
Age is just a number: managing relapsed or refractory diffuse large B-cell lymphoma (DLBCL) in older patients
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin’s lymphoma (NHL), and is curable with frontline RCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) chemoimmunotherapy in approximately 60–70% of all patients (1). It is generally a disease of older adults; the median age at diagnosis is 66, and nearly 30% of cases occur in patients over the age of 75 years (2). Age alone has prognostic implications; age greater than 60 years is included in the International Prognostic Index (IPI) model which is used to estimate overall survival (OS) (3). These older patients therefore do not enjoy the same rates of cure with upfront treatment compared to younger cohorts. They may be ineligible for intensive upfront chemotherapy if older than age 80 years or with otherwise limiting medical comorbidities and instead receive dose-attenuated regimens such as R-mini-CHOP (4). R-mini-CHOP resulted in a 2-year progression free survival (PFS) of 47% in patients over the age of 80 years, and the majority of patients who died on this study did so due to complications from lymphoma.
Following relapse, management is challenging as many older patients may not be candidates for the established standard of care of salvage chemotherapy followed by autologous stem cell transplant (ASCT) due to age or accumulating comorbidities. For those who are ineligible for ASCT or who progress following, there have been several recent advances in both cellular and targeted therapies that provide options for treatment in those fit enough to receive them, but they are not currently considered curative.
It is important to note that the older patient population is quite heterogeneous; fitness and ability to receive intensive therapies can vary drastically. Formal assessments of frailty can be helpful in guiding therapy choices, but the final treatment decisions need to be individualized to the patient’s preferences regarding route, administration schedule, side effects, and anticipated duration of response (DOR). With more options than ever before for patients with relapsed or refractory (R/R) DLBCL, the question remains how best to select and sequence these therapies in an aging patient population, particularly as these patients are underrepresented in clinical trials (5). This review will discuss the management of relapsed and refractory DLBCL in the older patient.
Assessment of fitness
Part of the challenge in treating this diverse population is the potential subjectivity in determining a patient’s ability to tolerate a specific therapy. Traditionally, oncologists have had to make this decision based on limited information: age, Eastern Cooperative Oncology Group (ECOG) performance status, and clinical judgment based on a patient’s appearance in the exam room. Comprehensive geriatric assessments (CGA) are multifaceted evaluations of a patient’s functional status, assessing several domains including medical comorbidities, cognition, nutrition, finances, social support, and potential polypharmacy (6). Ongoing research has shown the positive effects of CGAs, including alteration in treatment plans, recommendations for optimization of medications and nutritional status (7), and most recently a randomized trial which resulted in a reduction in toxic effects of therapy for those who underwent a geriatric assessment prior to treatment (8).
CGAs have been prospectively studied in frontline DLBCL and have been shown to be able to predict OS (9,10). Most recently, the Foundazione Italiana Linfomi (FIL) created a simplified geriatric assessment (sGA) based on age, ability to perform activities of daily living (ADLs) and instrumental activities of daily living (IADLs), and the Cumulative Illness Rating Scale for Geriatrics (CIRS-G) score. This assessment tool divided patients into three categories: fit, unfit, and frail, and patients in these categories had a 3-year OS of 75%, 58%, and 43% respectively when given treatment at the discretion of their physician (11). This information in combination with the hemoglobin level and IPI were used to build an Elderly Prognostic Index that also was predictive of OS. This simplified assessment can be completed quickly during a treatment planning visit by the primary hematologist-oncologist, which is particularly helpful in this aggressive malignancy where additional time to coordinate CGAs may not be possible prior to initiation of therapy. While not able to provide the more complete picture of CGAs, this does allow clinicians to determine the fitness of DLBCL patients more objectively, and as recently reviewed (12), several studies have used select geriatric assessment domains to guide frontline therapy choices.
Importantly, these assessments have not been as well studied in the relapsed DLBCL population, who may have the additional physical challenge of having recently completed multiagent chemoimmunotherapy. However, this framework can still be used to discuss our management of elderly patients who we conceptualize as falling into one of these three categories. As fitness assessments are covered more in-depth elsewhere in this review series, we will turn our attention to the management of the fit, unfit, and frail older patients with R/R DLBCL.
Fit patients: cellular therapy candidates
Autologous stem cell transplantation
The current standard of care for DLBCL patients who have relapsed more than 12 months from initial therapy is consideration of salvage chemotherapy and proceeding to ASCT if the disease is shown to be responsive to chemotherapy. This is based on the phase 3 PARMA trial, in which ASCT resulted in an improvement in OS compared to salvage therapy alone; however, this was studied in a young population of patients with a median age of 43 years (13). Previous data from the European Blood and Marrow Transplantation registry had suggested that ASCT for DLBCL came with a higher rate of non-relapse mortality in the elderly (4.4% vs. 2.8%) as well as lower 3-year PFS (51% vs. 62%) than in patients under the age of 60 years (14). Accordingly, candidacy for ASCT is determined based on age (typically reserved for those less than 70–75 years), medical comorbidities as determined by the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) score, and ultimate response to salvage chemotherapy, which itself can contribute toxicity.
Data on efficacy and safety outcomes in this population are conflicting. A series of 202 NHL patients over the age of 60 years including 73 with DLBCL showed that age was not associated with 3-year OS (60%) or PFS (73%); there was no difference in these outcomes between patients 70 and older versus those in their sixties. Similarly, high HCT-CI scores did not correlate with outcomes, and the rate of treatment related mortality was low at 4% (15).
