
Diffuse large B-cell lymphoma among the elderly: a narrative review of current knowledge and future perspectives
Introduction
Diffuse large B-cell lymphoma (DLBCL) is predominantly a disease of the elderly, with incidence increasing with age (1). The number of very old patients with DLBCL in the Western World is expected to rise significantly in coming years due to evolving demographics (2). The world population aged 80 years and above tripled in size between 1980 and 2010, an increase that is projected to continue (3). Although DLBCL is a potentially curable disease, and although there are robust clinical trial data of frontline therapy in the elderly (ages 60–80) (4-6), there is a paucity of data in the very elderly (>80 years of age). Patients with DLBCL >60 years of age are heterogeneous, which limits their widespread inclusion in clinical trials. The few trials focusing exclusively on the very elderly have generated a limited evidence base informing optimal therapies in this group of patients. For example, in a systematic review of frontline therapies in DLBCL, only 6 out of 41 phase II/III clinical trials included patients over the age of 80 years (7). Recent improvements in cancer and lymphoma care have primarily been achieved in younger patient groups (2,8), and it is unclear whether these also apply to the elderly population. The aim of this review is to provide an overview of the epidemiology, prognosis, frailty assessment and current and future treatment options for elderly DLBCL patients with a focus on the very elderly patients for whom evidence-based practice is limited.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://aol.amegroups.com/article/view/10.21037/aol-22-2/rc).
Methods
Relevant studies were identified using the PubMed database with the keywords reported above, searched alone and in combination. While there were no filters used to specify text language or publication dates, the evidence included in the review was limited to studies published in English in peer-reviewed international journals (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | Search conducted between November 4, 2021 and January 3, 2022 |
| Databases and other sources searched | PubMed database |
| Search terms used (including MeSH and free text search terms and filters) | DLBCL, elderly, chemotherapy, frailty, prognosis |
| Timeframe | None specified |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | Included literature were English-language papers published in peer-reviewed, international journals |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | All authors participated in literature selection, conducted independently |
Discussion
Epidemiology and survival in elderly DLBCL patients
DLBCL is the most common lymphoma subtype among adults in the Western World. With a median age at diagnosis of 70 years and increasing incidence with advanced age, the number of newly diagnosed elderly patients with DLBCL becomes larger every year as life expectancy increases. In a Swedish population-based study the incidence of DLBCL increased 2.2% per year between 2004 and 2016 (9). The definition of elderly has varied over time in the literature with no universally accepted age cut-off to define this population, although age ≥80 years is often used to define a very elderly subgroup (10,11).
Data describing patient characteristics and survival of older patients with lymphoma are mostly limited to retrospective and observational data. In published reports, patient characteristics for elderly patients with DLBCL are similar to those of younger patients with the exception of a higher fraction of patients with poor performance status (12,13). Females numerically exceed males in elderly cohorts due to the longer life expectancy of women, but male-to-female incidence rate ratio remains higher among older patients when calculating sex-specific incidence rates of DLBCL (14,15). Some studies report a higher proportion of stage III-IV disease among elderly patients, which may indicate a more protracted diagnostic workup with delayed diagnosis or a more aggressive disease biology (12,13,16). As expected, the comorbidity burden is high among elderly DLBCL patients (12,16,17). Prognostic factors among elderly patients with DLBCL are similar to those observed in other studies including a broader range of patients with DLBCL, including Eastern Cooperative Oncology Group performance status (ECOG PS) score 2–4, elevated lactate dehydrogenase (LDH), advanced stage and Charlson comorbidity index (CCI) >1 (14).
The addition of rituximab to standard chemotherapy has benefited both young and elderly DLBCL patients, although possibly to a lesser extent in the latter group (15,18-21). Three randomized clinical trials have demonstrated that R-CHOP is generally well tolerated and associated with improved outcomes exclusively in patients >60 years of age, although the proportion of patients >75 years of age included in these studies has ranged been 0 and 20% (4-6). A nation-wide study from the Netherlands included 4,737 DLBCL patients aged ≥80 years (10% ≥90 years) treated in the period 1989–2015. Five-year relative survival (RS) for patients who received treatment was 45%, 44% and 36% in age groups 80–84 years, 85–89 years and ≥90 years, respectively. Relative survival is the overall survival (OS) in a patient cohort divided by the expected survival (ES) of an equivalent group from the general population, matched to the patients with respect to age, sex, and period (15). The improvement was particularly pronounced in the cohort treated with immunochemotherapy, with a 5-year RS of 69% (15). A Surveillance, Epidemiology, and End Results (SEER) database study of DLBCL patients aged 80 years and above demonstrated improved 2-year OS of 39% in the rituximab era compared to 25–32% in the pre-rituximab era (22). In another SEER-study of 1,156 DLBCL patients aged 80 and above, R-CHOP was the only treatment associated with improved survival, but only 51% of the cohort received R-CHOP, indicating that elderly lymphoma patients may be at risk of undertreatment (12). Several other studies also report possible suboptimal treatment of elderly patients with DLBCL with as few as 30–50% receiving treatment in some studies, although it is difficult to retrospectively identify subgroups who may have benefited from more curative treatment approaches (16,23-25).
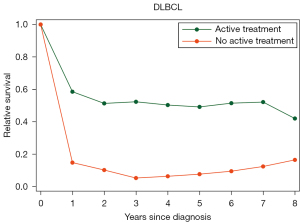
In a collaborative Nordic study of lymphoma patients aged 85 years and above, 2,347 Danish and Swedish patients were identified, of which 924 had DLBCL. Overall, 69% of DLBCL patients received active treatment which was associated with an improved survival with a 2-year RS of 51% for treated DLBCL patients as compared to 10% for untreated (Figure 1) (14). For patients who were selected for R-CHOP or R-CEOP (data on potential dose reductions not available), survival was even better with a 2-year RS of 64% (14). That treatment is beneficial also among the oldest old and should be administered when feasible is supported by other observational studies. A French retrospective study of 164 patients aged 90 years and above with all lymphoma subtypes showed that 56% of patients received systemic therapy which was associated with improved OS for aggressive subtypes (26). In another Swedish study of 1,194 DLBCL patients aged 80 years and above, curative intent treatment was associated with superior 2 year RS of 64% as compared to low-intensive treatment or palliative treatment (2-year RS 33% and 7%) (27). Similar results were seen in a retrospective cohort study of DLBCL patients aged 80 years and above from MD Anderson; 70% received treatment with R-CHOP, which was associated with a 3-year OS of 54% (28).
Is biology in elderly DLBCL different?
There is mounting evidence that tumour biology differs between younger and elderly patients with DLBCL. Klapper et al. demonstrated that cytogenetic complexity increases with age, and that the prognostically inferior ABC-subtype is more common among elderly patients (29). This was confirmed by other groups, where one also found a higher MYC expression among older DLBCL patients (30,31). A report that proposed a new subtype stratification of DLBCL based on genetic alterations, found that most mutations were caused by mutational processes associated with aging (32). Finally, a recent study from the Munich Leukemia laboratory with comprehensive genetic analyses on 3,069 patients with hematologic disorders, found a higher median number of mutations and different distribution of genetic aberrations among patients ≥60 years (33). Although this study only included a few cases of B-cell non-Hodgkin lymphoma (B-NHL), it indicates that biological differences may partially explain the worse survival of elderly DLBCL patients (33).
Frailty and prognostic scores in elderly DLBCL patients
The clinical evaluation of elderly patients with DLBCL can be complicated by poor performance status and higher frailty scores, often related to lymphoma-associated symptoms (34,35). Because the international prognostic index (IPI) (36) incorporates age >60 years as a factor, its applicability for elderly lymphoma patients may be suboptimal. The NCCN-IPI, proposed in 2014, enhanced stratification by extending age and LDH categorisation, with patients aged >75 years grouped separately, and differentiating between specific extranodal presentations (37). However, it has not yet proven to predict prognosis more accurately than the original IPI for elderly patients (19). A Danish study showed that an age-cut off of 70 years was more discriminatory in a population-based cohort of patients with DLBCL (38). However, neither the IPI nor the NCCN-IPI incorporate other measures of frailty.
Frailty is defined as a state of decreased physical reserves, affecting the body’s tolerance to stressors due to deteriorated physical systems (39). Comprehensive geriatric assessment (CGA) is considered a gold standard to assess for frailty in elderly cancer patients (40). In DLBCL, it has been shown to predict both survival and treatment toxicity in DLBCL and has potential to guide treatment choice (41,42). Still, its use has been limited in clinical practice as it is time consuming (40,43,44). Therefore, several screening tools to identify patients appropriate for a full CGA have been developed, including the Vulnerable-Elders Survey-13 (VES-13), Geriatric-8 (G8) and Triage Risk Screening Tool. These screening tools have been found to have high sensitivity to predict frailty and prognosis, but in a systematic review they had low specificity and poor positive prognostic values in identifying individual patients who might benefit from a full CGA (45,46). The G8-score has subsequently proved to predict prognosis among patients with hematological malignancies and could represent a useful tool in treatment choice (40,47,48). However, a Japanese group demonstrated that the cut-off value for when the G8 score should affect treatment choice was different among patients with DLBCL compared to solid malignancies, as it includes variables that may be affected by the lymphoma itself (48). That frailty according to the VES-13 is associated with poorer survival regardless of age has also been shown (49). Ultimately, the use of CGA and similar tools has not been adopted widely because (I) some of their components such as PS and nutritional scores may both be affected by the lymphoma itself, and (II) it is unclear how to best adapt therapy in response to results (48,50). Still, it is evident that CGA and simplified screening tools are associated with survival and need to be incorporated in future trials to evaluate the feasibility of these tools to guide treatment choice.
Several prognostic scores specifically developed to assess frailty and treatment tolerability for elderly patients with DLBCL have been proposed and are summarised in Table 2. The Fondazione Italiana Limfomi have previously demonstrated that a simplified version of geriatric assessment (sGA) correlates with DLBCL survival and identifies a group of frail patients who may not benefit from treatment (52). In a subsequent prospective observational study, sGA significantly predicted survival as did the IPI and hemoglobin level <12 g/L, which led to the construction of the Elderly prognostic index (EPI) (10). The authors found that patients with a low-risk EPI benefit from full-dose treatment with curative intent. In the group with intermediate EPI score, full-dose therapy with curative intent was feasible but reduced dose-intensity was not associated with worse outcomes. For patients with a high-risk EPI, treatment benefit remains uncertain (10). Data on socioeconomics and other factors such as marriage status, shown to be important for survival and treatment tolerability, were not accounted for. When stratifying patients into fit, unfit and frail using a frailty score proposed in a retrospective Norwegian study of 742 patients aged 70–100 (51), administration of R-CHOP was beneficial for all cohorts without significantly increased treatment related mortality. However, attenuated R-CHOP (defined as relative dose intensity ≤80%) was as effective as full dose R-CHOP among unfit and frail patients (51). Thus, both this and the EPI may function as tools to define treatment goals for elderly patients, although further prospective validations are needed (10,51).
