
Initial treatment of elderly population with aggressive lymphoma: a narrative review of current evidence and future directions
Introduction
As a result of an increase in life expectancy, the overall percentage of the elderly population with hematopoietic malignancies such as lymphoma is growing. Non-Hodgkin lymphoma (NHL) is the seventh most common malignancy in men and the sixth most common in women. Annually, more than 77,000 new NHL cases are diagnosed in the US, and more than 20,000 individuals die from this malignancy (1,2). Lymphomas can be broadly categorized as aggressive or indolent. The most common subtype of aggressive lymphoma (AL) is diffuse large B-cell lymphoma (DLBCL) (1-4).
DLBCL is potentially curable with combination immunochemotherapy but becomes distinctly more difficult to cure as patients get older and typically frailer. Frailty is a syndrome consisting of the physiological, psychological, functional, and social domains. Frailty in the elder population results in more vulnerability following a physiological stressor such as antineoplastic treatments, including chemotherapy, immunotherapy, and targeted agents. The coexistence of comorbidities such as heart failure and renal failure increases the risk of drug accumulation, toxicity, and organ damage. At the genomic level, the higher rate of DNA damage due to alteration in repair mechanisms and higher occurrence of hematopoietic malignancies with genetic complexity may result in malignancies more challenging to manage (1-5).
Hence, it is essential to consider the degree of frailty and the potential adverse effect (AE) of treatment on different organs’ function while planning the initial therapy in the elderly population with DLBCL. Further, as the result of this decline in elderly physiological reserve, it is pertinent to dynamically evaluate and monitor their organ function at baseline, during, and after treatment. Regardless of recent advancements in therapeutic modalities, guidelines for dose adjustment, and geriatric assessment tools, the baseline evaluation, treatment, and follow-up remain a challenge for physicians and represent a burden for elderly patients with DLBCL (4-6). This review article discusses challenges inherent in treating elderly patients with DLBCL and outlines potential strategies for better treatment outcomes. We explore different promising regimens investigated in this population base on the level of their functionality. We present the following article in accordance with the Narrative Review reporting checklist. We present the following article in accordance with the Narrative Review reporting checklist (available at https://aol.amegroups.com/article/view/10.21037/aol-22-9/rc).
Methods
We identified relevant studies to our topic using PubMed and Google Scholar databases. No filtration was implemented for article selection while most relevant peer reviewed international literatures were included in our narrative review (see Table 1).
Table 1
| Items | Specifications |
|---|---|
| Date of search | Literature search was conducted between January 12, 2022, and April 5, 2022 |
| Databases and other sources searched | PubMed, Google Scholar |
| Search terms used | Aggressive, lymphoma, DLBCL, elderly, immunotherapy, chemotherapy, frail, fit, unfit, |
| Timeframe | Not specified |
| Inclusion and exclusion criteria | Included international peer-reviewed papers in the English language |
| Selection process | All the authors were involved in the selection and reviewing of the relevant publications |
DLBCL, diffuse large B-cell lymphoma.
Preparatory measures in initiating treatment
The initial step for assessing comorbidities and choosing the best therapeutic strategies is to determine baseline organ functional status by conducting a thorough medical history (specifically drug history), physical exam, and using clinical apparatus for estimating cardiac, renal, and pulmonary functions. The next step is to measure and score the concomitant risk factors using comorbidity scoring tools. Geriatric assessment tools have been developed to screen multiple geriatric-related domains and provide more accurate frailty assessment and functional reserve. Several geriatric assessment modalities have been devised and implemented in hematology and oncology clinical settings to identify subjects with a higher risk of morbidity or mortality after treatment (7,8).
Charlson et al. were among the first to develop comorbidity assessment tools. The Charlson Comorbidity Index (CCI) is a comprehensive tool that evaluates comorbidities that can alter clinical outcomes in longitudinal studies (9,10). CCI scores have shown to be independently associated with clinical outcomes in both oncologic and hematologic settings. A recent retrospective study by Johnson et al. investigated clinical outcomes and treatment toxicity in newly diagnosed NHL adults (from 2000 to 2020) aged ≥65 years who received systemic therapy. The authors found that 42.4% of the patients experienced grade 3+ toxicity, with 8.1% who experienced grades 4 or 5. Moreover, the study’s results showed that the rates of unplanned hospitalization were 41.0% (6.1% of ICU admission). Among the investigated variables, patients with hypoalbuminemia and higher Charlson comorbidity score had significantly higher treatment-related toxicity and unplanned hospitalization (11). Another practical and comprehensive comorbidity scale is the Cumulative Illness Rating Scale-Geriatric (CIRS-G) which contains all organ functions, including psychiatric illnesses. Like CCI, CIRS-G has shown to be a valuable prognostic tool and independently correlates with outcomes in patients with NHL (12).
Besides organ function, specific attention must be paid to the physical status, psychological condition, cognitive function, and life expectancy of elderly patients with AL. The comprehensive geriatric assessment (CGA) is a multi-domain assessment tool implemented for the elderly and frail population. Besides organ function, it includes physical, psychological, mental health, cognitive function, nutritional status, socioeconomic status, and polypharmacy. Multiple studies have demonstrated the correlation of CGA score with mortality and morbidity in older patients with hematologic malignancies and further proof of the benefit of its usage in planning treatment strategies. However, the CGA requires clinician training and demands time and resources to administer (13,14).
Tucci et al. introduced a simplified version of CGA (SCGA) to further subcategorize frail elderly patients with DLBCL to adjust treatment intensity. Like CGA, SCGA has multi-domains, including the activity of daily living (ADL), Lawton-Brody Instrumental Activities of Daily Living (IADL), age, and CIRS-G, classifying patients into the fit, unfit, and frail categories. In their prospective multicenter observational study, Tucci and colleagues validated the SCGA as a predictive tool for outcome survival study in elderly populations with DLBCL. However, SCGA does not include patients’ cognitive function, a vital prognostic factor. Some studies have demonstrated a lack of consistency in integrating SCGA-based approaches and improved patient outcomes (15).