More recently, the same group assessed toxicities in those over the age of 60 years who received ASCT using BEAM (carmustine, etoposide, cytarabine and melphalan) which is the most common preparative regimen used for DLBCL (16). This showed a very high rate of serious (grade ≥3) adverse events; all patients over the age of 70 years experienced at least one, and the median number was 3. Patients between 60–69 years had a median of two toxicities, and only 8% of these patients experienced no severe toxicity. In particular, the rate of cardiovascular toxicity was particularly high in the >70 years cohort, with a hazard ratio of 3.36 compared to the younger patients, even after an adjustment for underlying cardiac risk factors. The most common severe toxicities were febrile neutropenia (63%), oral/gastrointestinal (GI) (51%) and infection (31%). Like their earlier analysis, HCT-CI score did not predict outcomes.
However, another analysis suggested that comorbidities do affect outcomes in DLBCL transplant patients. Fifty-nine patients over the age of 60 years who received BEAM were compared to 93 patients under 60 years (17). In this series, the Charleston Comorbidity Index (CCI) Score did impact both treatment-related morality and OS, while age alone did not. Older patients were also more likely than younger patients to experience severe oral mucositis.
In totality, the research suggests that ASCT should not be avoided in patients based on age alone given reasonably similar disease-control, particularly as this is potentially curative therapy. However, strong consideration should be given to an individual patient’s comorbidities, which may ultimately impact their outcomes. Disease risk, including timing of relapse must be taken into consideration as well given the modest benefit of transplant in refractory patients, especially given emerging data with cellular therapy. Older patients should be informed that they are more likely to experience grade 3 or higher adverse events, particularly oral mucositis. It is important to note that these analyses only represent patients whom clinical judgement has suggested are good candidates for ASCT, and as previously stated, clinical judgement is less predictive of outcomes in older patients than formal assessments. Prospective data utilizing geriatric assessments to risk stratify potential ASCT candidates would be welcome, but in its absence, one can consider a CGA in addition to the standard pre-transplant evaluation to uncover modifiable risk factors and optimize the fit, older patient’s chance of success (18,19).
Chimeric antigen receptor T-cell therapy (CAR-T)
One of the most successful developments in R/R DLBCL in the past decade has been the development of CAR-T. Three separate CAR-T therapies, axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), and lisocabtagene maraleucel (liso-cel) have been approved as third-line therapy in DLBCL (20-22) (Table 1). Most recently, practice-changing Phase 3 trials assessing these constructs versus ASCT in patients with primary refractory disease or relapse within twelve months of therapy were performed; axi-cel and liso-cel showed benefit in event free survival (EFS) (23,24) while tisa-cel did not (25). These products have never been compared head to head, and there are notable differences in the design of these trials that are beyond the scope of this paper, but have been recently well reviewed (26). Therefore, axi-cel or liso-cel may be considered as a new standard of care for primary refractory or early relapsing fit, older patients, and all three products can be considered in the third-line setting. Older patients were included in the CAR-T trials, and several analyses of this specific patient population demonstrate the safety and efficacy. Of particular concern in this patient population is the rate of cytokine release syndrome (CRS) and neurologic events.
Table 1
| Variables | Axicabtagene ciloleucel, n [%] | Tisagenlecleucel, n [%] | Lisocabtagene maraleucel, n [%] |
|---|---|---|---|
| Study | ZUMA-1 (21) | JULIET (22) | TRANSCEND NHL 001 (20) |
| Patients enrolled | 111 | 111 | 269 |
| Patients ≥65 years | 24 [22] | 25 [23] | 112 [42] |
| Patients <65 years | 87 [78] | 86 [77] | 157 [58] |
| ORR | 83 [82] | 48 [52] | 186 [73] |
| Patients ≥65 years | 22 [92] | 13 [59] | 82 [76] |
| Patients <65 years | 61 [79] | 35 [49] | 104 [70] |
| CRR | 59 [54] | 37 [40] | 136 [53] |
| Patients ≥65 years | 18 [75] | Subgroups not reported | 65 [60] |
| Patients <65 years | 41 [53] | 71 [48] | |
| Grade ≥3 CRS | 12 [11] | 20 [22] | 6 [2] |
| Patients ≥65 years | 2 [7] | Subgroups not reported | 1 [1] |
| Patients <65 years | 10 [12] | 5 [3] | |
| Grade ≥3 neuro toxicity | 35 [32] | 11 [12] | 27 [10] |
| Patients ≥65 years | 12 [44] | Subgroups not reported | 12 [11] |
| Patients <65 years | 23 [28] | 15 [10] |
These studies utilized different criteria to define severity of toxicity; ZUMA-1 and TRANSFORM utilized the Lee criteria and JULIET the Penn criteria which does limit direct comparison. CAR-T, chimeric antigen receptor T-cell therapy; R/R, relapsed or refractory; DLBCL, diffuse large B-cell lymphoma; ORR, overall response rate; CRR, complete response rate; CRS, cytokine release syndrome.
Axi-cel is an anti-CD19 CAR construct with a CD28 costimulatory domain. In a subgroup analysis of the ZUMA-1 trial of axi-cel as third-line or later therapy, patients older than 65 were compared to the younger patients. There were no major differences in efficacy or safety based on age alone; response rates, complete response (CR) and DOR were all similar in patients on either side of the age 65 years divide as were rates of CRS. There were higher rates of grade 3 or higher neurologic events (44% vs. 28%) in the older patients, including higher rates of delirium and encephalopathy, as may be expected in older, hospitalized patients (27). Similar results were seen in a US Lymphoma CAR-T consortium analysis of those older than 65 treated with axi-cel in the real world setting; where there was comparable safety and efficacy, but a higher rate of neurologic toxicity (28). An additional real world safety observational analysis of axi-cel did not show a difference in survival in older patients, and baseline cardiac, renal, and hepatic dysfunction also did not impact response rates. However, performance status did impact response and OS rates; those with an ECOG PS of ≥2 had a HR of 3.26 for survival (29).