Table 2
| Prognostic score | No. of patients | Age group developed for | Included variables | Validation strategy | Categorisation (% of patient with this risk score) | OS |
|---|---|---|---|---|---|---|
| sGA (10) | 1,207 | 65–94 years (median age 76) | ADL ≥/<5, IADL ≥/<6, CIRS-G, age </≥80 | Prospective study | Fit (54%) | NR |
| Unfit (28%) | NR | |||||
| Frail (18%) | NR | |||||
| EPI (10) | 1,065 | Training cohort: 65–94; external validation cohort: 65–99 | sGA: fit (0 p), unfit (3 p), frail (4 p); IPI: 1 (0 p), 2 (1 p), 3–5 (3 p); hemoglobin: >12 g/L (0 p), <12 g/L (1 p) | Patients in prospective study for training cohort; external validation of 456 patients, 328 with complete data | Low 0–1 points (23%) | 3-y OS 87% |
| Intermediate 2–5 points (48%) | 3-y OS 69% | |||||
| High-risk 6–8 points (29%) | 3-y OS 42% | |||||
| Norwegian score (51) | 784 | Training cohort: 70–100; validation cohort: 70–93 | Age: <85 (1 p), ≥85 (2 p); ADL: independent (1 p), dependent (2 p); CCI: 0–1 (1 p), 2 (1.5 p), ≥3 (2 p); GNRI: absent/low (1 p), moderate (2 p), severe (2.5 p) | Retrospective study. Training cohort from Norwegian cancer register (n=522); validation cohort from Norwegian cancer register (n=262) | Fit 1 point (31%) | 2-y OS 82% |
| Unfit 1.5–3 points (35%) | 2-y OS 47% | |||||
| Frail >3 points (34%) | 2-y OS 14% |
DLBCL, diffuse large B-cell lymphoma; OS, overall survival; sGA, simplified geriatric assessment; ADL, activities of daily living; IADL, instrumental activities of daily living; CIRS-G, cumulative illness rating scale-geriatric; NR, not reported; EPI, elderly prognostic index; IPI, international prognostic index; CCI, Charlson comorbidity index; GNRI, geriatric nutritional risk index.
Several other methods to assess frailty have been proposed. For example, gait speed, measured on a 4-meter walk, has been found to be associated with both mortality and need for in-hospital stays for patients with hematologic malignancies (29% with DLBCL) (53). With a mortality increase of 20% for every 0.1 m/s decrease in gait speed, this measure appears to capture several different physiological aspects such as function of the central and peripheral nervous system, perception, muscle mass and energy production (54). How these simple tests can guide treatment selection in elderly patients with DLBCL tolerability remains to be prospectively studied.
Optimal dosing of R-CHOP in elderly patients
R-CHOP has been the standard of care for patients over the age of 60 for the last two decades based on randomized data (4-6). However, elderly patients are at increased risk of toxicity from R-CHOP, often requiring dose reductions and/or premature treatment discontinuations (21). Standard doses of R-CHOP cannot be consistently used in all elderly patients outside of clinical trials, especially in the very elderly or frail. In a study of 3,149 Swedish DLBCL patients, age >75 years and comorbidity were predictors of premature treatment discontinuation. Patients unable to complete the full six cycles of R-CHOP experienced substantially worse OS even when discontinuations were unrelated to disease progression; five-year OS for 1–3 cycles was 26% (95% CI: 19–33%), 49% (95% CI: 41–57%) for 4–5 cycles, and 76% (74–77%) for completion of treatment (55).
Randomized controlled trials comparing full vs. reduced intensity R-CHOP in elderly/frail patients have not been performed. The efficacy of attenuated R-CHOP (mini-CHOP) was established in a single-arm trial of 150 DLBCL patients >80 years (11). The attenuated R-CHOP regimen (R-mini-CHOP) consisted of six cycles of R-CHOP-21 with 375 mg/m2 rituximab, 400 mg/m2 cyclophosphamide, 25 mg/m2 doxorubicin, and 1 mg vincristine on day 1 of each cycle, and 40 mg/m2 prednisone on days 1–5 (11). The median age of enrolled patients was 83 years (range 80–95 years) with intermediate-high to high risk by IPI in 71%. Functional limitations according to the IADL were documented in 54% of the patients. The relative median dose-intensity for the 108 patients completing six cycles was 97% for doxorubicin, suggesting that this dose is tolerable for many elderly. The 2-year OS was 59% (95% CI, 49–67%) (11). Despite the reduced intensity R-CHOP, 13 patients died between first and second cycle. Prophylactic granulocyte colony-stimulating factor (G-CSF) and/or antibiotics were left to the discretion of the treating physician, but later studies have shown reduction of early mortality from infectious complications if used consistently in elderly patients (56). Ongoing studies of novel agents against DLBCL in elderly patients have implemented the dose-attenuated R-CHOP-21 regimen as standard treatment, confirming that this regimen is now widely accepted as first line treatment in elderly patients.
A major challenge for clinicians is to identify elderly DLBCL patients for full dose R-CHOP. A recent systematic review included studies of DLBCL where survival was reported for at least 100 R-CHOP-21 treated patients with available data on dose intensity (57). Thirteen relevant studies including a total of 5,188 patients were identified, all retrospective and many single center. Relative dose intensity (RDI) was defined as the ratio of delivered dose to the planned dose multiplied by a time factor (the ratio of planned treatment time to the actual time taken to complete chemotherapy) in 10/13 studies (57). Seven of nine studies evaluating the impact of RDI on OS found that OS was reduced in patients receiving reduced RDI. This association was most evident in patients <80 years, whereas reduced dose intensity in patients 80 years or older did not consistently appear to compromise survival outcomes (57).
Four of the largest studies of dose intensity and outcomes are summarized in Table 3. The two studies that based treatment intensity of the dosing of first cycle of R-CHOP both consistently showed that full dose R-CHOP was not associated with better outcomes in patients ≥80 years (13,16). Thus, an intention to treat with less than full dose in this population did not adversely affect outcomes in this age group. Further, patients ≥80 years treated with R-CHOP at an intended dose intensity (IDI) of ≤80% did not experience more relapses suggesting that reduced dose is sufficient for durable remissions in elderly patients. This was not the case for patients <80 years, where reduced dose was associated with increased relapse risk (13). Importantly, R-CHOP of any dose appeared to be better than milder regimens such as R-CVP for patients up to 85 years, with exception of patients 80–84 with severe comorbidity (16). Therefore, R-CHOP should be pursued regardless of the need for dose reductions in healthy patients up to 85 years. For the very elderly ≥85 years, treatment decisions will have to be on individual case basis with careful evaluation of patient preferences and comorbidities, as results are conflicting for this group. In a Nordic study of lymphoma patients ≥85 years where dose intensity was not reported, standard treatment (R-CHOP or R-CEOP) for DLBCL was associated with a 2-year relative survival (RS) of 64% (95% CI, 60–73%) whereas low-intensive systemic therapies were associated with a 2-year RS of 40% (95% CI, 34–47%) (14). In a recent Japanese multi-center study, a linear relation between dose-intensity and OS was observed in 127 patients. Median follow-up was short at 15 months, 22/64 deaths were unrelated to lymphoma and some patients with treated with pirarubicin instead of doxorubicin. The results showed that patients in the <50% dose intensity group (based on anthracycline, cyclophosphamide, and vincristine) had poorer response rates and survival. The median dose of anthracycline in the <50% dose intensity group was 31%, and thus far below the mini-CHOP dosing schedule, which provides a dose intensity >50% due to cyclophosphamide of 400 mg/m2 (60). Main drivers for <50% dose intensity were, among others, patient specific factors such as dementia, comorbidity, and age. This study suggests that R-CHOP doses <50% are associated with worse outcomes in patients 80 years or older, despite adjustments for confounders such IPI and comorbidity, but do not provide evidence against the efficacy of R-miniCHOP in patients 80 years or older.
Table 3
| Study | Study design | Patients | Dose intensity evaluations | Crude outcomes analyses | Adjusted outcomes analyses | Additional findings |
|---|---|---|---|---|---|---|
| Juul et al. (16) | Population-based, retrospective | 1,011 DLBCL patients ≥75 years (median 81, range 75–101), 599 patients treated with CHOP +/− R | Cyclophosphamide and doxorubicin ≥80% of standard in first cycle was defined as full dose (n=403, of which 53% completed). Doses of <80% were defined as reduced dose (n=196) | Intended full dose associated with OS benefit in patients aged 75–79 years but not in patients ≥80 years | R-CHOP(-like) therapy (regardless of intended dose) associated with better outcomes in patients below 85 years in multivariable analyses adjusting for time period, age, sex, IPI and CCI score | Milder regimens (R-CVP or R-CEOP) were associated with similar survival in patients ≥85 years and for patients 80–84 years with high comorbidity score |
| Mörth et al. (58) | Multi-institutional, retrospective study | 541 DLBCL patients, median age 66 years (range 18–91) treated with at least one cycle of R-CHOP (full or mini-CHOP) or R-CHOEP | RDI for doxorubicin (≤70% vs. >70%). Omissions of vincristine at any time during therapy | Not reported for doxorubicin | RDI ≤70%: HR 1.88 (0.97–3.67) for DFS and 2.04 (1.15–3.61) for OS after adjustments for disease risk factors, BMI, performance status | Omission of vincristine had no impact on OS regardless of time of omission (cycle 1–3 vs. later) |
| Eyre et al. (13) | Multi-institutional, retrospective study | 690 DLBCL patients aged ≥70 years (median 77, range 70–96), all treated with R-CHOP with curative intent | IDI for R-CHOP was average dose of doxorubicin and cyclophosphamide in cycle 1 (IDI ≥80% vs. <80%). | Despite more frequent dose reductions in patients ≥80 years, cumulative relapse risk was similar (SHR =1.20, P=0.27). IDI ≥80% associated with better outcomes in age category 70–89 but not in +80 years | Higher cumulative relapse rate for patients aged 70–79 years with IDI <80% remained in multivariable analyses including ECOG-PS, CIRS-G, and disease related risk factors. IDI not associated with relapse risk in multivariable analysis restricted to patients ≥80 years. RDI/IDI ratio not predictive of outcome | IDI ≥80% was not associated with increased number of admissions in patients ≥80 years |
| Hwang et al. (59) | Single center, retrospective study | 608 DLBCL patients median age 53, 605 patients treated with R-CHOP | RDI (<85% vs. >85%) | RDI <85% associated with inferior PFS and OS in univariate analyses | RDI not associated with outcomes in multivariate analyses | Korean population where drug metabolism may differ from Caucasians. Only 6 patients ≥80 years |
DLBCL, diffuse large B-cell lymphoma; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; OS, overall survival; IPI, international prognostic index; CCI, Charlson comorbidity index; R-CVP, rituximab, cyclophosphamide, vincristine, and prednisone; R-CEOP, rituximab, cyclophosphamide, etoposide, vincristine, and prednisone; R-CHOEP, rituximab, cyclophosphamide, doxorubicin, etoposide, vincristine, and prednisone; RDI, relative dose intensity, equaling actual delivered dose per time multiplied by a time factor to account for treatment delays (planned treatment days divided with actual number of days); HR, hazard ratio; DFS, disease-free survival; BMI, body mass index; IDI, intended dose intensity; SHR, sub hazard ratio, estimated in competing risk analyses; ECOG PS, eastern cooperative oncology group performance status; CIRS-G, cumulative illness rating scale-geriatric; PFS, progression-free survival.