Considering CGA and SCGA weaknesses, Di et al. implemented the Surveillance, Epidemiology, and End Results (SEER)-Medicare database combined with the Outcome and Assessment Information Set (OASIS). They proposed a novel global risk indicator that includes cognitive function as well as age, comorbidities, and functional status. Their results suggest that the global risk indicator tool is an independent predictor of treatment approach, adverse events in short intervals, and overall survival (OS) in the long term in the geriatric population with DLBCL and was superior to single domain assessment tools (16).
Nutritional status is another crucial factor that needs to be evaluated before initiating therapy. Malnutrition is a common disorder in subjects with hematologic malignancies and is directly correlated with higher mortality (17). Hence several nutritional assessment tools have been developed for better risk stratification. The geriatric nutrition risk index (GNRI) is a modified version of the prognostic nutritional index (PNI) developed for the octogenarian population. PNI is calculated from albumin and absolute lymphocyte count. Besides albumin, GNRI includes weight, ideal weight, and height. Controlling nutritional status (CONUT) is another nutritional index calculated from albumin, absolute lymphocyte count, and cholesterol level (18). Nagata et al., in a recent retrospective study, investigated the effect of the CONUT score on OS in 472 DLBCL patients (median age of 68.5) who underwent initial treatment with either R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) or R-CHOP-like therapeutics. Study results showed that in patients >70 years old, high CONUT scores negatively affect OS (hazard ratio of 1.86 and P<0.01) (19).
DLBCL in elderly population
DLBCL is the most common AL in adults, specifically in the elderly. The median age of diagnosed cases of DLBCL is 66 years old, and almost one-third of them are 75 years old and above. Based on morphology, cytogenic and genomics, and molecular profiling, the 2017 World Health Organization (WHO) guideline updated DLBCL classifications into subtypes, including primary DLBCL of the CNS, primary cutaneous DLBCL leg-type, EBV positive DLBCL, T-cell/histiocyte-rich large B-cell lymphoma (LBLC), primary mediastinal or thymic LBCL, and intravascular LBCL (20). The rest of the DLBCL cases are categorized as DLBCL, not otherwise specified (NOS). Determining the cell of origin (COO) by implementing gene expression profiling, the NOS group is further divided into two subsets; germinal center B-cell-like (GCB) lymphomas that specifically express genes encoding CD10 and BCL6 and activated B-cell-like (ABC) group, that expresses IRF4 and BCL2 but not CD10 and BCL6. The prevalence of ABC increases with age, and it shares a high prevalence in primary DLBCL of the CNS and cutaneous leg-type; Studies have shown that ABC has a lower response rate to standard therapeutic regimens (20-22).
High-grade B-cell lymphoma (HGBL) is a DLBCL category introduced by WHO in the 2016 revision and replaced B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma. HGBL consists of two subgroups, HGBL with MYC and BCL2 or/and BCL6 rearrangements [double hits (DH) and triple-hit (TH) lymphoma] and HGBL NOS. Both categories can affect the geriatric population but with a higher median age in the HGBL NOS subgroup (21,22).
Initial treatment based on level of functionality and comorbidities
Fit category
Like other age groups, the standard treatment of choice for elderly DLBCL patients is R-CHOP. The standard protocol is 3 or 6 cycles (depending on stage) of CHOP combined with rituximab given every 21 days or R-CHOP-21 (rituximab 375 mg/m2, vincristine 1.4 mg/m2 (maximum dose, 2 mg), cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2 on day 1, and prednisone 100 mg days 1–5). Depending on the stage, the number of cycles may be decreased in lower-risk patients in the lower stages (I–II) (23,24). Although R-CHOP remains the standard frontline treatment for fit DLBCL patients, a significant proportion of patients are not cured by this modality (25). Multiple prospective trials have proposed and investigated similar protocols to R-CHOP, hoping to address its limitation in riskier subpopulations such as elderly patients with variable degrees of frailty. Modified and attenuated versions of the R-CHOP such as R-CHOP-14 (reducing R-CHOP-21 intervals to 14 days or two weeks), R-miniCHOP (around 50% dose reduction of CHOP portion), and CHOP-like regimens have been designed and investigated to alleviate potential side effects in the elderly with DLBCL (specifically in patients aged 80 and above) (24). When functionality and age were incorporated for further stratification, these regimens provided adequate disease control and represented rational alternative treatment options (25).
Delarue et al., in a randomized trial, compared R-CHOP-14 and R-CHOP-21 treatments in 602 elderly (aged 60–80 years) subjects with untreated DLBCL. Three hundred and four patients were assigned to the R-CHOP-14 group and 298 patients to the R-CHOP-21. With a median follow-up of 56 months, the 3-year event-free survival did not differ between the two arms. Comparing the two groups, no significant differences were found comparing subjects who experienced at least one adverse event. The authors concluded that R-CHOP-14 and R-CHOP-21 had no differences in efficacy and resulted in a similar frequency of adverse events (26).
DA-EPOCH-R is similar to R-CHOP but also includes dose-adjusted etoposide. Compared to R-CHOP, it has shown significantly better progression-free survival (PFS) (82% in DA-EPOCH-R vs. 43% in R-CHOP) and OS (90% in DA-EPOCH-R vs. 62% in R-CHOP), but in younger patients (age <65 years) (27). Adjusted dosed EPOCH-R is another approach implemented by Zhang et al. in elderly patients with untreated CD20+ DLBCL. They administered 70% standard EPOCH dose to patients aged 75 to 79 years and 50% to patients over 80 years with rituximab at a similar dosage to all patients. The complete response rate was 71%, and 3-year OS and PFS were 62.8% and 60.3%, respectively (28).
Phase III Intergroup Trial Alliance/CALGB 50303 study randomized 524 DLBCL patients in DA-EPOCH-R or R-CHOP arms (each received six cycles). Two-year PFS and OS were almost similar between the two cohorts (86.5% for DA-EPOCH-R vs. 85.7% for R-CHOP). On the other hand, grades 3 and 4 AE were significantly higher in the DA-EPOCH-R arm compared to the R-CHOP arm, including febrile neutropenia (35.0% in DA-EPOCH-R vs. 17.7% in R-CHOP), mucositis (8.4% in DA-EPOCH-R versus 2.1% in R-CHOP neuropathy (18.6% in DA-EPOCH-R vs. 3.3% in R-CHOP) and infection (16.9% in DA-EPOCH-R vs. 10.7% in R-CHOP). The Alliance study results showed that DA-EPOCH-R might be an alternative regimen limited to selected DLBCL subgroups (29). DA-EPOCH-R regimen has shown efficacy in treating DLBCL patients with high International Prognostic Index (IPI) and cytogenic features such as double-hit lymphoma. This treatment approach may be considered an alternative option in selected elderly fit DLBCL with double-hit or triple-hit rearrangements (careful dosing and toxicity profile consideration specifically in fit patients >70 years old) (30-32).