Tisa-cel is also an anti-CD19 CAR T-cell but has a 4-1BB costimulatory domain. The JULIET trial assessed its safety and efficacy for patients who had progressed on two prior therapies and 23% of the patients were over the age of 65 years (22). There are fewer data assessing outcomes in this specific population compared to the other CAR constructs; one real world analysis of CD19 CAR therapy in the older that included predominantly tisa-cel patients did not show toxicity differences compared to a younger population, but the small nature of this retrospective study limits definitive conclusions (30). There is an ongoing trial assessing tisa-cel in the second line setting for patients who are not considered transplant candidates (NCT04161118).
Liso-cel also utilizes a 4-1BB costimulatory domain, with an equal ratio of CD8+ and CD4+ T cells administered. This manufacturing method results in a less differentiated and less variable T-cell population which is thought to result in lower toxicity (20). The initial study of liso-cel as third line treatment, TRANSCEND NHL-001, included 10% of patients ≥75 and 42% of patients ≥65, most patients were ECOG PS 1 (58%). The overall response rate (ORR) was 73% including a 53% CR, with a low rate of grade 3/4 CRS by Lee criteria (2%) and neurologic events (10%). This CRS and neurologic rate is significantly lower than seen in the ZUMA-1 trial and JULIET TRIALS (Table 1). The safety and efficacy rates were comparable in patients >65 compared to the younger cohort, as well as those with moderate comorbidities compared to those without. When assessed as second line treatment in the TRANSFORM study, there were similarly low rates of high grade CRS (1% grade 3) and neurotoxicity (4% grade 3) (24). The ongoing PILOT study is assessing liso-cel as second line therapy in transplant ineligible patients over the age of 70 years, with ECOG PS2, or with impaired cardiac, pulmonary, renal, or hepatic function. In a preliminary report, there was no grade 3/4 CRS and only 8% had grade 3/4 neurologic events (31).
The research discussed above, much like the data on ASCT, continues to suggest that age itself should not be a contraindication for CAR-T therapy, with most evidence suggesting similar efficacy compared to younger cohorts, with performance status playing a much larger role in outcomes. However, the data for axi-cel consistently suggests higher rates of neurologic toxicity in the older population, and there is very limited data for tisa-cel. For these reasons, liso-cel is an attractive option given the seemingly comparable efficacy with lower toxicity rates and is our CAR-T construct of choice for older patients, particularly if it can be given in the outpatient setting (32). It should be noted that even if older patients are considered functional candidates for cellular therapy, logistical and social barriers such as insurance approval, family support, travel, and disease control during the collection process still exist and may ultimately limit eligibility.
An additional clinical scenario involves the patient with a late relapse who may be deemed ineligible for ASCT, typically based on medical comorbidities such as inadequate cardiac or pulmonary function, but who may be otherwise eligible for CAR-T therapy. As they would only be able to receive commercial CAR-T products in the third line setting, we would consider a trial of one of the below novel, yet palliative, agents and then proceed with CAR-T therapy on relapse.
Unfit patients: novel agents
For patients who have relapsed following transplant or cellular therapy, or who are deemed not to be cellular therapy candidates based on performance status or following CGAs, we next consider the evolving world of therapies that include targeted agents. Prior to these recent advances, the transplant-ineligible relapsed DLBCL population was treated with chemoimmunotherapy combinations, typically including gemcitabine, bendamustine or a platinum agent (33,34). These combinations remain available to patients, however four novel options gained FDA approval since 2019: polatuzumab vedotin in combination with bendamustine and rituximab (pola-BR), tafasitamab-cxix with lenalidomide (tafa-len), loncastuximab tesirine-lpyl (lonca), and selinexor (Table 2).
Table 2
| Novel agents | Median age | Median lines of previous therapies | Primary refractory patients (%) | ORR/CRR (%) | DOR, median (months) | PFS, median (months) | OS, median (months) | Hematologic toxicity, Grade 3/4 | Notable non-hematologic toxicity, all grades |
|---|---|---|---|---|---|---|---|---|---|
| Polatuzumab vedotin + BR (35) | 67 | 2 | 53 | 63/50 | 13 | 10 | 12 | Neutropenia (28.2%); thrombocytopenia (41%) | Peripheral neuropathy (42.6%) |
| Tafasitamab and lenalidomide (36) | 72 | 2 | 44 | 58/40 | 44 | 12 | 34 | Neutropenia (48%); thrombocytopenia (17%) | Diarrhea (33%); rash (36%); asthenia (23%) |
| Loncastuximab tesirine (37) | 66 | 3 | 20 | 48/24 | 10 | 5 | 10 | Neutropenia (26%); thrombocytopenia (18%) | Peripheral edema (20%); rash (13%); photosensitivity reaction (10%) |
| Selinexor (38) | 67 | 2 | 41 | 28/12 | 9 | 3 | 9 | Thrombocytopenia (46%); neutropenia (25%) | Nausea (58%); fatigue (47%); anorexia (37%) |
R/R, relapsed or refractory; DLBCL, diffuse large B-cell lymphoma; BR, bendamustine and rituximab; ORR, overall response rate; CRR, complete response rate; DOR, duration of response; PFS, progression free survival; OS, overall survival.
Polatuzumab vedotin with bendamustine and rituximab (BR)
Polatuzumab vedotin is an antibody drug conjugate targeting the CD79b component of the B-cell receptor that delivers the microtubule inhibitor monomethyl auristatin E (MMAE) to B-cells. This was initially studied as a single agent, and although the response rates were acceptable, the CR rates were less than 15% (39). It was therefore combined with BR in a phase 2 trial in transplant ineligible patients and compared with BR alone (35). Importantly, patients need to have ≤ grade 1 neuropathy due to the potential neurologic toxicity from pola which is an important consideration in older patients who may have underlying neuropathy from diabetes or other medical conditions.
