
The gamma delta lymphomas: an Australian multi-centre case series
Introduction
Gamma delta (γδ) lymphomas are an exceedingly rare and characteristically aggressive type of T-cell lymphomas that originate from γδ lymphocytes. These lymphocytes display a distinctive T-cell receptor (TCR) on their surface and are derived from CD4−/CD8− T cells. Normal γδ T lymphocytes represent only a small percentage of circulating lymphocytes (<5%) but are enriched in the splenic red pulp, mucosal sites and cutaneous tissues. Their function remains to be completely defined and they are thought to have a role in both innate and adaptive immunity. Their cytotoxic features enable them to act as early effectors allowing non-major histocompatibility complex (MHC) restricted antigen recognition and secretion of high levels of cytokines (1). The 2016 World Health Organization (WHO) classification of lymphoid neoplasms recognizes four entities of γδ lymphomas: hepatosplenic T-cell lymphoma (HSPTCL), primary cutaneous gamma delta lymphoma (PCGDTL), monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL), and large granular lymphocytic leukemia (T-LGL) (2). Furthermore, there are numerous case reports of lymphomas composed of γδ lymphocytes that are not able to be classified with the current WHO classification. With the exception of T-LGL which usually behaves in an indolent manner, the γδ lymphomas are typically aggressive with early relapses and chemo-refractory disease common. Standard of care treatment of γδ lymphomas has not been established with many centers employing aggressive front-line chemotherapy often followed by stem cell transplantation (SCT). Despite this, the outcomes remain dismal with most patients succumbing to the disease often within months of the diagnosis. Prospective trials are lacking with evidence limited to retrospective case series and database analyses.
The Australasian Lymphoma Alliance (ALA) is a collaborative working group of clinicians and scientists from centers across the Asia Pacific. All centers were invited to contribute data on patients with a confirmed diagnosis of γδ lymphoma as defined by the WHO criteria that were diagnosed between 2000 and 2019. Histological diagnoses were made by the local hematopathologist and were accepted without further review. Demographic, survival, and treatment data from five centers was submitted and there was a total of 21 cases included (10 HSPTCL, 5 MEITL, 5 PCGDTL and 1 non-classifiable). We report the management and clinical outcomes of the first Australian multi-centre retrospective case series of 21 patients with γδ lymphoma subtypes.
This project was approved by Peter MacCallum Cancer Centre governance with protocol approval by respective local ethics committees. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Waiver of consent was granted by a fully constituted Human Research Ethics Committee. We present the following article in accordance with the CARE reporting checklist (available at https://aol.amegroups.com/article/view/10.21037/aol-21-41/rc).
Case series clinical presentations and treatment
HSPTCL
Ten patients with HSPTCL were reported with a median follow-up of 31.5 months. The median age at diagnosis was 45 (range, 18–66) years; 80% were male; Eastern Cooperative Oncology Group (ECOG) was generally low, with B-symptoms (70%) and marrow involvement (90%) present in the majority. Prior use of immunosuppression for any indication was identified in three patients with one patient having prior immunosuppression with azathioprine for inflammatory bowel disease (Table 1).
Table 1
| Characteristics | HSPTCL | MEITL | PCGDTCL |
|---|---|---|---|
| Number of patients | 10 | 5 | 5 |
| Median age [years] | 44 [18–66] | 55 [34–67] | 38 [24–71] |
| Gender, n [%] | |||
| Male | 8 [80] | 3 [60] | 5 [100] |
| Female | 2 [20] | 2 [40] | 0 [0] |
| History of immunocompromise, n [%] | 3 [30] | 0 [0] | 0 [0] |
| Bone marrow involvement, n [%] | 9 [90] | 1 [20] | 0 [0] |
| Lymphadenopathy, n [%] | 1 [10] | 2 [40] | 3 [60] |
| Liver enzyme derangement, n [%] | 6 [60] | 1 [20] | 1 [20] |
| B-symptoms, n [%] | 7 [70] | 2 [40] | 2 [40] |
| Elevated LDH, n [%] | 7 [70] | 2 [40] | 2 [40] |
| Cytopenia, n [%] | |||
| Anaemia | 8 [80] | 2 [40] | 2 [40] |
| Thrombocytopenia | 8 [80] | 0 [0] | 2 [40] |
| Neutropenia | 7 [70] | 0 [0] | 2 [40] |
One patient with γδ not able to be classified not included. HSPTCL, hepatosplenic T-cell lymphoma; MEITL, monomorphic epitheliotropic intestinal T-cell lymphoma; PCGDTCL, primary cutaneous γδ T-cell lymphoma; LDH, lactate dehydrogenase.