Elderly fit with cardiac comorbidities
Non-pegylated liposomal doxorubicin (NPLD/Myocet™) was developed as an alternative drug to address doxorubicin’s potential cardiac toxicity (33,34). Multiple studies have investigated R-COMP (R-CHOP but with NPLD replacing doxorubicin) (33-37). The randomized phase 2 trial from the GELTAMO group compared R-COMP with R-CHOP (90 subjects and 45 patients in each group) as first-line therapy for DLBCL patients ≥60 years (ECOG <2 in more than 80% of the subjects). The study found no significant differences in 2-year event-free survival (EFS) and PFS and OS probabilities between the two cohorts (EFS of 46% in R-CHOP vs. 62% R-COMP and P=0.083; PFS of 59% in R-CHOP vs. 62% in R-COMP and P=0.505; OS 75% in R-CHOP vs. 73% R-COMP, P=0.751). However, a significantly higher percentage of the R-CHOP group experienced increased troponin levels than the R-COMP group in cycle 6 (100% vs. 63% and P=0.001) and one month after treatment (88% vs. 56%, respectively, P=0.015). Further, nine episodes of cardiovascular AEs were seen in five patients in the R-CHOP patients (four were grade ≥3), and five episodes were seen in four patients in the R-COMP cohort (all grade 1–2) (38). A recent systematic review compared the efficacy of R-COMP with R-CHOP and has shown significantly higher OS (85.9% versus 70%) and PFS (77.0% versus 60 %) pooled estimates in patients who received R-COMP regimens. The authors deduced that R-COMP might represent a safe and effective option for the elderly with DLBCL, specifically for those with cardiac impairment at baseline (39).
R-CEOP (substituting doxorubicin with etoposide in R-CHOP) is another modified regimen designed to lower cardiac risk. Prusila et al., in a recent matched-pair retrospective analysis, compared PFS among patients who received R-CHOP, R-CEOP, and R-CIOP (doxorubicin replaced by epirubicin) and found a reasonable 2-year PFS of 87.7% (40). Another recent retrospective study compared the safety and efficacy of R-CEOP treatment in 70 de novo DLBCL patients (median age of 73) with a matched control group of 140 subjects who received R-CHOP. The median follow-up time of the study was 12 years. No significant differences were seen between the two cohorts concerning the 10-year time to progression and disease-specific survival. However, the 10-year survival was significantly lower in the R-CEOP group than in the control group (30% vs. 49% and P=0.002) due to concomitant comorbidities. The study outcomes proposed that R-CEOP may only be considered an alternative curative option for elderly DLBCL patients with absolute contraindication for anthracycline administration (41).
Mitoxantrone is a synthetic derivative of doxorubicin that works against malignant cells by intercalating DNA. R-CNOP has all R-CHOP components, but doxorubicin is replaced by mitoxantrone. R-CNOP has been previously studied for the treatment of elderly DLBCL patients. Three randomized trials compared R-CNOP with R-CHOP in elderly patients (including DLBCL >80 years) and found it to be inferior for OS, complete remission (CR), and treatment failure (TTF) (42-44).
Unfit and frail category, candidate for curative intent therapy
The concept of lower toxicity and overall better or equivalent survival outcomes while using a lower dosage of CHOP portion or ‘R-miniCHOP’ has been investigated previously. GELA group investigated the safety and efficacy of R-miniCHOP in untreated elderly DLBCL subjects >80 years old (median age of 83 with a range of 80–95 years). The study included one hundred forty-nine patients; the median follow-up was 29 months. Study analysis showed a two-year PFS of 47% with a median PFS of 21 months. The most common side-effect was hematological toxicity [neutropenia in 95 (64%) of patients with grade 3 toxicity or higher in 59 patients and thrombocytopenia in 56 (38%) patients] (45). A recent retrospective study examined intended dose intensity (IDI) and relative dose intensity (RDI) in elderly DLBCL. IDI was defined as the average dose of doxorubicin and cyclophosphamide received in cycle 1. RDI was defined as the total cumulative dose of cyclophosphamide and doxorubicin patients received across all cycles. The study investigated the influence of IDI and RDI with factors including age, Eastern Cooperative Oncology Group performance status (ECOG PS), CIRS-G score, lactate dehydrogenase (LDH), tumor bulkiness, hemoglobulin level, and albumin on outcomes for DLBCL patients ≥70 years. Study findings showed that patients 70–79 years of age treated with IDI ≥80% had superior PFS and OS than those treated with an IDI <80%. However, comparing the same IDI range (IDI ≥80% vs. IDI <80%), no significant differences were seen in patients ≥80 years. Further, multivariable analysis showed that patients 70–79 years treated with IDI <80% had increased CRR, whereas this was not observed in patients 80 years and older treated with IDI <80% (46). Hounsome et al., in their recent population-based study, analyzed 3-year Real World data from Public Health England’s National Cancer Registration and Analysis Service and examined treatment and outcome patterns among patients >65 years who received R-CHOP (n=4,079) vs. R-miniCHOP (n=313 or 7%) between 2013 and 2015. The study results showed that the choice of R-CHOP or R-miniCHOP had no influence over 3-year OS (54% for both) in each group, and both regimens had similar efficacy, specifically in DLBCL patients aged ≥80 years (47).