The median age in this trial was 71, as would be expected in a transplant ineligible relapsed DLBCL cohort, and patients into their mid-80s were included. The patients in the pola-BR arm represented a high number of refractory (75%) and heavily pre-treated (45% ≥3 lines of therapy) patients. Pola-BR improved PFS (median 9.5 vs. 3.7 months) and OS (median 12.4 vs. 4.7 months) compared to BR alone. Subgroup analyses of patients older than 65 and with ECOG PS ≥2 showed similar PFS compared to younger and more fit patients. The adverse events were mostly cytopenias, including a 46.2% rate of grade 3/4 neutropenia, and neuropathy which was mostly low grade and reversible. A recently published update to this trial including an extension cohort showed a CR rate of 38.7% with ongoing survival benefit, with no new issues with safety, even though the extension cohort included a higher percentage of patients over the age of 65 years (73%) compared to the initial study (58%) (40).
This regimen is often our first choice for older patients who are relatively fit, but cellular therapy ineligible, or who have progressed following cellular therapy as it is effective and time limited. When giving with palliative intent, we attempt to improve tolerability by considering dose reduction of the bendamustine to 70 mg/m2 (vs. the 90 mg/m2 in the trial), and/or spacing out the cycles to every 4 weeks as opposed to every 3, and using granulocyte colony stimulating factor (G-CSF) to combat neutropenia depending on the patient’s tolerance. We do not use this regimen in patients with baseline grade 2 or higher peripheral neuropathy, and closely monitor for this toxicity while on treatment. We also do not anticipate using this regimen in patients who relapse following pola-R-CHP, which may soon be utilized in some patients instead of R-CHOP based on the pivotal POLARIX trial (41) and ongoing trials in older patients with mini-pola-R-CHP (NCT04594798 and NCT04332822).
Tafasitamab and lenalidomide
Tafasitamab and lenalidomide was approved as combination therapy based on the L-MIND trial (36). Tafasitamab is a CD19 antibody that has increased affinity for the Fcy receptor and mediates antibody-dependent cellular toxicity and phagocytosis. The phase 2 study of 81 transplant-ineligible patients following one to three lines of therapy included those with transformed or bulk disease but excluded patients with primary refractory disease and double or triple “hit” lymphomas. The median age was 72 years, including 46% of patients over the age of 70 years. Tafasitamab has a rather burdensome initial schedule of weekly infusions for the first three 28-day cycles, including an additional loading dose on day 4 of cycle 1. Following cycle 4, it is spaced to every other week; 25 mg of lenalidomide is given on days 1–21 of each cycle for the first 12 cycles. Tafasitamab can then be continued as monotherapy maintenance. 60% of patients had a response, including 43% with a CR, and the median DOR was 21.7 months. Cytopenias were the most common adverse events, including 48% grade 3 neutropenia, which could be managed with G-CSF. Non-hematologic events included diarrhea and rash.
As discussed below, lenalidomide is an active monotherapy in R/R DLBCL, so a patient-matched analysis called RE-MIND was performed comparing patients who were treated on L-MIND vs. those who received lenalidomide monotherapy in the real world setting (42). This analysis showed significantly improved outcomes for patients who received the combination, suggesting synergistic effects to dual therapy. A similar retrospective cohort study, RE-MIND2 (43), compared the L-MIND patients to matched patients treated with pola-BR, lenalidomide and rituximab (R2) or CAR-T. Acknowledging the limitations of this type of data, tafa-len did have a higher ORR than pola-B2 and R2, and a similar ORR to CAR-T therapy.
While separate analyses have not been performed specifically in frail older patients, the population in L-MIND had a significant portion of patients over the age of 70 years, so we feel this regimen is likely tolerable. We therefore consider this combination of agents for those who are not bothered by initially frequent infusion appointments, and we closely monitor for neutropenia and consider dose reduction of lenalidomide. If a patient is considered to be a potential future CAR-T candidate, we would typically avoid this product immediately prior to CAR-T given the shared CD19 target. If a patient has already received CD19 directed CAR-T therapy, we would assure continued CD19 expression on a repeat biopsy sample prior to use of tafasitamab.
Loncastuximab tesirine
Like polatuzumab vedotin, loncastuximab tesirine (lonca) is an antibody drug conjugate, but instead targets CD19 with a pyrrolobenzodiazepine cytotoxin which causes interstrand DNA crosslinks. It is Food and Drug Administration (FDA) approved for R/R DLBCL based on the LOTIS-2 trial (37), a single-arm phase 2 trial in which lonca was given on day 1 of each 21-day cycle. The population included refractory and patients who had transformed disease from an indolent lymphoma. Most patients were over the age of 65 years, including 14% of patients older than 75. There was an ORR of 48.3%, evenly divided between complete and partial responses, and the median DOR was 10.3 months. The most common grade 3/4 adverse events were neutropenia (26%) and thrombocytopenia (18%). Other side effects attributed to the cytotoxic payload include skin rashes, photosensitivity reactions, liver function abnormalities, and edema and pleural effusions which required treatment with spironolactone. There was no increase in toxicity in the patients over the age of 65 years compared to younger patients.
Patient reported outcomes for LOTIS-2 are available using the EQ-5D (patient reported health status) and FACT-Lym surveys (physical, emotional, social, and function well-being and lymphoma-specific symptom questions) (44). Overall, patients reported their healthcare related quality of life was stable or improved while on treatment. One question in the FACT-Lym asks patients to rate on a Likert scale “I am bothered by side effects of treatment”. Consistently across cycles, at least 60% of patients were able to answer “not at all” or “a little bit” suggesting true tolerability. Results of these surveys were similar for the older population as compared to the overall population.