In addition to showing a general adverse impact of reduced dose-intensity of doxorubicin (<70% of full dose) on OS, a Swedish study of 541 patients treated with CHOP or CHOEP showed that omission of vincristine, was not associated with inferior outcomes (HR for OS 1.30, 95% CI, 0.53–3.16) (58). This is important, as neurotoxicity is seen in 20% of elderly patients and this group is likely less able to cope with the neurological decline which can have a detrimental impact on physical functioning (11).
Most of the patients analyzed in the UK study of IDI in 690 DLBCL patients were also analyzed by the authors for infection-related morbidity and mortality in a subsequent study; among 331 patients receiving an IDI ≥80%, 33% were hospitalized with ≥1 infection(s) as compared with 23.3% of 355 patients receiving an IDI of <80% (OR, 1.61; 95% CI, 1.15–2.25; P=0.006). The use of primary quinolone prophylaxis also independently reduced the risk of infection-related hospital admission (61). Therefore, less than full dose in patients ≥80 years as well as prophylactic antibiotics can reduce risk of hospitalizations in this fragile elderly group and thereby likely increase quality of life during treatment.
Recent real-world data from England comparing R-mini-CHOP to standard R-CHOP in 904 patients ≥80 years have confirmed equivalent survival with the two regimens in this age-group with a completely overlapping 3-year OS of 54%. Interestingly, patients treated with non-anthracycline (mostly R-CEOP but also R-GCVP) had a similar 3-year OS to patients treated with R-mini-CHOP of 49% (95% CI, 38–60%) suggesting that elderly with risk factor for anthracycline-induced cardiotoxicity can be managed successfully without anthracyclines (62).
For future research in dose-intensity in DLBCL and its impact on outcomes, randomized trials would be of major importance to establish the benefit/risk of full-dose R-CHOP in patients ≥80 years, although this trial may never be performed due to the increasing number of novel therapies that may prove to be particularly relevant in elderly patients at risk of chemo-induced toxicity. Based on available data, we recommend reduced intensity R-CHOP for the majority of patients ≥80 years, as lymphoma outcomes are encouraging and risk of complications are less than with full dose. Patients ≥85 years need to be carefully evaluated, but reduced intensity R-CHOP should be considered in the healthy and fit subpopulation.
How to treat elderly patients with DLBCL despite frailty and comorbidities: alternatives to R-CHOP in 1st line
As reviewed above, older patients with DLBCL are more likely to have multiple comorbidities, associated with increased rates of treatment toxicity and risk of severe adverse events (28). Safe and effective options in those patients unable to tolerate standard or reduced doses of R-CHOP are limited. Table 4 lists single-arm studies of modified R-CHOP for first-line treatment of DLBCL using dose-reduced chemotherapy and/or anthracycline alternatives.
Table 4
| Study | Regimen | n | Eligibility | Outcomes | Toxicities | Comments |
|---|---|---|---|---|---|---|
| NCT00244127. Phase II EUR018 trial (63) | R-COMP [standard R-CHOP, replacing doxorubicin with nonpegylated liposomal doxorubicin (NPLD)]: cyclophosphamide 750 mg/m2, vincristine 1.4 mg/m2 (max 2 mg), and NPLD 50 mg/m2 on day 1; prednisone 100 mg/day on days 1–5, and rituximab 375 mg/m2 on day 3 of cycle 1; day 1 of subsequent cycles. 3 cycles, then 5 more cycles if response | 72 | Age ≥60, untreated DLBCL, ECOG PS ≤2, LVEF ≥50% | CR rate 57% (95% CI, 43–67%). Estimated 3-y OS, FFS, and PFS rates were 72% (95% CI, 58–82%), 39% (95% CI, 28–51%), and 69% (95% CI, 56–79%), respectively | 9 treatment-related deaths; 18% of patients had grade 3–4 FN. 7 patients discontinued treatment for cardiac events (1 fatal) | Prophylactic G-CSF was not included in the protocol; given per institutional policies. 34 patients received G-CSF. 19 had treatment delays, mostly for hematologic toxicity. 38 patients had a pre-existing cardiovascular condition, most frequently hypertension. Median age 72 with 60% of patients >70 years |
| NCT01009970. Phase II HEART01 (64) | R-COMP (standard R-CHOP, replacing doxorubicin with NPLD): cyclophosphamide 750 mg/m2, vincristine 1.4 mg/m2 (max 2 mg), and NPLD 50 mg/m2 on day 1; prednisone 100 mg/day on days 1–5, and rituximab 375 mg/m2 on day 3 of cycle 1; day 1 of subsequent cycles. Limited stage: 4 cycles. Limited stage bulky and advanced stage: 6 cycles | 50 | Untreated DLBCL or follicular grade III b; age ≥18 years; and at least one cardiac disorder | CR rate 56% (95% CI, 41–70%). 3-y OS, FFS, and PFS rates were 50% (95% CI, 34–65%), 27% (95% CI, 15–40%) and 38% (95% CI, 24–51%) respectively | 9 treatment-related deaths; 6 cardiac events grade ≥3 during treatment, 2 deaths from CHF in the follow-up period. There was a signal towards decreased LVEF by end of treatment (−4%) but it was not significant (P=0.116) | Primary end-points were CR rate and rate of cardiac events. Cardiac events defined as LVEF decrease ≥20% from baseline or absolute LVEF <25% at end of treatment or clinical evidence of heart failure. Median baseline LVEF 60%. 7 patients included with a baseline LVEF<50%. Median age was 76 with 94% of patients >60 years |
| Retrospective, single-arm, single-centre (65) | R-CVP (R-CHOP with doxorubicin omitted), planned for 8 cycles | 43 | Age ≥80, newly diagnosed stage II bulky or advanced stage DLCBL, and comorbidities: (creatinine clearance <60 ml/min, or LVEF <50%, or ECOG PS 3, or total bilirubin >30 mmol/L or transaminases >2.5× ULN) | 2-y OS 31.9%. Median OS 12.6 mo. Median OS of patients who received 8 cycles was 26.4 mo. Median OS of those who had <8 cycles was 3.4 mo. Median PFS 11.2 mo | Neutropenia in 46.5% of patients. FN in 18.6% of patients. TRM 34.8% | G-CSF prophylaxis per clinician’s choice; G-CSF protocol included for all patients with grade 4 neutropenia or FN |
DLBCL, diffuse large B-cell lymphoma; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; ECOG PS, eastern cooperative oncology group performance status; LVEF, left ventricular ejection fraction; CR, complete remission; OS, overall survival; FFS, failure-free survival; PFS, progression-free survival; FN, febrile neutropenia; G-CSF, granulocyte colony stimulating factor; CHF, congestive heart failure; ULN, upper limit of normal; TRM, treatment-related mortality.
The R-COMP studies, substituting non-pegylated liposomal doxorubicin (NPLD) in patients with both normal heart function (63) and those with pre-existing significant cardiac comorbidities (64), showed similar efficacy to R-CHOP (66). It is important to note, however, that even in the population selected for their history of cardiac dysfunction, median left ventricular ejection fraction (LVEF) was 60% (IQR 12) and only 7 patients (12%) had a LVEF<50% (64). These results may therefore not be readily applicable to older adults with underlying cardiac dysfunction.
In a retrospective analysis of R-CVP (R-CHOP without doxorubicin) in elderly, comorbid DLBCL patients with contraindications to anthracyclines, outcomes were inferior to those in studies of R-COMP, R-miniCHOP, and R-CEOP (65). Those poor outcomes may indeed be related to the very elderly and comorbid patients enrolled, and it is uncertain if this group would have had more favorable outcomes with the addition of etoposide or NPLD to R-CVP.
Table 5 summarizes studies comparing frontline DLBCL treatment alternatives to full-dose R-CHOP in elderly and comorbid patients. None of the various R-CHOP alternatives out-performed full-dose R-CHOP. The less intensive regimens of bendamustine plus rituximab (BR) or R-CVP had inferior efficacy. In a retrospective study comparing BR to R-CHOP as first-line therapy in elderly patients with DLBCL, BR was an independent poor prognostic factor for OS (71). Subgroup analyses of a retrospective study comparing BR to R-CHOP showed that patients age 80 and older benefitted from R−CHOP compared to BR, regardless of performance status (median PFS 59 vs. 11 months) (70). In a large retrospective, multi-centre study of DLBCL treatment in adults age ≥80 that compared R-CHOP, R-CVP, CHOP, and CVP, use of R-CHOP was independently associated with improved OS (69).