Bataillard et al. conducted a systematic review investigating the challenge of treating elderly DLBCL cases with a full dose versus reduced dose intensity (DI) of R-CHOP. Thirteen retrospective trials (4,499 subjects) were included in the study. Most of the included high-quality studies showed an association between reduced DI and poorer outcomes in fit patients aged <80. However, in the subgroup population aged ≥80, survival was not consistently affected by reduced DI, and dose-reduced R-CHOP did not compromise survival (48). A recent systematic review analyzed 633 articles exploring the best R-CHOP-based treatment for elderly fit patients with DLBCL. From 2007 and 2020, 64 trials were deemed eligible for the analysis. Most R-CHOP/modified R-CHOP-based studies, including R-miniCHOP, had a CR of over 60% compared to anthracycline-free trials. Moreover, elderly patients >80 years in this subgroup and other groups that received immunochemotherapy with an alternative anthracycline had the highest OS range (46% to 64.7%). Evidence from this systematic review and analysis favored R-miniCHOP or reduced-dose R-CHOP implementation for elderly fit patients >80 years (49).
Ofatumumab is a second-generation, fully human, anti-CD20 monoclonal that inhibits early-stage B lymphocyte activation. It has been used alone or combined with other regimens to treat leukemia and lymphoma (50). Eyre et al., in a phase two randomized trial, investigated CHOP-21 with ofatumumab induction/maintenance therapy (six cycles) in the treatment of forty-three patients (73% aged >60) with Richter transformation of chronic lymphocytic leukemia (CLL). The overall response rate (ORR) was 46%, and the median PFS and median OS were 6.2 and 11.4 months, respectively. The most common adverse effects were fever and infection, and no treatment-related mortality was observed. Comparing the historical outcome authors concluded minimal benefit in the administration of ofatumumab after R-CHOP in treatment for this type of AL (51). A recent metanalysis compared the efficacy and safety of rituximab and anti-CD20 monoclonal antibodies as induction therapy for NHL. Comparing ofatumumab and rituximab, study results did not show any superiority concerning ORR, OS, and CRR but a higher incidence of AEs for patients who received ofatumumab (52). The phase 2 trial from the LYSA group investigated the safety and efficacy of ofatumumab in combination with reduced-dose CHOP in elderly DLBCL patients. The study included 120 DLBCL patients aged 80 years or older (53% had intermediate or high CGA scores). The study results found comparable OS (64.7%) to R-miniCHOP in the past (OS 59%) and the most common adverse effect was grade 3–4 neutropenia (24 subjects or 21%). These results were suggestive that ofatumumab + miniCHOP is safe and effective in elderly DLBCL patients (>80 years old), although it is not superior to R-miniCHOP (53).
Spina et al., in their prospective trial, examined the efficacy and safety of modulated chemotherapeutics based on modified CGA in the newly diagnosed DLBCL elderly population (aged 70 years). Using CGA, they stratified 100 subjects into the fit, unfit, and frail cohorts but administered modulated regimens or doses based on comorbidities and ADL and IADL scores. Step one of stratification was based on the severity of cardiomyopathy using the New York Heart Association or NYHA classification, diabetes, and neutropenia. Patients without comorbidities received either CHOP or R-CHOP regimens. Patients with mild cardiopathy (NYHA class II or CIRS-G grade 2) received CEOP (epirubicin, 70 mg/m2 i.v. on day 1 replaced doxorubicin in the CHOP regimen) or R-CEOP (epirubicin, 70 mg/m2 i.v. on day two instead of doxorubicin). Anthracyclines were omitted for patients with moderate or severe cardiopathy (NYHA class III or class IV or CIRS-G grade 3 or 4), and they were treated with either CVP (cyclophosphamide, vincristine, and prednisone) or R-CVP (rituximab plus CVP regimen). In step two, the dosage of chemotherapies modulated based on ADL or IADL (full dose in ADL score of 6 and/or an IADL score of 7 or 8, 75% of the full dose in patients with an ADL score of 5 and/or an IADL score of 5 or 6 and 50% full dose in patients with ADL score five or an IADL score 5). The treatment resulted in an 81% complete response rate and 80% of 5-year disease-free survival (with 5-year OS rates of 76%, 53%, and 29% (P=0.001), in fit, unfit, and frail subjects, respectively, and similar relapse rate in all three subcategories). Moreover, the rate of grade 3 or 4 hematological and non-hematological toxicities was not significant among the fit, unfit, and frail cohorts. However, frail patients experienced significantly more episodes of febrile neutropenia (33%) than unfit (13%) and fit patients (5%). Further, no significant difference concerning deaths related to toxicity was seen among the fit, unfit and frail cohorts. It was concluded that adjusting R-CVP based on CGA scoring is associated with promising survival outcomes with manageable toxicity in elderly DLBCL patients, especially in fit and unfit subcategories (54).
Unfit and frail category, non-candidate for curative intent therapy
Very old patients with comorbidities and low functionality are considered the most challenging population for treatment. In the unfit and frail elderly for whom a curative regimen is not an option, less intensive treatments with palliative intention are considered a better approach. Patients receiving palliative treatment burden a high risk of death and an overall dismal prognosis (55).
Rituximab mono or in combination have been tested as a palliative treatment. Rituximab, in combination with trofosfamide (alkylating agent), was tested in 11 patients >75 years old (median of 83). CR and PR were seen in 45% and 27% of patients, respectively and the one-year estimated OS was 54.5%. Rituximab-DEVEC is another regimen that showed promising results as a palliative approach. Cox et al. investigated oral regimen, DEVEC (Deltacortene®, etoposide, vinorelbine, cyclophosphamide, +/− rituximab), in 51 elderly DLBCL (including both R/R and treatment naïve frail or unfit) subjects. The treatment naïve (17/51 or 33% of subjects) had a one-year OS of 67% and PFS of 61% (56,57).
For extremely elderly and frail patients, who cannot tolerate curative intent therapy, the lack of application of universal assessment tools in different trials has made the selection of treatment strategies more challenging. A phase II multicenter study in Italy used rituximab combined with bendamustine (BR), an alkylating DNA crosslinker, as front-line therapy in frail DLBCL patients aged >70. Based on the CGA assessment, 78% of subjects were unfit, and 22% were frail. Treatment protocol comprised bendamustine (90 mg/m2, day 1–2) with rituximab (375 mg/m2, day 1) every 28 days. Patients with age-adjusted IPI (aaIPI) =0 and without bulky disease received four cycles of BR followed by two cycles of rituximab. The rest of the patients received six cycles of BR followed by two cycles of rituximab. During the median follow-up of 33 months, the overall CR was 54% (24 patients), the overall response rate was 62%, and the median PFS was ten months. The most frequent adverse event was neutropenia (37.8% of which were grades 3–4) (58). Zeremski et al., in their retrospective multicenter study, ECOG PS as the assessment tool for patient inclusion, comparing BR with R-CHOP. They included patients aged ≥65 with ECOG with PS ≥2 or ≥75 years regardless of PS. One hundred forty patients were included in the study. The study results showed that BR was associated with marked inferior OS (16.3 vs. 75.4 months; P=0.006) and PFS (11.0 vs. 62.3 months; P<0.001). Incorporating multivariate analysis, they concluded that only the high age-adjusted Charlson Comorbidity Index (aaCCI) was associated with inferior PFS in the R-CHOP cohort. Hence, R-CHOP did not show any superiority in older DLBCL patients with comorbidities, and the authors concluded that BR might be an alternative option for elderly DLBCL patients with comorbidities (59).