We consider lonca for use in patients whose peripheral neuropathy limits pola-BR, or who desire the convenient every 3-week dosing schedule. We would avoid this agent in patients with comorbidities that make them susceptible to fluid overload, or in patients with disease bulk as they were excluded from the trial.
Selinexor
Selinexor is an oral agent that works by inhibiting transport of proteins from the nucleus into the cytoplasm that ultimately results in cell-cycle arrest. The SADAL trial assessed selinexor in patients with relapsed DLBCL, including 45% over the age of 70 years (38). However, this was very highly-selected patient group as the study required a 2–3-month washout period depending on prior response to assure patients had an adequate life expectancy, which was not a feature in the other relapsed DLBCL studies discussed here and selects for more indolent-behaving disease. There was a modest 28% ORR and 12% CR rate which was unaffected by age in sub-group analyses; median DOR was 9.3 months. Grade 3/4 adverse events included thrombocytopenia (46%) and neutropenia (24%), and lower grade nausea (52%) was common. Although this study did include a large population of older patients, we reserve this option for the select patient who strongly prefers an oral therapy and who has disease that is behaving in a non-aggressive manner.
Frail patients: palliative therapies
There will be some older patients who, either at first relapse or following later lines of therapy, are unable to tolerate these novel agents or who desire a more palliative approach. For these frail patients, we consider monotherapy, radiation therapy (RT), or steroids. The hope is to provide meaningful additional time for patients while controlling their disease and limiting symptoms.
Lenalidomide with or without rituximab
Lenalidomide and rituximab (R2) is an active regimen in follicular and mantle cell lymphomas (45,46). While it does have less impressive efficacy in R/R DLBCL, it remains an option for frail patients given its relative tolerability and ease of administration. Several small phase 2 studies have assessed this, with responses around 30% (47,48). There is also evidence to suggest improved benefit in non-germinal center B-cell (GCB) DLBCL (49,50), and high risk (transformed disease or translocations in MYC, BLC2, and/or BLC6) (51) so we consider this regimen particularly in these situations. The most common adverse events are neutropenia, thrombocytopenia, rash, diarrhea, and fatigue, for which dose modifications of lenalidomide can be helpful for frail patients. We typically combine lenalidomide with rituximab except in patients who are rituximab-refractory.
Ibrutinib
Ibrutinib, a Bruton’s tyrosine kinase inhibitor that affects B-cell receptor signaling pathways, has also been studied in R/R DLBCL. It appears to be most effective in activated B-cell (ABC) subtype disease; with a 37% response rate in one study compared to only 5% in GCB subtype. There are small subgroups in ABC DLBCL, particularly those with MYD88 mutations who have high response rates to ibrutinib (52). It should be noted that study utilized gene expression profiling to identify the cell of origin, which is known to be intermittently discordant with the Hans criteria for immunohistochemistry (IHC). A separate analysis of ibrutinib use in patients whose disease was characterized by IHC did not show a significant difference in response rates for ABC vs. GCB patients (53). Nevertheless, this remains an option for frail patients who are unable to tolerate novel combination therapies, but close monitoring should be undertaken for atrial fibrillation and bleeding. Ibrutinib can also be considered in patients who have central nervous system (CNS) involvement, particularly as options are quite limited for older patients who experience this unfortunate relapse pattern (54).
Brentuximab vedotin (BV)
Less than 25% of DLBCL expresses CD30, but treatment with BV can be considered in those that do. BV is an antibody drug conjugate targeting CD30+ cells with the tubulin toxin MMAE. A study assessing BV monotherapy in CD30+ (by local IHC) DLBCL showed a 44% response rate, with a DOR of 16.6 months, and responses did not correlate with level of CD30 expression (55). Although BV can have neuropathy and the risk for ileus, which can be problematic in older patients who may be prone to constipation, we still consider this agent for frail patients who disease expresses CD30.
RT
RT has a variety of uses in relapsed and refractory DLBCL, including sites of bulk in the peri-transplant or CAR-T period, or in CNS disease (56). We involve our radiation oncologists for patients with pain or symptomatic disease resulting in neurologic changes, obstruction, or bleeding. Even frail patients can typically tolerate palliative RT and achieving local disease control may improve the quality of their remaining time.
Palliative care and steroids
For older patients who are no longer candidates for treatment, attention turns toward symptomatic management and palliative care. For patients experiencing symptoms from their disease while nearing the end of their life, several days of prednisone or dexamethasone can provide relief.
There are many helpful frameworks to guide oncologists in goals-of-care discussions with their patients (57,58). These discussions are particularly important to initiate for the unfit and frail for whom curative-intent therapy is no longer an option, and who are likely to succumb to their disease. Admittedly, these can be challenging as the prognosis for patients with R/R DLBCL can vary wildly depending on DORs to systemic therapy, and many advanced lymphoma patients underestimate the severity of their illness (59). However, when it is clear that a patient has limited treatment options discussing their wishes for their remaining time is crucial.
Future therapies
As R/R DLBCL remains a substantial unmet need, there is significant ongoing research in additional therapies. One class of drug likely to gain approval are the bispecific antibodies which work by engaging two separate antigens, typically a B-cell and a T-cell antigen, resulting in activation of the T-cell and lymphoma cell death. Potential toxicities are similar to CAR T-cells in that there is a risk of CRS and neurotoxicity, however, the bispecifics appear to be more manageable and the goal is for most to be able to be delivered in the outpatient setting.