Table 5
| Study | Regimen | n | Median age [range] | Outcomes | Toxicities | Comments |
|---|---|---|---|---|---|---|
| NCT01148446. Randomized, two-arm ANZINTER3 trial (67) | RminiCEOP (rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, vinblastine 5 mg/m2, and epirubicin 50 mg/m2 on day 1; plus prednisone 50 mg/m2 on days 1–5) vs. R-CHOP | 114 vs. 110 | 71 [65–86] vs. 73 [64–84] | RminiCEOP: CR 73% (95% CI, 63–81%), 5-y OS 62% (95% CI, 51–71%); R-CHOP: CR 68% (95% CI, 58–76%), 5-y OS 63% (95% CI, 52–72%); aaIPI and age >72 were independent negative prognostic factors for OS | 17 treatment-related deaths, 10 in the R-CHOP arm and 7 in the RminiCEOP arm. TRM of 7.5%, no significant difference between arms. Grade 3–4 adverse events were not significantly different between groups, including rates of neutropenia and infectious complications | G-CSF prophylaxis included in the protocol. Rate of neutropenia (23%) lower than in comparable trials. R-CHOP arm patients slightly older |
| Retrospective cohort study using matched controls (68) | R-CEOP (R-CHOP with etoposide substituted for doxorubicin: etoposide 50 mg/m2 IV on day 1 and 100 mg/m2 orally on days 2 and 3) vs. R-CHOP | 70 vs. 140 | 73 [34–93] vs. 73 [21–92] | R-CEOP: 5-y OS 47%, 10-y OS 30%, 5-y DSS 62%, 10-y DSS 58%; R-CHOP: 5-y OS 65%, 10-y OS 49% (P=0.002), 5-y DSS 74%, 10-y DSS 67% (P=0.250) | TRM 4% in each group. Specific toxicities not reported; retrospective data | 2:1 randomly selected sequential patient controls in the same time period, matched for age, stage, and IPI. Study included 8 patients with primary mediastinal B-cell lymphoma and 4 patients with intermediate-grade B-cell lymphoma not otherwise specified |
| Retrospective, multi-centre, patients age ≥80 (69) | R-CVP (R-CHOP with doxorubicin omitted) vs. R-CHOP | 335 vs. 258 | 83 [NP] vs. 85 [NP] | R-CVP: median OS 8.95 mo; R-CHOP: median OS 21.82 mo | Specific toxicities not addressed; retrospective data | Use of R-CHOP, younger age, and lower stage independently associated with improved OS |
| Retrospective, multi-centre (70) | BR (bendamustine 90 mg/m2 days 1 and 2, rituximab 375 mg/m2 day 1; optional reduction bendamustine 70 mg/m2) vs. R-CHOP (standard or dose-reduced) | 68 vs. 72 | 80 [68–91] vs. 77 [65–93] | BR: median OS 16.3 mo (95% CI, 10.6–22.0 mo), median PFS 11.0 mo (95% CI, 5.0–17.0 mo); R-CHOP: median OS 75.4 mo (95% CI, 49.0–101.8 mo) (P=0.006), median PFS 62.3 mo (95% CI, 50.2–74.4 mo) (P<0.001) | Specific toxicities not addressed; retrospective data | BR cohort older with 53% age ≥80 (vs. 28% in R-CHOP group); more often advanced stage and higher median IPI |
| Retrospective, single centre (71) | BR vs. reduced-dose R-CHOP (standard R-CHOP with cyclophosphamide IV 450–600 mg/m2 and doxorubicin IV 30–40 mg/m2 on day 1) | 26 vs. 34 | 81 [75–93] vs. 80 [75–87] | BR: CR 42%, median OS 11.2 mo, median PFS 8 mo; R-CHOP: CR 71% (P=0.036), median OS 39 mo (P=0.035), median PFS 17.5 mo (P=0.035) | Specific toxicities not addressed; retrospective data | G-CSF prophylaxis recommended but not mandatory |
DLBCL, diffuse large B-cell lymphoma; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete remission; OS, overall survival; aaPI, age-adjusted international prognostic index score; TRM, treatment-related mortality; G-CSF, granulocyte colony stimulating factor; NP, not published.
The long-term follow up of R-CEOP, substituting etoposide for doxorubicin, compared to matched controls treated with R-CHOP shows encouraging results with comparable disease-specific and progression-free survival between groups (68). Despite the limitations of this retrospective study, the >10 years of follow-up suggest R-CEOP may be a reasonable alternative to R-CHOP in patients with cardiac comorbidity.
Treatment of relapsed DLBCL in elderly patients
While DLBCL is a curable disease, a large cohort study of 4,805 patients published in 2021 showed that 25% of patients aged 70 or older experienced relapse of their disease or had disease that was refractory to first-line therapy (72). Relapsed disease is that which recurs after an initial response to treatment. Thirty-one percent of those either relapsed or refractory (R/R) patients exhibited primary refractory disease, defined as stable disease as the best response to treatment at post-treatment assessment (73).
To achieve durable disease remissions in the R/R setting, the standard of care therapy includes intensive salvage chemotherapy followed by autologous stem cell transplant (ASCT). However, more than half of relapsing patients will not be eligible for ASCT, and the best next treatment step for transplant-ineligible patients is less clear (73). This is not least true for the cohort of elderly patients with DLBCL where very few will be eligible for ASCT and data on ASCT in elderly patients is limited. A retrospective study from Japan looked at 484 R/R DLBCL patients age 60 or older treated with salvage chemotherapy and ASCT (66). To examine outcomes, the authors stratified patients by ages 60 to 64, 65 to 69, and 70 years or older. Non-relapse mortality at day 100 was not significantly different between age groups, but age >65 was associated with decreased OS. The relapse rate was significantly higher among patients age ≥70, as well as for those not in remission, and those with ECOG PS 2–4 at time of transplant (66). ASCT may be a good option in fit elderly patients, but the toxicity profile requires careful risk assessment due to the likely diminishing returns of transplant above the age of 70 (74).
Patients deemed ineligible for ASCT have a variety of therapeutic options, but there is no accepted standard of care. Table 6 outlines therapeutic options for elderly patients with R/R DLBCL ineligible for or relapsed post ASCT. The combination R-gemcitabine and oxaliplatin (R-GemOx) is often applied and has demonstrated a median OS of 10 months in a retrospective French cohort of 196 patients ineligible for transplant (75). In a phase II study of 49 patients with R/R DLBCL R-GemOx led to complete remission for 44% of patients (76).
Table 6
| Study | Regimen | n | Median age [range] | CR rate | Outcomes | Comments |
|---|---|---|---|---|---|---|
| Retrospective population-based (75) | R-GemOx | 194 | 72 [24–89] | 33% (23% in pts age ≥75) | Median PFS 5 mo, 2-y PFS 18% (95% CI, 13–25%); median OS 10 mo, 2-y OS 32% (95% CI, 26–40%) | Median OS significantly shorter in patients age ≥75 (median 7 vs. 16 mo, P<0.001). Cytopenias were the most common high-grade toxicity. There were 7 cases of grade 5 FN |
| Multi-centre, phase II (76) | R-GemOx | 49 | 69 [41–77] | 38% | Median PFS 5 mo, 5-y PFS 13% (95% CI, 5–24%); median OS 11 mo, 5-y OS 14% (95% CI, 6–26%) | Median PFS significantly worse in those previously exposed to rituximab (4 vs. 11 mo, P=0.02). Response rates were not stratified by age. Cytopenias were the most common high-grade toxicity |
| Multi-centre, phase II (77) | CAR-T cell therapy with axicabtagene ciloleucel | 101 | 58 [25–76] | 54% | PFS 15 mo 41% (95% CI, 31–50%); OS 18 mo 52% (95% CI, 41–62%) | There was no significant relationship between age and response. There was no associated with age and treatment-related adverse events discussed |
| Multi-centre, phase II (78) | CAR-T cell therapy with tisagenlecleucel | 93 | 56 [22–76] | 40% | Median PFS 2.9 mo (95% CI, 2.3–5.2 mo), median OS 11.1 mo (95% CI, 6.6–23.9 mo) | There was no significant relationship between age and response. There was no associated with age and treatment-related adverse events discussed |
| Multi-centre, phase II (79) | BR: rituximab 375 mg/m2 IV day 1, bendamustine 120 mg/m2 IV days 2 and 3 | 59 | 67 [36–75] | 37.3% | Median PFS 6.7 mo (95% CI, 3.6–13.7 mo); OS NP | Cytopenias were a dose/cycle-limiting toxicity |
| Multi-centre, phase II (80) | BR: rituximab 375 mg/m2 IV day 1, bendamustine 90 mg/m2 or 120 mg/m2 days 1 and 2 | 59 | 74 [25–90] | 15.3% | Median PFS 3.6 mo (95 % CI, 2.7–7.2 mo), OS NP | Cytopenias were a dose/cycle-limiting toxicity |
| Multi-centre, phase Ib/II (phase II randomized) (81) | Polatuzumab + BR: polatuzumab vedotin 1.8 mg/kg IV day 2 of cycle 1 and day 1 of subsequent cycles PLUS bendamustine 90 mg/m2 IV days 1 and 2, rituximab IV 375 mg/m2 on day 1 vs. BR: bendamustine 90 mg/m2 IV days 1 and 2, rituximab IV 375 mg/m2 on day 1 | 40 vs. 40 | 67 [33–86] vs. 71 [30–84] | 40% vs. 17.5%, P=0.026 | Pola-BR: median PFS 9.5 mo (95% CI, 6.2–13.9 mo), median OS 12.4 mo (95% CI, 9.0 mo–NE); BR: median PFS 3.7 mo (95% CI, 2.1–4.5 mo) (P<0.001), median OS 4.7 mo (95% CI, 3.7–8.3 mo) (P=0.002) | Pola arm had higher rates of grade 3–4 neutropenia, but similar rates of infection |
| Single centre, phase II (82) | Lenalidomide + rituximab: 20 mg oral lenalidomide daily for 21 of each 28-day cycle and 375 mg/m2 IV rituximab weekly for 4 weeks only during cycle 1 | 32 | 66 [24–84] | 22% | Median PFS 2.8 mo (95% CI, 1.8–11.1 mo); median OS 10.2 mo (95% CI, 6.6 mo–NR) | Toxicities include cytopenias and asthenia. There were no episodes of febrile neutropenia |
| Single centre, phase II (83) | Lenalidomide + rituximab, then lenalidomide maintenance: 20 mg/day oral lenalidomide for 21 of each 28-day cycle, and rituximab 375 mg/m2 IV on day 1 and day 21 of each 28-day cycle for four cycles. After 4 cycles, patients in a CR, PR, or SD continued lenalidomide for 8 more cycles | 23 | 74 [NP] | 35% | 1-y estimated DFS 34.8% (95% CI, 14.4–56.2%); OS 18 mo 55.1% (95% CI, 32.3–72.9%) | Adverse events included cytopenias and asthenia. There were no hospitalizations for febrile neutropenia. There was no use of G-CSF |
| Randomized, multi-centre, phase II/III (84) | Lenalidomide: oral 25 mg/day, 21 of each 28-day cycle vs. investigator’s choice: of single-agent gemcitabine, rituximab, etoposide, or oxaliplatin | 51 vs. 51 | 69 [28–84] vs. 65 [20–84] | 9.8% vs. 2% | Lenalidomide: median PFS 13.6 wks, median OS 31 wks; investigator’s choice: median PFS 7.9 wks (P=0.041), median OS 24.6 wks (P=0.673) | Comparator is single-agent, palliative regimen. There was a signal for improved response to lenalidomide in ABC-type DLBCL, but it did not reach significance |
| Single-arm, multi-centre phase IIa (85) | Tafasitamab monotherapy: tafasitamab 12 mg/kg IV weekly ×8 weeks. Patients with at least SD could continue ×4 weeks. Those with a PR or CR after 12 weeks could continue until progression | 35 | 71 [35–90] | 6% | Median PFS 2.7 mo (95% CI 2.1–15.4 mo), OS NP | Activity similar in relapsed patients and those considered refractory to rituximab. Infusion reactions occurred in 12% of patients, one grade 4 |
| Single-arm, multi-centre, phase II L-MIND (86) | Tafasitamab + lenalidomide: tafasitamab 12 mg/kg IV and oral lenalidomide 25 mg/day for up to 12×28 day cycles, then tafasitamab monotherapy until progression | 81 | 72 (41–86) | 43% | Median PFS 11.6 mo (95% CI: 6.3–45.7 mo), median OS 33.5 mo (95% CI: 18.3–NR) | Activity was similar in refractory compared to relapsed disease. TRM was 5% (4 patients) |
| Multicentre, retrospective, historical matched control comparison RE-MIND (87) | Tafasitamab + lenalidomide (L-MIND data, regimen as above) vs. lenalidomide monotherapy (historical control cohort): received a starting dose of lenalidomide 25 mg/day | 76 vs. 76 | 71.5 [41–86] vs. 71 [41–86] | 39.5% vs. 13.2% | Tafasitamab + lenalidomide: median PFS 12.1 mo (95% CI, 5.9 mo–NE), median OS NE (NE; 95% CI, 15.5 mo–NE); lenalidomide monotherapy: median PFS 4 mo (95% CI, 3.1–7.4 mo) (P=0.0026), median OS 9.4 mo (95% CI, 5.1–20 mo) (P=0.0002) | Study generates an historical control group treated with lenalidomide monotherapy and compares outcomes with results of the L-MIND study. Demonstrates the incremental value of adding tafasitamab to lenalidomide |
DLBCL, diffuse large B-cell lymphoma; CR, complete response; RGemOx, rituximab, gemcitabine, oxaliplatin; PFS, progression free survival; OS, overall survival; FN, febrile neutropenia; NP, not published; NE, not estimable; NR, not reached; PR, partial response; SD, stable disease; G-CSF, granulocyte colony stimulating factor; TRM, treatment-related mortality.