Palliative radiation (PT) is another modality that may be considered in patients with bulky tumors and multiple comorbidities such as cognitive and physical dysfunction making them poor candidates for any regimen. The dosage and number of fractions can be adjusted based on the type of lymphoma (higher dose in DH/TH DLBCL), site of the tumor, and overall patient functionality and prognosis. While implementing PT in patients with poor prognosis it is crucial to alleviate symptoms while considering factors such as toxicity, quality of life, and patient’s goals of care (60).
Post treatment multidisciplinary care and follow-ups
Aging results in a decrease in renal function and liver volume and consequently alteration of therapies’ pharmacokinetics. In addition, if present, concomitant comorbidities decrease the threshold of therapy-related toxicity and organ damage. DLBCL patients may experience a higher risk of morbidity and mortality from organ impairments and noncancer disorders during and after treatment (1). Close clinical and laboratory monitoring and follow-ups by the outpatient care team have vital roles in risk management during and after treatment.
In a recent cohort population-matched study, Jull et al. investigate the cardiovascular risks in elderly DLBCL patients after treatment. After analyzing data from 1,009 patients and comparison to matched cohort, the authors concluded that fit DLBCL patients aged >75 who received doxorubicin in the context of R-CHOP or attenuated R-CHOP regimen are at increased risk of heart failure. Further, the study found a higher risk of venous thromboembolism (VTE) during the six months after diagnosis (61). These findings justify routine monitoring of elderlies’ cardiac functions [including echocardiogram and electrocardiogram (EKGs)] and assessing any anticoagulation indication after treatment (61,62).
Using US SEER database, Howlader et al. assessed the noncancer causes of mortality among DLBCL patients after immunochemotherapy. From 2002 to 2011, 8,274 deaths from 18,047 DLBCL patients were recorded and included in the analysis. Within the five years after DLBCL diagnosis, infections had the highest standardized mortality ratios (SMRs) after blood disorders (63). A recent retrospective study examined the morbidity and mortality among 690 DLBCL geriatric patients in 8 different centers in the United Kingdom. Study results showed that cumulative incidences of death due to infections were directly associated with IPI scores (3 to 5), CIRS-G scores (≥6), and low albumin, and it increased up to 5 years after DLBCL diagnosis (3.3% at six months to 11.1% at five years) (64). Besides infection, a higher rate of other immune disorders such as humoral deficiencies and autoimmune cytopenias have been detected in DLBCL survivors. These findings on immune system disorders post-DLBCL treatments motivate close surveillance and long-term follow-up.
Besides organ function, nutritional status at baseline, during, and after cancer treatment directly affects survival outcomes. Studies showed that losing weight during the treatment period is associated with a decrease in the number of treatments received and a lower survival rate (65). Chemotherapy by itself affects weight and calorie intake. A recent retrospective study investigated protein-energy malnutrition (PEM) in cancer-related mortality. Data from 76,425 DLBCL cases also diagnosed with PEM were collected using the National Inpatient Sample database. Study results showed that PEM was directly associated with a higher length of hospital stay, neutropenia, candida sepsis, septic shock, bacteremia, and acute kidney injury. These results encourage close and adequate nutritional monitoring and follow-up during and after DLBCL treatment (66).
Future direction
Currently, there is a clear need for a standard and accepted regimen for the elderly DLBCL. Toxicity is one of the major dilemmas with full dose R-CHOP (67,68). Trials incorporating novel targeting agents with attenuated dosing of R-CHOP, such as R-miniCHOP are being evaluated. R-miniCHOP has been studied in multiple trials in the elderly population and is a regimen that is deliverable. The POLAR BEAR trial is currently examining the efficacy and safety of R-miniCHOP and polatuzumab vedotin (antibody-drug conjugate) in frail DLBCL patients >75 or fit >80 years old (NCT04332822). SWOG 1918 is another randomized phase II/III trial that is investigating R-miniCHOP plus/minus oral azacitidine (synergistic effect by DNA hypomethylation) in elderly DLBCL >75 years while incorporating FIL Tool and GCA for frailty assessment (69).
The results of these studies may change the clinical approach and standard treatments in elderly DLBCL patients with or without comorbidities. Considering the effect of novel multi-target therapeutics, prospective treatment strategies also require careful patient stratification with a focus on both elderly’s quality of life and survival outcomes.
Conclusions
AL survival rates have increased in the elderly population during the last three decades. Initial treatment of elderly patients with DLBCL requires a multidisciplinary approach. Multiple functional domains such as physiological, psychological, social, and environmental factors (including support and polypharmacy) must be considered, investigated, and addressed before initiating trials or treatments. Integrative geriatric assessment tools are adjunctive modalities developed for further risk stratification and prognostic evaluation of treatment outcomes. When combined with genotyping categorizations, implementing these multi-domain assessment guidelines has resulted in less biased patient selection, better optimization of the protocols, and more consistent outcomes.