There are several CD20/CD3 bispecific antibodies currently being studied in DLBCL. Mosunetuzumab was combined with polatuzumab vedotin in an expansion cohort of patients with aggressive lymphomas (60). There was an ORR of 73%, including in those with prior CART therapy. While there is no specific data available yet for older patients, this agent is being investigated in the frontline setting as a monotherapy for patient who were deemed too frail for chemoimmunotherapy. There were no grade 3 or higher adverse events reported, suggesting remarkable tolerability even in frail or medically complex patients (61). Glofitamab showed an ORR of 53.7% in aggressive NHL including DLBCL patients (62), and is also being studied in combination with polatuzumab vedotin (63). Epcoritamab which is delivered subcutaneously, is involved in a phase 3 trial compared to investigator’s choice of rituximab with gemcitabine and oxaliplatin or BR in R/R DLBCL (64), the results of which are awaited given its impressive ORR of 66.7% in heavily-pretreated DLBCL patients (65).
Several antibody-drug conjugates also have ongoing trials in DLBCL. Zilovertamab vedotin (VLS-101) conjugates an antibody to ROR1 (receptor tyrosine kinase-like orphan receptor) to MMAE. Four out of five DLBCL patients treated in the phase 1 study had a response (66), and there is an ongoing cohort expansion study (NCT03833180). Toxicities are preliminarily like other MMAE-containing ADCs, suggesting this might be a tolerable option for older patients. Naratuximab emtansine, an anti-CD37 antibody conjugated to DM1, was combined with rituximab in a phase 2 trial in R/R NHL including DLBCL, 44.6% of which had a response (67). Safety data suggest cytopenias are the most common severe toxicities.
Our approach
When considering how best to treat older patients with DLBLC that is relapsed or refractory, we first determine if they are eligible for any clinical trials, particularly given their historic underrepresentation. We then assess their functional status (Figure 1). We consider a CGA prior to treatment, which we find particularly helpful in identifying modifiable risk factors for adverse treatment outcomes. However, there may not be time to coordinate this prior to treatment, or it may not be available to all oncologists. In that case, we use clinical judgement and the FIL framework which divides patients into the categories of fit, unfit, or frail based on an sGA. For patients who are fit, regardless of chronologic age, we evaluate them for candidacy for ASCT or CAR-T therapy if they have a quick relapse or refractory disease. If proceeding with CAR-T therapy, our preference is for liso-cel because of lower rates of severe toxicity.
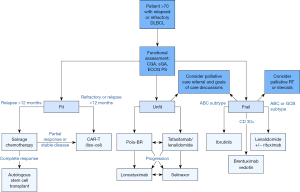
For patients who are deemed not fit enough to receive cellular therapy, we consider the novel agents discussed above. We typically select either polatuzumab-BR or tafasitamab combined with lenalidomide, making the ultimate decision based on patient preference for time-limited vs. ongoing therapy and taking anticipated side effects into consideration. Lonca or selinexor remain options in later lines of therapy. Frail patients may benefit from lenalidomide and rituximab, BV, or ibrutinib for systemic options, and we consider whether RT might provide local control and symptom improvement. We engage patients in goals-of-care discussions at the time of relapse to assure we are proceeding with therapy choices that keep their preferences in mind, particularly when discussing non-curative options.
Conclusions
In conclusion, older patients with R/R DLBLC remain a significant unmet clinical and research need given their risk for adverse effects and frequent limitations in receiving curative therapy. However, it is critically important to recognize that age is truly just a number, and more standardized metrics of assessing fitness should be used to assure that older patients with DLBCL have the best chance of having their disease controlled for as long as possible with the best quality of life.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jonathon B. Cohen and Craig Portell) for the series “Management of Elderly Patients with HL and NHL” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aol.amegroups.com/article/view/10.21037/aol-22-3/coif). The series “Management of Elderly Patients with HL and NHL” was commissioned by the editorial office without any funding or sponsorship. PMR received consulting fees from Kite Pharma and Caribou Biosciences, support from Kite Pharma, and received materials from Seagen and Genentech. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet 2013;381:1817-26. [Crossref] [PubMed]
- Cancer Stat Facts: NHL — Diffuse Large B-Cell Lymphoma (DLBCL). NCI, SEER. Accessed January 21, 2022.
- International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 1993;329:987-94. [Crossref] [PubMed]
- Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol 2011;12:460-8. [Crossref] [PubMed]
- Sedrak MS, Freedman RA, Cohen HJ, et al. Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA Cancer J Clin 2021;71:78-92. [Crossref] [PubMed]
- Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007;25:1824-31. [Crossref] [PubMed]