Recently, chimeric antigen receptor T-cell (CAR-T) therapy has emerged as an attractive, curative-intent option for patients who are refractory to salvage regimens, relapse post-ASCT, and for select older patients deemed transplant-ineligible due to age or comorbidities. Patients with R/R DLBCL who received CAR-T cells targeting CD19 were studied in the ZUMA-1 and JULIET trials and, based on those results, CAR-T products were approved for R/R DLBCL following failure of two or more lines of therapy, including prior stem cell transplant. In the ZUMA-1 and JULIET trials, 27 patients (25%) and 25 patients (23%), respectively, were age 65 or older. CAR-T activity did not seem to be impacted by age, therefore it may be an option for older patients with R/R DLBCL (77,78).
In older transplant-ineligible patients for whom CAR-T cell therapy is not yet accessible or not appropriate, several newer approaches may better balance efficacy and tolerability. Polatuzumab vedotin (pola) in combination with BR has activity in R/R DLBCL and is approved for transplant-ineligible patients after two lines of therapy (88). This regimen demonstrates significantly improved efficacy over BR alone with a similar toxicity profile, making it an attractive choice in the R/R setting (81). Interestingly, in the study comparing pola-BR to BR alone, when analyzed by age group pola-BR demonstrated a particular improvement in OS and PFS in patients age ≥65 (81).
Lenalidomide (len) has modest single-agent activity in R/R DLBCL and can be considered as a bridging therapy to ASCT or CAR-T, or as a palliative treatment with or without the addition of rituximab (82). The regimen is well tolerated with a favorable toxicity profile, though somewhat limited effectiveness (84). A study comparing len to single-agent palliative-intent chemotherapy suggests the efficacy of len may be more pronounced in non-germinal centre B-cell (GCB) type DLBCL, and demonstrated a minor benefit over chemo monotherapies, but none of the responses were durable, making an argument for preferential use of len in combination with another agent (84). For example, the addition of anti-CD19 monoclonal antibody tafasitamab (tafa) has offered improved efficacy over lenalidomide monotherapy without significantly increasing toxicity (86). Tafa has demonstrated activity as a single agent, but by itself it has not shown a robust disease response (85). Using tafa and len together achieves a significantly more impressive response than either agent alone (86). This synergistic combination represents a tolerable and effective chemotherapy-free option for R/R disease.
While there are second- and later-line therapies available for older, transplant-ineligible patients with DLBCL, there is no established standard of care therapy. CAR-T cell therapy can offer a potentially curative option to patients considered ineligible for ASCT. Newer agents in combination with established regimens, like pola-BR, and novel combinations such as tafa-len are emerging as potential therapeutic options in R/R DLBCL.
Novel therapies in DLBCL with potential in elderly patients
Many novel therapies with activity in R/R DLBCL, especially those with favourable single-agent toxicity profiles, represent opportunities to improve frontline therapy in the elderly. There are four strategies to potentially integrate novel therapies into frontline therapy: (I) as additions to the chemoimmunotherapy (CIT) backbone, most commonly in the form of R-CHOP or R-miniCHOP, (II) as replacements of some or all the components of CIT, (III) as maintenance or consolidation therapy after CIT, or (IV) as lead-in therapy prior to CIT.
Table 7 lists various novel agents tested in combination with standard doses of R-CHOP or R-EPOCH in adult patients. Approximately half of the patients accrued in these studies were ≥65 years old, although few patients were >80 years old. For example, in the ROBUST trial, 33% patients receiving lenalidomide + R-CHOP were age ≥70, but only 3% were age ≥80 (93). These studies show that a proportion of robust elderly patients are eligible for novel agents in combination with CIT, although efficacy and toxicity were not evaluated in elderly subgroups in most of these studies. These studies also highlight the heterogeneity of elderly patients with DLBCL, and do not inform management of the very old or the very frail.
Table 7
| Novel agent added to R-CHOP | Study name | N receiving novel agent | Median age [range] in patients receiving novel agent | Comments |
|---|---|---|---|---|
| Bortezomib | REMoDL-B (89) | 459 | 64 [20–84] | Efficacy/toxicity not described according to age groups |
| PYRAMID (90) | 101 | 65 [20–83] | Efficacy/toxicity not described according to age groups | |
| Polatuzumab vedotin |
POLARIX (91) | 440 | 65 [19–80]* | Addition of polatuzumab had no impact on PFS according to age in age groups ≤60 vs. >60 years of age. Toxicity not described according to age groups |
| Ibrutinib | PHOENIX (92) | 419 | 63 [19–88] | Patients ≥60 years of age in the ibrutinib arm had worse outcomes including OS and there were significantly more serious adverse events (63%) and rates of treatment discontinuation (26%) compared to the R-CHOP alone arm |
| Lenalidomide | ROBUST (93) | 285 | 65 [21–82] | Addition of lenalidomide had no impact on PFS or OS in age groups <60 vs. ≥60 years of age. Toxicity not described according to age groups |
| EA1412 (94) | 145 | 67 [24–88] | Efficacy/toxicity not described according to age groups | |
| Ibrutinib + lenalidomide | Smart Start (95) | 60 | 64 [29–83] | 2 cycles of lenalidomide + ibrutinib + rituximab lead-in, followed by 6 additional cycles in combination with CHOP or DA-EPOCH. Efficacy/toxicity not described according to age groups |
| Venetoclax | CAVALLI (96) | 206 | 65 [18–85] | Efficacy/toxicity not described according to age groups |
| Glofitamab | NP40126 (97) | 57** | 62 [34–78] | Efficacy/toxicity not described according to age groups |
| Axicabtagene ciloleucel | ZUMA-12 (98) | 42 | 61 [23–86] | Axicabtagene ciloleucel given after 2 cycles of R-CHOP or R-EPOCH. Efficacy/toxicity not described according to age groups |
| Atezolizumab | Phase I/II (99) | 42 | 65 [22–84] | Efficacy/toxicity not described according to age groups |
| Pembrolizumab | Phase I/II (100) | 30 | 62 [22–78] | Efficacy/toxicity not described according to age groups |
*, 80 years was the upper age limit of eligibility; **, included DBLCL (n=26) and various relapsed/refractory B-cell non-Hodgkin lymphomas (n=31). DLBCL, diffuse large B-cell lymphoma; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; PFS, progression-free survival; OS, overall survival.
Table 8 lists clinical trials evaluating novel agents, with or without CIT, exclusively in elderly patients although eligibility criteria and definitions of elderly or frailty were variable. Two studies have evaluated novel agents in combination with R-miniCHOP. In the randomized phase III SENIOR study, which only enrolled patients over the age of 80, the addition of lenalidomide to R-miniCHOP was associated with increased toxicity compared to R-miniCHOP alone (102). This combination was not associated with improved PFS or OS, in line with the ROBUST trial in adult patients (93). Similarly, in the Australasian Leukaemia & Lymphoma Group NHL29 phase II study, which only enrolled patients over the age of 75, the addition of ibrutinib to R-miniCHOP was associated with significant toxicity requiring treatment modifications or interruptions (103). A similar observation was made in the subgroup of patients over the age of 60 randomized to ibrutinib + R-CHOP in the PHOENIX trial, in whom the greater toxicity and treatment discontinuation rates may have abrogated the impact of this combination on outcomes (92).