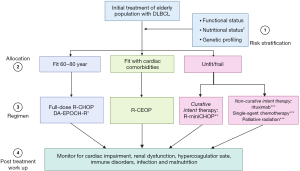
Despite developments of newer chemoimmunotherapeutic regimens that can be applied for octogenarian DLBCL subgroups, R-CHOP and R-CHOP modified regiments remain the best initial options. R-CEOP may be considered an alternative option for fit elderly populations with significant cardiac impairment. When dealing with the very elderly, R-miniCHOP has shown promising results for fit patients >80 years old. Before administering such treatment, one must determine if potential curative treatment can be administered, and if it can, R-miniCHOP is worth considering. Patients for whom curative intent therapy is too toxic, then one must consider simple palliative maneuvers like external beam radiation, single-agent rituximab, or R-CVP (see Figure 1). Post-treatment close laboratory, clinical monitoring, and follow-up are critical concepts for achieving optimum outcomes. For future perspectives, clinical trials investigating new initial regimens need to implement equally reliable integrative assessment tools, perform biological profiling, and plan long-term follow-ups to achieve the most favorable outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jonathon B. Cohen and Craig Portell) for the series “Management of Elderly Patients with HL and NHL” published in Annals of Lymphoma. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at available at https://aol.amegroups.com/article/view/10.21037/aol-22-9/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aol.amegroups.com/article/view/10.21037/aol-22-9/coif). The series “Management of Elderly Patients with HL and NHL” was commissioned by the editorial office without any funding or sponsorship. BSK reports grants to institution from Genentech, Abbvie, BeiGene, consulting fees received from Genentech, PCYC, ADCT, Kite, BeiGene, AstraZeneca, TG, Epizyme, Abbvie, BMS, Genmab, Incyte, Lilly, payments or honoraria from Research to Practice, and he participates on advisory board for MEI, Celgene and Takeda. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ulu BU, Yiğenoğlu TN, Başcı S, et al. Factors affecting survival in elderly patients with diffuse large B-Cell lymphoma. Leuk Res 2021;110:106700. [Crossref] [PubMed]
- Thieblemont C, Bernard S, Molina T. Management of aggressive lymphoma in very elderly patients. Hematol Oncol 2017;35:49-53. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Morrison VA, Hamlin P, Soubeyran P, et al. Diffuse large B-cell lymphoma in the elderly: impact of prognosis, comorbidities, geriatric assessment, and supportive care on clinical practice. An International Society of Geriatric Oncology (SIOG) expert position paper. J Geriatr Oncol 2015;6:141-52. [Crossref] [PubMed]
- Goede V. Hematological Cancer in Older Adults with Frailty. In: Frailty in Older Adults with Cancer. Springer, Cham; 2022:481-93.
- Cordoba R, Luminari S, Eyre TA. The use of frailty assessments in treating older adults with aggressive lymphomas. Br J Haematol 2021;194:677-85. [Crossref] [PubMed]
- Akhtar OS, Huang LW, Tsang M, et al. Geriatric assessment in older adults with non-Hodgkin lymphoma: A Young International Society of Geriatric Oncology (YSIOG) review paper. J Geriatr Oncol 2022;13:572-81. [Crossref] [PubMed]
- Schmittlutz K, Marks R. Current treatment options for aggressive non-Hodgkin lymphoma in elderly and frail patients: practical considerations for the hematologist. Ther Adv Hematol 2021;12:2040620721996484. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Kobayashi Y, Miura K, Hojo A, et al. Charlson Comorbidity Index is an independent prognostic factor among elderly patients with diffuse large B-cell lymphoma. J Cancer Res Clin Oncol 2011;137:1079-84. [Crossref] [PubMed]
- Johnson PC, Yi A, Horick N, et al. Clinical Outcomes, Treatment Toxicity, and Health Care Utilization in Older Adults with Aggressive Non-Hodgkin Lymphoma. Oncologist 2021;26:965-73. [Crossref] [PubMed]
- Nagl L, Koinig K, Hofer F, et al. Comorbidities cluster with impaired functional capacities and depressive mood and predict adverse outcome in older patients with hematological malignancies. Leuk Lymphoma 2020;61:1954-64. [Crossref] [PubMed]
- Hamaker ME, Schiphorst AH, ten Bokkel Huinink D, et al. The effect of a geriatric evaluation on treatment decisions for older cancer patients--a systematic review. Acta Oncol 2014;53:289-96. [Crossref] [PubMed]
- Yamasaki S, Matsushima T, Minami M, et al. Clinical impact of comprehensive geriatric assessment in patients aged 80 years and older with diffuse large B-cell lymphoma receiving rituximab-mini-CHOP: a single-institute retrospective study. Eur Geriatr Med 2022;13:195-201. [Crossref] [PubMed]
- Tucci A, Martelli M, Rigacci L, et al. Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B-cell lymphoma: a prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL). Leuk Lymphoma 2015;56:921-6. [Crossref] [PubMed]
- Di M, Keeney T, Belanger E, et al. Global Risk Indicator and Therapy for Older Patients With Diffuse Large B-Cell Lymphoma: A Population-Based Study. JCO Oncol Pract 2022;18:e383-402. [Crossref] [PubMed]
- Zhang Y, Chen Q, Lu C, et al. Prognostic role of controlling nutritional status score in hematological malignancies. Hematology 2022;27:653-8. [Crossref] [PubMed]
- Yilmaz M, Atilla FD, Sahin F, et al. The effect of malnutrition on mortality in hospitalized patients with hematologic malignancy. Support Care Cancer 2020;28:1441-8. [Crossref] [PubMed]
- Nagata A, Kanemasa Y, Sasaki Y, et al. Clinical impact of controlling nutritional status score on the prognosis of patients with diffuse large B-cell lymphoma. Hematol Oncol 2020;38:309-17. [Crossref] [PubMed]