- Hamaker ME, Te Molder M, Thielen N, et al. The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients - A systematic review. J Geriatr Oncol 2018;9:430-40. [Crossref] [PubMed]
- Mohile SG, Mohamed MR, Xu H, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet 2021;398:1894-904. [Crossref] [PubMed]
- Tucci A, Ferrari S, Bottelli C, et al. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer 2009;115:4547-53. [Crossref] [PubMed]
- Tucci A, Martelli M, Rigacci L, et al. Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B-cell lymphoma: a prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL). Leuk Lymphoma 2015;56:921-6. [Crossref] [PubMed]
- Merli F, Luminari S, Tucci A, et al. Simplified Geriatric Assessment in Older Patients With Diffuse Large B-Cell Lymphoma: The Prospective Elderly Project of the Fondazione Italiana Linfomi. J Clin Oncol 2021;39:1214-22. [Crossref] [PubMed]
- Akhtar OS, Huang LW, Tsang M, et al. Geriatric assessment in older adults with non-Hodgkin lymphoma: A Young International Society of Geriatric Oncology (YSIOG) review paper. J Geriatr Oncol 2022;13:572-81. [Crossref] [PubMed]
- Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med 1995;333:1540-5. [Crossref] [PubMed]
- Jantunen E, Canals C, Rambaldi A, et al. Autologous stem cell transplantation in elderly patients (> or =60 years) with diffuse large B-cell lymphoma: an analysis based on data in the European Blood and Marrow Transplantation registry. Haematologica 2008;93:1837-42. [Crossref] [PubMed]
- Dahi PB, Tamari R, Devlin SM, et al. Favorable outcomes in elderly patients undergoing high-dose therapy and autologous stem cell transplantation for non-Hodgkin lymphoma. Biol Blood Marrow Transplant 2014;20:2004-9. [Crossref] [PubMed]
- Dahi PB, Lee J, Devlin SM, et al. Toxicities of high-dose chemotherapy and autologous hematopoietic cell transplantation in older patients with lymphoma. Blood Adv 2021;5:2608-18. [Crossref] [PubMed]
- Wildes TM, Augustin KM, Sempek D, et al. Comorbidities, not age, impact outcomes in autologous stem cell transplant for relapsed non-Hodgkin lymphoma. Biol Blood Marrow Transplant 2008;14:840-6. [Crossref] [PubMed]
- Wildes TM, Stirewalt DL, Medeiros B, et al. Hematopoietic stem cell transplantation for hematologic malignancies in older adults: geriatric principles in the transplant clinic. J Natl Compr Canc Netw 2014;12:128-36. [Crossref] [PubMed]
- Muffly LS, Boulukos M, Swanson K, et al. Pilot study of comprehensive geriatric assessment (CGA) in allogeneic transplant: CGA captures a high prevalence of vulnerabilities in older transplant recipients. Biol Blood Marrow Transplant 2013;19:429-34. [Crossref] [PubMed]
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 2020;396:839-52. [Crossref] [PubMed]
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017;377:2531-44. [Crossref] [PubMed]
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380:45-56. [Crossref] [PubMed]
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N Engl J Med 2022;386:640-54. [Crossref] [PubMed]
- Kamdar M, Solomon SR, Arnason JE, et al. Lisocabtagene Maraleucel (liso-cel), a CD19-Directed Chimeric Antigen Receptor (CAR) T Cell Therapy, Versus Standard of Care (SOC) with Salvage Chemotherapy (CT) Followed By Autologous Stem Cell Transplantation (ASCT) As Second-Line (2L) Treatment in Patients (Pts) with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL): Results from the Randomized Phase 3 Transform Study. Blood 2021;138:91. [Crossref]
- Bishop MR, Dickinson M, Purtill D, et al. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. N Engl J Med 2022;386:629-39. [Crossref] [PubMed]
- Westin J, Sehn LH. CAR T cells as a second-line therapy for large B-cell lymphoma: a paradigm shift? Blood 2022;139:2737-46. [Crossref] [PubMed]
- Neelapu SS, Jacobson CA, Oluwole OO, et al. Outcomes of older patients in ZUMA-1, a pivotal study of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood 2020;135:2106-9. [Crossref] [PubMed]
- Sano D, Lekakis L, Feng L, et al. Safety and efficacy of axicabtagene ciloleucel (axi-cel) in older patients: Results from the US Lymphoma CAR-T Consortium. Hematol Oncol 2019;37:304-5. [Crossref]
- Locke FL, Jacobson C, Ma L, et al. Real-World Outcomes of Axicabtagene Ciloleucel (Axi-cel) for the Treatment of Large B-Cell Lymphoma (LBCL): Impact of Age and Specific Organ Dysfunction. Blood 2021;138:530. [Crossref]
- Ram R, Grisariu S, Shargian-Alon L, et al. Toxicity and efficacy of chimeric antigen receptor T-cell therapy in patients with diffuse large B-cell lymphoma above the age of 70 years compared to younger patients - a matched control multicenter cohort study. Haematologica 2022;107:1111-8. [PubMed]
- Sehgal AR, Hildebrandt G, Ghosh N, et al. Lisocabtagene maraleucel (liso-cel) for treatment of second-line (2L) transplant noneligible (TNE) relapsed/refractory (R/R) aggressive large B-cell non-Hodgkin lymphoma (NHL): Updated results from the PILOT study. J Clin Oncol 2020;38:8040. [Crossref]
- Godwin JE, Mattar BI, Maris MB, et al. Outreach: Preliminary safety and efficacy results from a phase 2 study of lisocabtagene maraleucel (liso-cel) in the nonuniversity setting. J Clin Oncol 2021;39:e19513. [Crossref]
- Network NCC. B-Cell Lymphomas (Version 5.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf. Accessed February 10, 2022.