HyperCVAD (hyperfractionated cyclophosphamide, doxorubicin, vincristine, prednisolone, cytarabine and methotrexate) (20%) and CHOEP (cyclophosphamide, doxorubicin, vincristine, etoposide and prednisolone) (20%) were the most common first-line therapies with the remainder being purine or platinum-based regimens. Only four patients had a complete remission (CR) to first line therapy but all three patients that obtained a partial response (PR) and two of three patients with stable (SD) or progressive disease (PD) were then able to obtain a CR with salvage therapy. Salvage regimens were heterogenous with four patients receiving ICE (ifosfamide, carboplatin and etoposide) while the others were treated high dose methotrexate combined with high dose cytarabine and the novel agent pralatrexate alone or in combination with romidepsin. Notably, one patient who was diagnosed with HSPTCL incidentally after splenectomy has survived >32 months with no evidence of relapse after observation alone.
Frontline consolidative SCT was used in seven cases (70%) of which four were allogenic (alloSCT). All patients who proceeded to SCT were reported to be in CR at the time. The 2-year overall survival (OS) was 60% and declined to 30% at 4-year. Two of three with a progression-free-survival (PFS) >3 years had received alloSCT (Table 2).
Table 2
| Patient | Age (years) | Treatment line | Treatment regimen | Response | Response duration (months) | Survival from diagnosis (months) | Current status |
|---|---|---|---|---|---|---|---|
| 1 | 52 | 1 | ICE | SD | – | 4 | Disease related death |
| 2 | Pralatrexate | SD | – | ||||
| 2 | 33 | 1 | DICE + alloSCT | CR | 11 | 11 | Alive in remission |
| 3 | 39 | 1 | HyperCVAD | PD | – | 15 | Died of treatment related toxicity |
| 2 | ICE + alloSCT | CR | 13 | ||||
| 4 | 53 | 1 | CHOEP | CR | 17 | 26 | Disease related death |
| 2 | ICE + autoSCT | PR | 1 | ||||
| 5 | 18 | 1 | Pentostatin and alemtuzumab | PR | 3 | 66 | Alive in remission |
| 2 | ICE + alloSCT | CR | 63 | ||||
| 6 | 65 | 1 | CHOEP | PD | – | 11 | Disease related death |
| 2 | Pralatrexate and romidepsin + autoSCT | CR | 8 | ||||
| 7 | 55 | 1 | HyperCVAD + autoSCT | CR | 67 | 79 | Died of treatment related toxicity |
| 2 | VGF | SD | 7 | ||||
| 3 | Romidepsin | SD | 4 | ||||
| 8 | 66 | 1 | FCM + alloSCT | CR | 67 | 93 | Disease related death |
| 2 | Romidepsin | PR | 12 | ||||
| 3 | CHOP | PD | – | ||||
| 4 | ICE | PD | – | ||||
| 9 | 31 | 1 | DHAC | PR | 3 | 36 | Disease related death |
| 2 | High dose MTX and ara-C + autoSCT | CR | 10 | ||||
| 3 | VGF | SD | – | ||||
| 4 | Radiotherapy to spleen | PR | 14 | ||||
| 10 | 24 | 1 | Splenectomy | CR | 32 | 32 | Alive in remission |
ICE, ifosfamide, carboplatin, etoposide; SD, stable disease; DICE, dexamethasone, ifosfamide, carboplatin and etoposide; alloSCT, allogenic stem cell transplantation; HyperCVAD, hyperfractionated cyclophosphamide, doxorubicin, vincristine, prednisolone, cytarabine and methotrexate; CHOEP, CHOP and etoposide; CHOP, cyclophosphamide, doxorubicin, vincristine and, prednisolone; PD, progressive disease; CR, complete remission; PR, partial response; VGF, vinorelbine, gemcitabine and filgrastim; FCM, fludarabine, cyclophosphamide and mitoxantrone; DHAC, dexamethasone, cytarabine and carboplatin.
Primary cutaneous gamma delta T-cell lymphoma (PCGDTCL)
Five patients with PCGDTCL were identified with a median follow-up of 18 months. The median age of diagnosis was 38 (range, 24–71) years. The three patients aged <40 years had more aggressive disease characteristics with local nodal involvement and an elevated lactate dehydrogenase (LDH) and were treated front-line with anthracycline-based multi-agent chemotherapy. The two older patients (>65 years) were treated with non-chemotherapy modalities typically employed for indolent cutaneous T-cell lymphomas such as interferon and localized radiotherapy (Table 3).