- Olszewski A, Kurt H, Evens AM. Defining and Treating High-grade B-cell lymphoma, NOS. Blood 2021. [Epub ahead of print]. pii: blood.2020008374. doi:
10.1182/blood.2020008374 .10.1182/blood.2020008374 - Ok CY, Medeiros LJ. High-grade B-cell lymphoma: a term re-purposed in the revised WHO classification. Pathology 2020;52:68-77. [Crossref] [PubMed]
- Nowakowski GS, Czuczman MS. ABC, GCB, and Double-Hit Diffuse Large B-Cell Lymphoma: Does Subtype Make a Difference in Therapy Selection? Am Soc Clin Oncol Educ Book 2015;e449-57. [Crossref] [PubMed]
- Candelaria M, Dueñas-Gonzalez A. Rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in diffuse large B-cell lymphoma. Ther Adv Hematol 2021;12:2040620721989579. [Crossref] [PubMed]
- Pfreundschuh M, Trümper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 2006;7:379-91. [Crossref] [PubMed]
- Goy A. Succeeding in Breaking the R-CHOP Ceiling in DLBCL: Learning From Negative Trials. J Clin Oncol 2017;35:3519-22. [Crossref] [PubMed]
- Delarue R, Tilly H, Mounier N, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol 2013;14:525-33. [Crossref] [PubMed]
- Dodero A, Guidetti A, Tucci A, et al. Dose-adjusted EPOCH plus rituximab improves the clinical outcome of young patients affected by double expressor diffuse large B-cell lymphoma. Leukemia 2019;33:1047-51. [Crossref] [PubMed]
- Zhang WH, Li GY, Ma YJ, et al. Reduced-dose EPOCH-R chemotherapy for elderly patients with advanced stage diffuse large B cell lymphoma. Ann Hematol 2018;97:1809-16. [Crossref] [PubMed]
- Bartlett NL, Wilson WH, Jung SH, et al. Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303. J Clin Oncol 2019;37:1790-9. [Crossref] [PubMed]
- Nelles R, Morris K, Scott A, et al. Dose-adjusted EPOCH-R is a safe and well tolerated outpatient treatment regimen in double-hit lymphoma. Intern Med J 2022; Epub ahead of print. [Crossref] [PubMed]
- Dunleavy K, Fanale M, LaCasce A, et al. Preliminary report of a multicenter prospective phase II study of DA-EPOCH-R in MYC-rearranged aggressive B-cell lymphoma. Blood 2014;124:395. [Crossref]
- Dodero A, Guidetti A, Marino F, et al. Dose-adjusted EPOCH and rituximab for the treatment of double expressor and double-hit diffuse large B-cell lymphoma: impact of TP53 mutations on clinical outcome. Haematologica 2022;107:1153-62. [PubMed]
- Zhou D, Li L, Bao C, et al. Replacement of conventional doxorubicin by pegylated liposomal doxorubicin in standard RCHOP chemotherapy for elderly diffuse large B-Cell lymphoma: a retrospective study in China. Int J Clin Exp Med 2015;8:22497-502. [PubMed]
- Fridrik MA, Petzer AL, Keil F, et al. Non-pegylated liposomal encapsulated doxorubicin reduces cardiotoxicity in 1st line treatment of diffuse large B-cell lymphoma (DLBCL). Final results of a randomized trial. Blood 2011;118:2676. [Crossref]
- Picardi M, Giordano C, Pugliese N, et al. Liposomal doxorubicin supercharge-containing front-line treatment in patients with advanced-stage diffuse large B-cell lymphoma or classical Hodgkin lymphoma: Preliminary results of a single-centre phase II study. Br J Haematol 2022;198:847-60. [Crossref] [PubMed]
- Luminari S, Viel E, Ferreri AJM, et al. Nonpegylated liposomal doxorubicin combination regimen in patients with diffuse large B-cell lymphoma and cardiac comorbidity. Results of the HEART01 phase II trial conducted by the Fondazione Italiana Linfomi. Hematol Oncol 2018;36:68-75. [Crossref] [PubMed]
- Merli F, Spina M, Tucci A, et al. Comparison between R-COMP and R-CHOP in Older Patients with Diffuse Large B-Cell Lymphoma (DLBCL): A Substudy of the Elderly Project by the Fondazione Italiana Linfomi. Blood 2021;138:2487. [Crossref]
- Sancho JM, Fernández-Alvarez R, Gual-Capllonch F, et al. R-COMP versus R-CHOP as first-line therapy for diffuse large B-cell lymphoma in patients ≥60 years: Results of a randomized phase 2 study from the Spanish GELTAMO group. Cancer Med 2021;10:1314-26. [Crossref] [PubMed]
- Visco C, Pregnolato F, Ferrarini I, et al. Efficacy of R-COMP in comparison to R-CHOP in patients with DLBCL: A systematic review and single-arm metanalysis. Crit Rev Oncol Hematol 2021;163:103377. [Crossref] [PubMed]
- Prusila REI, Peroja P, Jantunen E, et al. Treatment of diffuse large B-cell lymphoma in elderly patients: Replacing doxorubicin with either epirubicin or etoposide (VP-16). Hematol Oncol 2019;37:136-42. [Crossref] [PubMed]
- Moccia AA, Schaff K, Freeman C, et al. Long-term outcomes of R-CEOP show curative potential in patients with DLBCL and a contraindication to anthracyclines. Blood Adv 2021;5:1483-9. [Crossref] [PubMed]
- Sonneveld P, de Ridder M, van der Lelie H, et al. Comparison of doxorubicin and mitoxantrone in the treatment of elderly patients with advanced diffuse non-Hodgkin's lymphoma using CHOP versus CNOP chemotherapy. J Clin Oncol 1995;13:2530-9. [Crossref] [PubMed]
- Osby E, Hagberg H, Kvaløy S, et al. CHOP is superior to CNOP in elderly patients with aggressive lymphoma while outcome is unaffected by filgrastim treatment: results of a Nordic Lymphoma Group randomized trial. Blood 2003;101:3840-8. [Crossref] [PubMed]
- Pangalis GA, Vassilakopoulos TP, Michalis E, et al. A randomized trial comparing intensified CNOP vs. CHOP in patients with aggressive non-Hodgkin's lymphoma. Leuk Lymphoma 2003;44:635-44. [Crossref] [PubMed]
- Peyrade F, Jardin F, Gisselbrecht C, et al. Rituximab and reduced dose CHOP (R-mini-CHOP) for patients over 80 years with diffuse large B-cell lymphoma (DLBCL)–Groupe d'Etude Des Lymphomes De l'Adulte (GELA) Study LNH03-7B. Blood 2010;116:853. [Crossref]