- Ohmachi K, Niitsu N, Uchida T, et al. Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2013;31:2103-9. [Crossref] [PubMed]
- Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J Clin Oncol 2020;38:155-65. [Crossref] [PubMed]
- Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol 2020;21:978-88. [Crossref] [PubMed]
- Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 2021;22:790-800. [Crossref] [PubMed]
- Kalakonda N, Maerevoet M, Cavallo F, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol 2020;7:e511-22. [Crossref] [PubMed]
- Morschhauser F, Flinn IW, Advani R, et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol 2019;6:e254-e265. [Crossref] [PubMed]
- Sehn LH, Hertzberg M, Opat S, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv 2022;6:533-43. [Crossref] [PubMed]
- Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N Engl J Med 2022;386:351-63. [Crossref] [PubMed]
- Nowakowski GS, Rodgers TD, Marino D, et al. RE-MIND study: A propensity score-based 1:1 matched comparison of tafasitamab + lenalidomide (L-MIND) versus lenalidomide monotherapy (real-world data) in transplant-ineligible patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). J Clin Oncol 2020;38:8020. [Crossref]
- Nowakowski GS, Yoon DH, Mondello P, et al. Tafasitamab Plus Lenalidomide Versus Pola-BR, R2, and CAR T: Comparing Outcomes from RE-MIND2, an Observational, Retrospective Cohort Study in Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood 2021;138:183. [Crossref]
- Spira A, Zhou X, Chen L, et al. Health-Related Quality of Life, Symptoms, and Tolerability of Loncastuximab Tesirine in Patients With Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Clin Lymphoma Myeloma Leuk 2022;22:158-68. [Crossref] [PubMed]
- Leonard JP, Trneny M, Izutsu K, et al. AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J Clin Oncol 2019;37:1188-99. [Crossref] [PubMed]
- Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol 2012;13:716-23. [Crossref] [PubMed]
- Zinzani PL, Pellegrini C, Gandolfi L, et al. Combination of lenalidomide and rituximab in elderly patients with relapsed or refractory diffuse large B-cell lymphoma: a phase 2 trial. Clin Lymphoma Myeloma Leuk 2011;11:462-6. [Crossref] [PubMed]
- Wang M, Fowler N, Wagner-Bartak N, et al. Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: a phase II clinical trial. Leukemia 2013;27:1902-9. [Crossref] [PubMed]
- Czuczman MS, Trněný M, Davies A, et al. A Phase 2/3 Multicenter, Randomized, Open-Label Study to Compare the Efficacy and Safety of Lenalidomide Versus Investigator's Choice in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Clin Cancer Res 2017;23:4127-37. [Crossref] [PubMed]
- Li J, Zhou J, Guo W, et al. Efficacy and Safety of Lenalidomide Monotherapy for Relapsed/Refractory Diffuse Large B Cell Lymphoma: Systematic Review and Meta-Analysis. Front Oncol 2021;11:756728. [Crossref] [PubMed]
- Rodgers TD, Baran A, Reagan PM, et al. Efficacy of lenalidomide in high-risk diffuse large B-cell lymphoma. Br J Haematol 2020;188:e33-6. [Crossref] [PubMed]
- Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 2015;21:922-6. [Crossref] [PubMed]
- Winter AM, Landsburg DJ, Mato AR, et al. A multi-institutional outcomes analysis of patients with relapsed or refractory DLBCL treated with ibrutinib. Blood 2017;130:1676-9. [Crossref] [PubMed]
- Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II 'proof-of-concept' iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer 2019;117:121-30. [Crossref] [PubMed]
- Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood 2015;125:1394-402. [Crossref] [PubMed]
- Ng AK, Yahalom J, Goda JS, et al. Role of Radiation Therapy in Patients With Relapsed/Refractory Diffuse Large B-Cell Lymphoma: Guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 2018;100:652-69. [Crossref] [PubMed]
- Odejide OO. Strategies for introducing palliative care in the management of relapsed or refractory aggressive lymphomas. Hematology Am Soc Hematol Educ Program 2020;2020:148-53. [Crossref] [PubMed]
- Horowitz RK, Hogan LA, Carroll T. MVP-Medical Situation, Values, and Plan: A Memorable and Useful Model for All Serious Illness Conversations. J Pain Symptom Manage 2020;60:1059-65. [Crossref] [PubMed]
- Trevino KM, Rutherford SC, Marte C, et al. Illness Understanding and Advance Care Planning in Patients with Advanced Lymphoma. J Palliat Med 2020;23:832-7. [Crossref] [PubMed]
- Budde LE, Ghosh N, Chavez JC, et al. Promising tolerability and efficacy results from dose-escalation in an ongoing phase Ib/II study of mosunetuzumab (M) with polatuzumab vedotin (Pola) in patients (pts) with relapsed/refractory (R/R) B-cell non-Hodgkin’s lymphoma (B-NHL). J Clin Oncol 2021;39:7520. [Crossref]
- Olszewski AJ, Avigdor A, Babu S, et al. Single-Agent Mosunetuzumab Is a Promising Safe and Efficacious Chemotherapy-Free Regimen for Elderly/Unfit Patients with Previously Untreated Diffuse Large B-Cell Lymphoma. Blood 2020;136:43-5. [Crossref]
- Dickinson M, Carlo-Stella C, Morschhauser F, et al. Glofitamab Monotherapy Provides Durable Responses after Fixed-Length Dosing in Relapsed/Refractory (R/R) Non-Hodgkin Lymphoma (NHL) Patients (pts). Blood 2021;138:2478. [Crossref]
- Hutchings M, Sureda A, Terol MJ, et al. Glofitamab (Glofit) in Combination with Polatuzumab Vedotin (Pola): Phase Ib/II Preliminary Data Support Manageable Safety and Encouraging Efficacy in Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL). Blood 2021;138:525. [Crossref]
- Thieblemont C, Clausen MR, Balari AS, et al. Phase 3 trial (GCT3013-05) of epcoritamab versus standard of care in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). J Clin Oncol 2021;39:TPS7569. [Crossref]
- Hutchings M, Mous R, Clausen MR, et al. Subcutaneous Epcoritamab Induces Complete Responses with an Encouraging Safety Profile across Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma Subtypes, Including Patients with Prior CAR-T Therapy: Updated Dose Escalation Data. Blood 2020;136:45-6. [Crossref]
- Wang M, Barrientos JC, Furman RR, et al. VLS-101, a ROR1-Targeting Antibody-Drug Conjugate, Demonstrates a Predictable Safety Profile and Clinical Efficacy in Patients with Heavily Pretreated Mantle Cell Lymphoma and Diffuse Large B-Cell Lymphoma. Blood 2020;136:13-4. [Crossref]
- Levy MY, Jagadeesh D, Grudeva-Popova Z, et al. Safety and Efficacy of CD37-Targeting Naratuximab Emtansine PLUS Rituximab in Diffuse Large B-Cell Lymphoma and Other NON-Hodgkin'S B-Cell Lymphomas - a Phase 2 Study. Blood 2021;138:526. [Crossref]
Cite this article as: Wallace DS, Reagan PM. Age is just a number: managing relapsed or refractory diffuse large B-cell lymphoma (DLBCL) in older patients. Ann Lymphoma 2022;6:10.