Table 8
| Study | n | Eligibility | Intervention | Efficacy results | Toxicity results |
|---|---|---|---|---|---|
| REMARC phase III (101) | 650 | Age 60–80 years in CR/PR after full-dose R-CHOP | Randomization to maintenance lenalidomide (25 mg) for 24 months vs. placebo | ~30% conversion from PR to CR in both arms; median PFS not reached for lenalidomide vs. 59 months placebo; 2-year PFS 80% lenalidomide vs. 75% placebo; OS similar between both arms | Grade 3–4 AE with lenalidomide: neutropenia 56%, infection 8%, cardiac 6%, rash 5%; SPM rates similar in both arms ~10% |
| SENIOR phase III (102) | 249 | Age ≥80 years | Randomization to lenalidomide (10 mg) + R-miniCHOP vs. R-miniCHOP | ORR 82% (CR 58%) lenalidomide arm vs. ORR 73% (CR 53%) standard arm; 2-year PFS ~55% both arms; 2-year OS 66% both arms | ≥25% required lenalidomide dose reductions and/or discontinuation; 83% grade 3–4 AE with lenalidomide, especially cytopenias and infections despite GCSF (vs. 74% grade 3–4 AE in the standard arm) |
| ALLG NHL29 phase II (103) | 80 | Age ≥75 years | Ibrutinib 560 mg daily + R-miniCHOP | ORR 76% (CR 70%); 2-year OS 68%, PFS 60%, DFS 85%; cell of origin did not impact response or survival, improvement in QOL scores over time | Ibrutinib interrupted in 62% and discontinued in 25% for AE; 64% had ≥1 grade 3–4 AE; 67% SAE mostly infection, atrial fibrillation, fever; 30 (38%) deaths, 16 PD, 14 AE; TRM 6% |
| FIL ReRi phase II (104) | 68 | ≥70 years and frail as defined by the simplified CGA | Lenalidomide (20 mg) plus rituximab every 28 days. CR/PR after 6 cycles continue for up to 12 cycles | ORR 50% (CR 25%); 56% completed six cycles; 2-year OS 45%, PFS 28% | Treatment discontinued in 28 patients: PD [11], non-hematologic toxicity [7], hematologic toxicity [1], lost [1], death [7]; greater than expected incidence of grade 3–4 AE |
| GO40554 phase I/II (105) | 40 | Age ≥80 years or 60–79 with comorbidities or reduced ADL/IADL performance unfit for full dose CIT | Single agent mosunetuzumab 13.5 mg or 30 mg IV given in 21-day cycles. CR stop at 8 cycles, PR/SD continue up to 17 cycles | ORR 68% (CR 42%); ongoing CR in 11/13 patients, including 4 with sustained response ≥12 months from mosunetuzumab initiation (and off therapy); follow-up too short for outcomes | 38% mosunetuzumab-related grade 3–4 AE; CRS 23%; all grade 1–2 and successfully treated; n=1 grade 2 neurotoxicity; neutropenia 13%, including n=1 febrile neutropenia |
DLBCL, diffuse large B-cell lymphoma; CR, complete response; PR, partial response; PFS, progression-free survival; OS, overall survival; AE, adverse event; SPM, second primary; ORR, overall response rate; QOL: quality of life; TRM, treatment-related mortality; CGA, comprehensive geriatric assessment; PD, progressive disease; ADL, activities of daily living; IADL, instrumental activities of daily living; CIT: chemoimmunotherapy; SD, stable disease; SAE, severe adverse event; CRS, cytokine release syndrome.
Table 8 shows two studies evaluating upfront strategies that do not include CIT. The combination of lenalidomide + rituximab appears to be active although response rates and outcomes were lower than expected in this study, likely related to the selection of frail patients as defined by the sCGA (104). Single agent mosunetuzumab also appears to be active and well-tolerated in those unfit for CIT and may represent an opportunity to improve frontline therapy in the elderly, although the data currently available require larger sample size and longer follow-up (105).
Because of the additional toxicity of novel agents when combined with CIT, strategies employing them after or before CIT could improve outcomes while reducing toxicity. In the randomized phase III REMARC trial, maintenance lenalidomide for 2 years after full-dose R-CHOP was associated with improved PFS but not OS, and with expected toxicity for this agent (101). However, only patients aged 60–80 and fit for full-dose R-CHOP were included in this study, limiting applicability across the broader range of elderly patients. Approximately half of the patients with high-risk large B-cell lymphoma enrolled in the ZUMA-12 study were over the age of 60 and able to tolerate axicabtagene ciloleucel after two cycles of CIT and achieve excellent early responses (98).
Novel agents could also be used to establish initial disease control and improve performance status, allowing patients to better tolerate CIT. In the Start Smart trial, the ORR was 86% (CR 36%) after two cycles of lenalidomide + ibrutinib + rituximab prior to CIT. Even though only 28% patients were over the age of 70, this study suggests this strategy could be feasible in a broader elderly population (95). Not all novel agents might be suitable for this approach, especially those which elicit slow tumor responses with the passage of time such as single agent BTK inhibitors or PD1 inhibitors.
Novel agents alone or in combinations will play an increasingly important role in the management of DLBCL in the elderly, especially in those unable to tolerate R-CHOP or R-miniCHOP. Selection of appropriate novel agent strategies will need to incorporate assessments based on frailty/organ function, patient preferences, and the biologic heterogeneity of DLBCL.
Conclusions
Age itself is not a contraindication to standard treatment of DLBCL, but its association with frailty and presence of comorbidity means that individual assessment is necessary to determine treatment feasibility and safety. Several novel scoring systems have been proposed to improve frailty assessment in the clinical setting, although the optimal algorithm to adjust therapy based on these results, especially in newly diagnosed patients, remains unclear. In elderly patients with newly diagnosed DLBCL, R-CHOP with or without modifications, is the most appropriate first-line therapy even for the very old patients. However, for patients older than 80 years, there is no clear benefit of full-dose R-CHOP over reduced dose R-CHOP and reduced dose R-CHOP is associated with a lower risk of complications. In patients with contraindications to anthracyclines, R-CEOP seems to result in comparable long-term outcomes, although with the limitation of small sample sizes and observational data. Less intensive therapies such as BR or R-CVP are generally associated with poor outcomes but can be considered in the very old (≥85 years) or in those who are not eligible for reduced doses of R-CHOP or R-CEOP. In the relapsed setting, data regarding optimal treatment for older patients with DLBCL are sparse although several novel agents with favorable single-agent activity may provide options that balance efficacy against toxicity. Moving forward, prospective, randomised studies performed exclusively in elderly patients, ideally with easily applicable assessments of frailty and disease biology, will identify the most appropriate treatment options in this population.
Acknowledgments
We wish to thank the Danish Cancer Society (TCEG). TW was supported by Region Stockholm (clinical postdoctoral appointment).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://aol.amegroups.com/article/view/10.21037/aol-22-2/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aol.amegroups.com/article/view/10.21037/aol-22-2/coif). DV has participated in advisory boards and has received honoraria from Roche, Janssen, Kite/Gilead, BMS/Celgene, Kyowa Kirin, Abbvie, AstraZeneca. DV has received research funding from Roche and AstraZeneca (institutional funding). TCEG serves as an unpaid editorial board member of Annals of Lymphoma from October 2020 to September 2022, and was previously employed by Roche Ltd (Basel) and has received a speaker’s fee from Abbvie. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morrison VA, Hamlin P, Soubeyran P, et al. Diffuse large B-cell lymphoma in the elderly: impact of prognosis, comorbidities, geriatric assessment, and supportive care on clinical practice. An International Society of Geriatric Oncology (SIOG) expert position paper. J Geriatr Oncol 2015;6:141-52. [Crossref] [PubMed]
- Ocias LF, Larsen TS, Vestergaard H, et al. Trends in hematological cancer in the elderly in Denmark, 1980-2012. Acta Oncol 2016;55:98-107. [Crossref] [PubMed]
- Wan He DGaPK. An Aging world: 2015. In: Bureau USC. editor. International Population Reports, P95/16-1. Washington, DC: U.S. Government Publishing Office, 2016.
- Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 2006;24:3121-7. [Crossref] [PubMed]
- Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood 2010;116:2040-5. [Crossref] [PubMed]
- Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 2008;9:105-16. [Crossref] [PubMed]
- Beygi S, Sadashiv S, Reilly JB, et al. Frontline treatment of diffuse large B-cell lymphoma in elderly: a systematic review of clinical trials in post-rituximab era. Leuk Lymphoma 2018;59:2847-61. [Crossref] [PubMed]
- Marcos-Gragera R, Allemani C, Tereanu C, et al. Survival of European patients diagnosed with lymphoid neoplasms in 2000-2002: results of the HAEMACARE project. Haematologica 2011;96:720-8. [Crossref] [PubMed]
- Ekberg S, E, Smedby K, Glimelius I, et al. Trends in the prevalence, incidence and survival of non-Hodgkin lymphoma subtypes during the 21st century - a Swedish lymphoma register study. Br J Haematol 2020;189:1083-92. [Crossref] [PubMed]
- Merli F, Luminari S, Tucci A, et al. Simplified Geriatric Assessment in Older Patients With Diffuse Large B-Cell Lymphoma: The Prospective Elderly Project of the Fondazione Italiana Linfomi. J Clin Oncol 2021;39:1214-22. [Crossref] [PubMed]
- Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol 2011;12:460-8. [Crossref] [PubMed]
- Williams JN, Rai A, Lipscomb J, et al. Disease characteristics, patterns of care, and survival in very elderly patients with diffuse large B-cell lymphoma. Cancer 2015;121:1800-8. [Crossref] [PubMed]
- Eyre TA, Martinez-Calle N, Hildyard C, et al. Impact of intended and relative dose intensity of R-CHOP in a large, consecutive cohort of elderly diffuse large B-cell lymphoma patients treated with curative intent: no difference in cumulative incidence of relapse comparing patients by age. J Intern Med 2019;285:681-92. [Crossref] [PubMed]
- Wästerlid T, Oren Gradel K, Eloranta S, et al. Clinical characteristics and outcomes among 2347 patients aged ≥85 years with major lymphoma subtypes: a Nordic Lymphoma Group study. Br J Haematol 2021;192:551-9. [Crossref] [PubMed]
- Dinmohamed AG, Issa DE, van der Poel MWM, et al. Treatment and relative survival in very elderly patients with DLBCL in The Netherlands: a population-based study, 1989 to 2015. Blood Adv 2017;1:1839-41. [Crossref] [PubMed]
- Juul MB, Jensen PH, Engberg H, et al. Treatment strategies and outcomes in diffuse large B-cell lymphoma among 1011 patients aged 75 years or older: A Danish population-based cohort study. Eur J Cancer 2018;99:86-96. [Crossref] [PubMed]
- Wästerlid T, Mohammadi M, Smedby KE, et al. Impact of comorbidity on disease characteristics, treatment intent and outcome in diffuse large B-cell lymphoma: a Swedish lymphoma register study. J Intern Med 2019;285:455-68. [Crossref] [PubMed]
- Hasselblom S, Stenson M, Werlenius O, et al. Improved outcome for very elderly patients with diffuse large B-cell lymphoma in the immunochemotherapy era. Leuk Lymphoma 2012;53:394-9. [Crossref] [PubMed]
- Gobba S, Moccia AA, Gulden-Sala W, et al. Outcome of patients older than 80 years with diffuse large B-cell lymphoma (DLBCL) treated with "standard" immunochemotherapy: A large retrospective study from 4 institutions. Hematol Oncol 2018;36:84-92. [Crossref] [PubMed]
- Boslooper K, Kibbelaar R, Storm H, et al. Treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone is beneficial but toxic in very elderly patients with diffuse large B-cell lymphoma: a population-based cohort study on treatment, toxicity and outcome. Leuk Lymphoma 2014;55:526-32. [Crossref] [PubMed]
- Eyre TA, Salisbury R, Eyre DW, et al. Results of a large retrospective analysis of the effect of intended dose intensity of R-CHOP on outcome in a cohort of consecutive, unselected elderly patients with de novo diffuse large B cell lymphoma. Br J Haematol 2016;173:487-91. [Crossref] [PubMed]
- Giri U, Martin MG. Survival outcomes in the very elderly with DLBCL prior to and after the introduction of rituximab: a US population-based study. Blood Adv 2017;1:615-8. [Crossref] [PubMed]
- van de Schans SAM, Wymenga ANM, van Spronsen DJ, et al. Two sides of the medallion: poor treatment tolerance but better survival by standard chemotherapy in elderly patients with advanced-stage diffuse large B-cell lymphoma. Ann Oncol 2012;23:1280-6. [Crossref] [PubMed]
- Thieblemont C, Grossoeuvre A, Houot R, et al. Non-Hodgkin's lymphoma in very elderly patients over 80 years. A descriptive analysis of clinical presentation and outcome. Ann Oncol 2008;19:774-9. [Crossref] [PubMed]
- Carson KR, Riedell P, Lynch R, et al. Comparative effectiveness of anthracycline-containing chemotherapy in United States veterans age 80 and older with diffuse large B-cell lymphoma. J Geriatr Oncol 2015;6:211-8. [Crossref] [PubMed]
- Trebouet A, Marchand T, Lemal R, et al. Lymphoma occurring in patients over 90 years of age: characteristics, outcomes, and prognostic factors. A retrospective analysis of 234 cases from the LYSA. Ann Oncol 2013;24:2612-8. [Crossref] [PubMed]
- Sonnevi K, Wästerlid T, Melén CM, et al. Survival of very elderly patients with diffuse large B-cell lymphoma according to treatment intensity in the immunochemotherapy era: a Swedish Lymphoma Register study. Br J Haematol 2021;192:75-81. [Crossref] [PubMed]
- Chihara D, Westin JR, Oki Y, et al. Management strategies and outcomes for very elderly patients with diffuse large B-cell lymphoma. Cancer 2016;122:3145-51. [Crossref] [PubMed]
- Klapper W, Kreuz M, Kohler CW, et al. Patient age at diagnosis is associated with the molecular characteristics of diffuse large B-cell lymphoma. Blood 2012;119:1882-7. [Crossref] [PubMed]
- Paul U, Richter J, Stuhlmann-Laiesz C, et al. Advanced patient age at diagnosis of diffuse large B-cell lymphoma is associated with molecular characteristics including ABC-subtype and high expression of MYC. Leuk Lymphoma 2018;59:1213-21. [Crossref] [PubMed]
- Mareschal S, Lanic H, Ruminy P, et al. The proportion of activated B-cell like subtype among de novo diffuse large B-cell lymphoma increases with age. Haematologica 2011;96:1888-90. [Crossref] [PubMed]
- Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 2018;24:679-90. [Crossref] [PubMed]
- Stengel A, Baer C, Walter W, et al. Mutational patterns and their correlation to CHIP-related mutations and age in hematological malignancies. Blood Adv 2021;5:4426-34. [Crossref] [PubMed]
- Bron D, Aurer I, André MPE, et al. Unmet needs in the scientific approach to older patients with lymphoma. Haematologica 2017;102:972-5. [Crossref] [PubMed]
- Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations†. Ann Oncol 2015;26:288-300. [Crossref] [PubMed]
- International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 1993;329:987-94. [Crossref] [PubMed]
- Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014;123:837-42. [Crossref] [PubMed]
- Gang AO, Pedersen M, d'Amore F, et al. A clinically based prognostic index for diffuse large B-cell lymphoma with a cut-off at 70 years of age significantly improves prognostic stratification: population-based analysis from the Danish Lymphoma Registry. Leuk Lymphoma 2015;56:2556-62. [Crossref] [PubMed]
- Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011;27:17-26. [Crossref] [PubMed]
- Hamaker ME, Prins MC, Stauder R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy--a systematic review. Leuk Res 2014;38:275-83. [Crossref] [PubMed]
- Spina M, Balzarotti M, Uziel L, et al. Modulated chemotherapy according to modified comprehensive geriatric assessment in 100 consecutive elderly patients with diffuse large B-cell lymphoma. Oncologist 2012;17:838-46. [Crossref] [PubMed]
- Yoshida M, Nakao T, Horiuchi M, et al. Analysis of elderly patients with diffuse large B-cell lymphoma: aggressive therapy is a reasonable approach for 'unfit' patients classified by comprehensive geriatric assessment. Eur J Haematol 2016;96:409-16. [Crossref] [PubMed]
- Ong DM, Ashby M, Grigg A, et al. Comprehensive geriatric assessment is useful in an elderly Australian population with diffuse large B-cell lymphoma receiving rituximab-chemotherapy combinations. Br J Haematol 2019;187:73-81. [Crossref] [PubMed]
- Lin RJ, Behera M, Diefenbach CS, et al. Role of anthracycline and comprehensive geriatric assessment for elderly patients with diffuse large B-cell lymphoma. Blood 2017;130:2180-5. [Crossref] [PubMed]
- Hamaker ME, Jonker JM, de Rooij SE, et al. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol 2012;13:e437-44. [Crossref] [PubMed]
- Briand M, Gerard S, Gauthier M, et al. Impact of therapeutic management and geriatric evaluation on patient of eighty years and older with diffuse large B-cell lymphoma on survival: A systematic review. Eur J Haematol 2022;108:3-17. [Crossref] [PubMed]
- Hamaker ME, Mitrovic M, Stauder R. The G8 screening tool detects relevant geriatric impairments and predicts survival in elderly patients with a haematological malignancy. Ann Hematol 2014;93:1031-40. [Crossref] [PubMed]
- Lee S, Fujita K, Morishita T, et al. Association of the Geriatric 8 with treatment intensity and prognosis in older patients with diffuse large B-cell lymphoma. Br J Haematol 2021;194:325-35. [Crossref] [PubMed]
- Fama A, Martin P, Allmer C, et al. Vulnerable Elders Survey-13 (VES-13) Predicts 1-Year Mortality Risk in Newly Diagnosed Non-Hodgkin Lymphoma (NHL). Blood 2019;134:69. [Crossref]
- Moccia AA, Thieblemont C. Curing diffuse large B-cell lymphomas in elderly patients. Eur J Intern Med 2018;58:14-21. [Crossref] [PubMed]
- Isaksen KT, Mastroianni MA, Rinde M, et al. A simplified frailty score predicts survival and can aid treatment-intensity decisions in older patients with DLBCL. Blood Adv 2021;5:4771-82. [Crossref] [PubMed]
- Tucci A, Martelli M, Rigacci L, et al. Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B-cell lymphoma: a prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL). Leuk Lymphoma 2015;56:921-6. [Crossref] [PubMed]
- Liu MA, DuMontier C, Murillo A, et al. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood 2019;134:374-82. [Crossref] [PubMed]
- Wildes TM. Make time for gait speed: vital to staging the aging. Blood 2019;134:334-6. [Crossref] [PubMed]
- Wästerlid T, Harrysson S, Andersson TM, et al. Outcome and determinants of failure to complete primary R-CHOP treatment for reasons other than non-response among patients with diffuse large B-cell lymphoma. Am J Hematol 2020;95:740-8. [Crossref] [PubMed]
- Yokoyama M, Kusano Y, Takahashi A, et al. Incidence and risk factors of febrile neutropenia in patients with non-Hodgkin B-cell lymphoma receiving R-CHOP in a single center in Japan. Support Care Cancer 2017;25:3313-20. [Crossref] [PubMed]
- Bataillard EJ, Cheah CY, Maurer MJ, et al. Impact of R-CHOP dose intensity on survival outcomes in diffuse large B-cell lymphoma: a systematic review. Blood Adv 2021;5:2426-37. [Crossref] [PubMed]
