell transplantation: case study. Neuro-oncology 2005;7:508. [Crossref] [PubMed]
Cite this article as: Calimeri T, Lopedote P, Ferreri AJM. Risk stratification and management algorithms for patients with diffuse large B-cell lymphoma and CNS involvement. Ann Lymphoma 2019;3:7.