- Eyre TA, Martinez-Calle N, Hildyard C, et al. Impact of intended and relative dose intensity of R-CHOP in a large, consecutive cohort of elderly diffuse large B-cell lymphoma patients treated with curative intent: no difference in cumulative incidence of relapse comparing patients by age. J Intern Med 2019;285:681-92. [Crossref] [PubMed]
- Hounsome L, Eyre TA, Ireland R, et al. Diffuse large B cell lymphoma (DLBCL) in patients older than 65 years: analysis of 3 year Real World data of practice patterns and outcomes in England. Br J Cancer 2022;126:134-43. [Crossref] [PubMed]
- Bataillard EJ, Cheah CY, Maurer MJ, et al. Impact of R-CHOP dose intensity on survival outcomes in diffuse large B-cell lymphoma: a systematic review. Blood Adv 2021;5:2426-37. [Crossref] [PubMed]
- Tavares A, Moreira I. Diffuse large B-cell lymphoma in very elderly patients: Towards best tailored treatment - A systematic review. Crit Rev Oncol Hematol 2021;160:103294. [Crossref] [PubMed]
- Soe ZN, Allsup D. The use of ofatumumab in the treatment of B-cell malignancies. Future Oncol 2017;13:2611-28. [Crossref] [PubMed]
- Eyre TA, Clifford R, Bloor A, et al. NCRI phase II study of CHOP in combination with ofatumumab in induction and maintenance in newly diagnosed Richter syndrome. Br J Haematol 2016;175:43-54. [Crossref] [PubMed]
- Luo C, Wu G, Huang X, et al. Efficacy and safety of new anti-CD20 monoclonal antibodies versus rituximab for induction therapy of CD20+ B-cell non-Hodgkin lymphomas: a systematic review and meta-analysis. Sci Rep 2021;11:3255. [Crossref] [PubMed]
- Peyrade F, Bologna S, Delwail V, et al. Combination of ofatumumab and reduced-dose CHOP for diffuse large B-cell lymphomas in patients aged 80 years or older: an open-label, multicentre, single-arm, phase 2 trial from the LYSA group. Lancet Haematol 2017;4:e46-55. [Crossref] [PubMed]
- Spina M, Balzarotti M, Uziel L, et al. Modulated chemotherapy according to modified comprehensive geriatric assessment in 100 consecutive elderly patients with diffuse large B-cell lymphoma. Oncologist 2012;17:838-46. [Crossref] [PubMed]
- Juul MB, Jensen PH, Engberg H, et al. Treatment strategies and outcomes in diffuse large B-cell lymphoma among 1011 patients aged 75 years or older: A Danish population-based cohort study. Eur J Cancer 2018;99:86-96. [Crossref] [PubMed]
- Cox MC, Pelliccia S, Marcheselli L, et al. The metronomic all-oral DEVEC is an effective schedule in elderly patients with diffuse large b-cell lymphoma. Invest New Drugs 2019;37:548-58. [Crossref] [PubMed]
- Schelker RC, Herr W, Reichle A, et al. Low-dose trofosfamide plus rituximab is an effective and safe treatment for diffuse large B-cell lymphoma of the elderly: a single center experience. BMC Cancer 2018;18:1000. [Crossref] [PubMed]
- Storti S, Spina M, Pesce EA, et al. Rituximab plus bendamustine as front-line treatment in frail elderly (>70 years) patients with diffuse large B-cell non-Hodgkin lymphoma: a phase II multicenter study of the Fondazione Italiana Linfomi. Haematologica 2018;103:1345-50. [Crossref] [PubMed]
- Zeremski V, Jentsch-Ullrich K, Kahl C, et al. Is bendamustine-rituximab a reasonable treatment in selected older patients with diffuse large B cell lymphoma? Results from a multicentre, retrospective study. Ann Hematol 2019;98:2729-37. [Crossref] [PubMed]
- Wright CM, Koroulakis AI, Baron JA, et al. Palliative Radiotherapy for Diffuse Large B-cell Lymphoma. Clin Lymphoma Myeloma Leuk 2021;21:650-8. [PubMed]
- Juul MB, Jelicic J, Anru PL, et al. Cardiovascular diseases in elderly survivors of diffuse large B-cell lymphoma: a Danish population-based cohort study. Leuk Lymphoma 2022; Epub ahead of print. [Crossref] [PubMed]
- Gangaraju R, Davis ES, Bhatia S, et al. Venous-thromboembolism and associated health care utilization in elderly patients with diffuse large B cell lymphoma. Cancer 2022;128:2348-57. [Crossref] [PubMed]
- Howlader N, Mariotto AB, Besson C, et al. Cancer-specific mortality, cure fraction, and noncancer causes of death among diffuse large B-cell lymphoma patients in the immunochemotherapy era. Cancer 2017;123:3326-34. [Crossref] [PubMed]
- Eyre TA, Wilson W, Kirkwood AA, et al. Infection-related morbidity and mortality among older patients with DLBCL treated with full- or attenuated-dose R-CHOP. Blood Adv 2021;5:2229-36. [Crossref] [PubMed]
- Suzuki H, Asakawa A, Amitani H, et al. Cancer cachexia--pathophysiology and management. J Gastroenterol 2013;48:574-94. [Crossref] [PubMed]
- Deenadayalan V, Kumi DD, Shah M, et al. Impact of protein-energy malnutrition on outcomes among patients with diffuse large B cell lymphoma admitted for inpatient chemotherapy. J Clin Oncol 2022;40:e19539. [Crossref]
- Hamlin PA, Satram-Hoang S, Reyes C, et al. Treatment patterns and comparative effectiveness in elderly diffuse large B-cell lymphoma patients: a surveillance, epidemiology, and end results-medicare analysis. Oncologist 2014;19:1249-57. [Crossref] [PubMed]
- Hershman DL, Eisenberger A, Wang J, et al. Doxorubicin, cardiac risk factors and cardiac toxicity in elderly patients with diffuse b-cell non-Hodgkin's lymphoma. J Clin Oncol 2007;25:9050. [Crossref] [PubMed]
- Brem EA, Li H, Beaven AW, et al. SWOG 1918: A phase II/III randomized study of R-miniCHOP with or without oral azacitidine (CC-486) in participants age 75 years or older with newly diagnosed aggressive non-Hodgkin lymphomas - Aiming to improve therapy, outcomes, and validate a prospective frailty tool. J Geriatr Oncol 2022;13:258-64. [Crossref] [PubMed]
Cite this article as: Amoozgar B, Kahl BS. Initial treatment of elderly population with aggressive lymphoma: a narrative review of current evidence and future directions. Ann Lymphoma 2022;6:9.