- Mörth C, Valachis A, Sabaa AA, et al. Does the omission of vincristine in patients with diffuse large B cell lymphoma affect treatment outcome? Ann Hematol 2018;97:2129-35. [Crossref] [PubMed]
- Hwang HS, Kim M, Park CS, et al. Prognostic Impact of Age at the Time of Diagnosis in Korean Patients with Diffuse Large B-cell Lymphoma in the Rituximab Era: A Single Institution Study. Cancer Res Treat 2021;53:270-8. [Crossref] [PubMed]
- Lee S, Fujita K, Negoro E, et al. Impact of relative dose intensity of standard regimens on survival in elderly patients aged 80 years and older with diffuse large B-cell lymphoma. Haematologica 2020;105:e415-8. [Crossref] [PubMed]
- Eyre TA, Wilson W, Kirkwood AA, et al. Infection-related morbidity and mortality among older patients with DLBCL treated with full- or attenuated-dose R-CHOP. Blood Adv 2021;5:2229-36. [Crossref] [PubMed]
- Hounsome L, Eyre TA, Ireland R, et al. Diffuse large B cell lymphoma (DLBCL) in patients older than 65 years: analysis of 3 year Real World data of practice patterns and outcomes in England. Br J Cancer 2022;126:134-43. [Crossref] [PubMed]
- Luminari S, Montanini A, Caballero D, et al. Nonpegylated liposomal doxorubicin (MyocetTM) combination (R-COMP) chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL): results from the phase II EUR018 trial. Ann Oncol 2010;21:1492-9. [Crossref] [PubMed]
- Luminari S, Viel E, Ferreri AJM, et al. Nonpegylated liposomal doxorubicin combination regimen in patients with diffuse large B-cell lymphoma and cardiac comorbidity. Results of the HEART01 phase II trial conducted by the Fondazione Italiana Linfomi. Hematol Oncol 2018;36:68-75. [Crossref] [PubMed]
- Laribi K, Denizon N, Bolle D, et al. R-CVP regimen is active in frail elderly patients aged 80 or over with diffuse large B cell lymphoma. Ann Hematol 2016;95:1705-14. [Crossref] [PubMed]
- Allen P. Diffuse Large B-Cell Lymphoma in the Elderly: Current Approaches. Curr Oncol Rep 2020;22:114. [Crossref] [PubMed]
- Merli F, Luminari S, Rossi G, et al. Cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab versus epirubicin, cyclophosphamide, vinblastine, prednisone and rituximab for the initial treatment of elderly "fit" patients with diffuse large B-cell lymphoma: results from the ANZINTER3 trial of the Intergruppo Italiano Linfomi. Leuk Lymphoma 2012;53:581-8. [Crossref] [PubMed]
- Moccia AA, Schaff K, Freeman C, et al. Long-term outcomes of R-CEOP show curative potential in patients with DLBCL and a contraindication to anthracyclines. Blood Adv 2021;5:1483-9. [Crossref] [PubMed]
- Huang HH, Ko BS, Chen HM, et al. Frontline treatments in extremely elderly patients with diffuse large B-cell lymphoma: a population-based study in Taiwan, 2010-2015. Immun Ageing 2020;17:17. [Crossref] [PubMed]
- Zeremski V, Jentsch-Ullrich K, Kahl C, et al. Is bendamustine-rituximab a reasonable treatment in selected older patients with diffuse large B cell lymphoma? Results from a multicentre, retrospective study. Ann Hematol 2019;98:2729-37. [Crossref] [PubMed]
- Cheng CL, Liu JH, Chou SC, et al. Retrospective analysis of frontline treatment efficacy in elderly patients with diffuse large B-cell lymphoma. Eur J Haematol 2018;101:28-37. [Crossref] [PubMed]
- Harrysson S, Eloranta S, Ekberg S, et al. Incidence of relapsed/refractory diffuse large B-cell lymphoma (DLBCL) including CNS relapse in a population-based cohort of 4243 patients in Sweden. Blood Cancer J 2021;11:9. [Crossref] [PubMed]
- Sarkozy C, Sehn LH. Management of relapsed/refractory DLBCL. Best Pract Res Clin Haematol 2018;31:209-16. [Crossref] [PubMed]
- Chihara D, Izutsu K, Kondo E, et al. High-dose chemotherapy with autologous stem cell transplantation for elderly patients with relapsed/refractory diffuse large B cell lymphoma: a nationwide retrospective study. Biol Blood Marrow Transplant 2014;20:684-9. [Crossref] [PubMed]
- Cazelles C, Belhadj K, Vellemans H, et al. Rituximab plus gemcitabine and oxaliplatin (R-GemOx) in refractory/relapsed diffuse large B-cell lymphoma: a real-life study in patients ineligible for autologous stem-cell transplantation. Leuk Lymphoma 2021;62:2161-8. [Crossref] [PubMed]
- Mounier N, El Gnaoui T, Tilly H, et al. Rituximab plus gemcitabine and oxaliplatin in patients with refractory/relapsed diffuse large B-cell lymphoma who are not candidates for high-dose therapy. A phase II Lymphoma Study Association trial. Haematologica 2013;98:1726-31. [Crossref] [PubMed]
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017;377:2531-44. [Crossref] [PubMed]
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380:45-56. [Crossref] [PubMed]
- Ohmachi K, Niitsu N, Uchida T, et al. Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2013;31:2103-9. [Crossref] [PubMed]
- Vacirca JL, Acs PI, Tabbara IA, et al. Bendamustine combined with rituximab for patients with relapsed or refractory diffuse large B cell lymphoma. Ann Hematol 2014;93:403-9. [Crossref] [PubMed]
- Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J Clin Oncol 2020;38:155-65. [Crossref] [PubMed]
- Wang M, Fowler N, Wagner-Bartak N, et al. Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: a phase II clinical trial. Leukemia 2013;27:1902-9. [Crossref] [PubMed]
- Zinzani PL, Pellegrini C, Gandolfi L, et al. Combination of lenalidomide and rituximab in elderly patients with relapsed or refractory diffuse large B-cell lymphoma: a phase 2 trial. Clin Lymphoma Myeloma Leuk 2011;11:462-6. [Crossref] [PubMed]
- Czuczman MS, Trněný M, Davies A, et al. A Phase 2/3 Multicenter, Randomized, Open-Label Study to Compare the Efficacy and Safety of Lenalidomide Versus Investigator's Choice in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Clin Cancer Res 2017;23:4127-37. [Crossref] [PubMed]
- Jurczak W, Zinzani PL, Gaidano G, et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin's lymphoma. Ann Oncol 2018;29:1266-72. [Crossref] [PubMed]
- Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol 2020;21:978-88. [Crossref] [PubMed]
- Zinzani PL, Rodgers T, Marino D, et al. RE-MIND: Comparing Tafasitamab + Lenalidomide (L-MIND) with a Real-world Lenalidomide Monotherapy Cohort in Relapsed or Refractory Diffuse Large B-cell Lymphoma. Clin Cancer Res 2021;27:6124-34. [Crossref] [PubMed]
- Amaya ML, Jimeno A, Kamdar M. Polatuzumab vedotin to treat relapsed or refractory diffuse large B-cell lymphoma, in combination with bendamustine plus rituximab. Drugs Today (Barc) 2020;56:287-94. [Crossref] [PubMed]
- Davies A, Cummin TE, Barrans S, et al. Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large B-cell lymphoma (REMoDL-B): an open-label, randomised, phase 3 trial. Lancet Oncol 2019;20:649-62. [Crossref] [PubMed]
- Leonard JP, Kolibaba KS, Reeves JA, et al. Randomized Phase II Study of R-CHOP With or Without Bortezomib in Previously Untreated Patients With Non-Germinal Center B-Cell-Like Diffuse Large B-Cell Lymphoma. J Clin Oncol 2017;35:3538-46. [Crossref] [PubMed]
- Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N Engl J Med 2022;386:351-63. [Crossref] [PubMed]
- Younes A, Sehn LH, Johnson P, et al. Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. J Clin Oncol 2019;37:1285-95. [Crossref] [PubMed]
- Nowakowski GS, Chiappella A, Gascoyne RD, et al. ROBUST: A Phase III Study of Lenalidomide Plus R-CHOP Versus Placebo Plus R-CHOP in Previously Untreated Patients With ABC-Type Diffuse Large B-Cell Lymphoma. J Clin Oncol 2021;39:1317-28. [Crossref] [PubMed]
- Nowakowski GS, Hong F, Scott DW, et al. Addition of Lenalidomide to R-CHOP Improves Outcomes in Newly Diagnosed Diffuse Large B-Cell Lymphoma in a Randomized Phase II US Intergroup Study ECOG-ACRIN E1412. J Clin Oncol 2021;39:1329-38. [Crossref] [PubMed]
- Westin JR, Nastoupil LJ, Fayad L, et al. Smart Start: Rituximab, Lenalidomide, and Ibrutinib Alone and in Combination with Standard Chemotherapy for Patients with Newly Diagnosed Diffuse Large B-Cell Lymphoma: Final Phase II Results. Blood 2019;134:1581. [Crossref]
- Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood 2021;137:600-9. [Crossref] [PubMed]
- Ghosh N, Townsend W, Dickinson M, et al. Glofitamab Plus R-CHOP Induces High Response Rates with Minimal Cytokine Release Syndrome (CRS) in Patients (pts) with Relapsed/Refractory (R/R) Non-Hodgkin Lymphoma (NHL) and Previously Untreated (1L) Diffuse Large B-Cell Lymphoma (DLBCL): Preliminary Results from a Dose-Escalation and Safety Run-in Phase Ib Study. Blood 2021;138:2479. [Crossref]
- Neelapu SS, Dickinson M, Munoz J, et al. Primary Analysis of ZUMA-12: A Phase 2 Study of Axicabtagene Ciloleucel (Axi-Cel) As First-Line Therapy in Patients with High-Risk Large B-Cell Lymphoma (LBCL). Blood 2021;138:739. [Crossref]
- Younes A, Burke JM, Cheson BD, et al. Safety and Efficacy of Atezolizumab in Combination with Rituximab Plus CHOP in Previously Untreated Patients with Diffuse Large B-Cell Lymphoma (DLBCL): Updated Analysis of a Phase I/II Study. Blood 2019;134:2874. [Crossref]
- Smith SD, Till BG, Shadman MS, et al. Pembrolizumab with R-CHOP in previously untreated diffuse large B-cell lymphoma: potential for biomarker driven therapy. Br J Haematol 2020;189:1119-26. [Crossref] [PubMed]
- Thieblemont C, Tilly H, Gomes da Silva M, et al. Lenalidomide Maintenance Compared With Placebo in Responding Elderly Patients With Diffuse Large B-Cell Lymphoma Treated With First-Line Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone. J Clin Oncol 2017;35:2473-81. [Crossref] [PubMed]
- Oberic L, Peyrade F, Puyade M, et al. Subcutaneous Rituximab-MiniCHOP Compared With Subcutaneous Rituximab-MiniCHOP Plus Lenalidomide in Diffuse Large B-Cell Lymphoma for Patients Age 80 Years or Older. J Clin Oncol 2021;39:1203-13. [Crossref] [PubMed]
- Verner E, Johnston A, Pati N, et al. Efficacy of Ibrutinib, Rituximab and Mini-CHOP in Very Elderly Patients with Newly Diagnosed Diffuse Large B Cell Lymphoma: Primary Analysis of the Australasian Leukaemia & Lymphoma Group NHL29 Study. Blood 2021;138:304. [Crossref]
- Gini G, Tani M, Bassan R, et al. Lenalidomide and Rituximab (ReRi) As Front-Line Chemo-Free Therapy for Elderly Frail Patients with Diffuse Large B-Cell Lymphoma. a Phase II Study of the Fondazione Italiana Linfomi (FIL). Blood 2021;138:305. [Crossref]
- Olszewski AJ, Avigdor A, Babu S, et al. Mosunetuzumab monotherapy in elderly/unfit pts with first-line diffuse large b-cell lymphoma (DLBCL): safety and efficacy remain promising with durable complete responses. Hematological Oncol 2021;39:EP503. [Crossref]
Cite this article as: Wästerlid T, Murphy S, Villa D, El-Galaly TC. Diffuse large B-cell lymphoma among the elderly: a narrative review of current knowledge and future perspectives. Ann Lymphoma 2022;6:6.