Table 3
| Patient | Age (years) | Treatment line | Treatment regimen | Response | Duration of response (months) | Survival from diagnosis (months) | Current status |
|---|---|---|---|---|---|---|---|
| 1 | 24 | 1 | IVAC + alloSCT | CR | 12 | 20 | Disease related death |
| 2 | ICE | PD | – | ||||
| 3 | CODOX-M | PD | – | ||||
| 4 | Pralatrexate and romidepsin | PD | – | ||||
| 2 | 24 | 1 | Cyclosporin | PR | 3 | 7 | Disease related death |
| 2 | ICE | N/A | – | ||||
| 3 | HyperCVAD | PD | – | ||||
| 4 | Romidepsin | PD | – | ||||
| 3 | 68 | 1 | Interferon + radiotherapy (30 Gy) | PR | 4 | 8 | Disease related death |
| 2 | VGF + radiotherapy (35 Gy) | PR | 3 | ||||
| 4 | 38 | 1 | HyperCVAD + alloSCT | CR | 18 | 18 | Alive in remission |
| 5 | 71 | 1 | TSEB radiotherapy (30 Gy) | PR | 6 | 133 | Death unrelated to disease or treatment |
| 2 | Vorinostat | PR | 13 | ||||
| 3 | Interferon | PR | 61 | ||||
| 4 | Low dose methotrexate | SD | 7 | ||||
| 5 | Denileukin diftitox | PR | 3 |
IVAC, ifosfamide, etoposide and cytarabine; alloSCT, allogenic stem cell transplantation; CR, complete remission; ICE, ifosfamide, carboplatin, etoposide; PD, progressive disease; CODOX-M, cyclophosphamide, vincristine, doxorubicin, methotrexate; PR, partial response; HyperCVAD, hyperfractionated cyclophosphamide, doxorubicin, vincristine, prednisolone, cytarabine and methotrexate; VGF, vinorelbine, gemcitabine and filgrastim; TSEB, total skin electron beam.
In our series, two patients received three courses of radiation therapy (30–35 Gy) with durable infield control achieved in one patient. The first, a 68-year-old male, received 30 Gy to a significant lesion on his right buttock in combination with pegylated interferon. Despite an impressive response with almost complete resolution to the buttock lesion he had rapid progression of lesions elsewhere and the development of nodal disease necessitating further systemic therapy with chemotherapy. Due to the excellent response to radiotherapy of the buttock lesion further radiotherapy was utilized at two further sites (shoulder and upper arm) in combination with vinorelbine, gemcitabine and filgrastim (VGF) chemotherapy. Using a dose of 35 Gy both sites demonstrated significant PRs, but the patient ultimately had rapidly PD outside of the prior radiotherapy fields. The second patient, a 71-year-old male, had widespread skin lesions and received total skin electron beam (TSEB) at a dose of 30 Gy with 10 Gy boosts to sites of tumor disease. Good disease control was achieved with resolution of the tumor disease but there was relapse 6 months with widespread tumor disease progression whereupon systemic therapy with the histone acetylase inhibitor vorinostat was implemented.
Upfront allogeneic transplant was utilized in two patients. One patient relapsed at 16-month post-transplant and died of infective complications; the other patient remains in remission 10-months post-transplant.
MEITL
Five patients with MEITL were identified, with a median follow-up of 14.5 months and a median age of 55 (range, 34–67) years. Three patients (60%) presented with B-symptoms, lymphadenopathy was seen in two patients (40%), marrow involvement was seen in only one patient (20%). Cytopenias were uncommon at diagnosis with anaemia present in only two patients. Front-line treatment was most frequently cyclophosphamide, doxorubicin, vincristine and, prednisolone (CHOP)-based (60%) with hyperCVAD and the Newcastle regimen of IVE/MTX (ifosfamide, vincristine, etoposide and methotrexate) also being utilized. Despite all patients achieving an initial early complete response, four patients (80%) relapsed within 60 days of frontline therapy. Consolidative allogeneic stem cell transplant was employed in two patients with one patient allografted in first CR (CR1) and the other in second CR (CR2) after early relapse post frontline chemotherapy. The survival post-transplant was 14 and 102 months, respectively. The 2-year OS of all five patients was poor at 20% with most patients succumbing to disease within 1 year (Table 4).
Table 4
| Patient | Age (years) | Treatment line | Treatment regimen | Response | Duration of response (months) | Survival from diagnosis (months) | Current status |
|---|---|---|---|---|---|---|---|
| 1 | 58 | 1 | CHOEP | CR | 1 | 11 | Disease related death |
| 2 | GDP | PD | – | ||||
| 3 | DHAC | PD | – | ||||
| 2 | 62 | 1 | CHOP | CR | 2 | 20 | Disease related death |
| 2 | GDP | CR | 7 | ||||
| 3 | 34 | 1 | HyperCVAD + AlloSCT | CR | 6 | 20 | Died of treatment related toxicity |
| 4 | 51 | 1 | CHOP | CR | 1 | 105 | Alive in remission |
| 2 | VGF + AlloSCT | CR | 100 | ||||
| 5 | 67 | 1 | IVE/MTX | CR | 2 | 7 | Disease related death |
| 2 | Romidepsin | PD | – |
CHOEP, CHOP and etoposide; CR, complete remission; GDP, gemcitabine, dexamethasone, cisplatin; PD, progressive disease; DHAC, dexamethasone, cytarabine and carboplatin; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisolone; CR, complete remission; HyperCVAD, hyperfractionated cyclophosphamide, doxorubicin, vincristine, prednisolone, cytarabine and methotrexate; alloSCT, allogenic stem cell transplantation; VGF, vinorelbine, gemcitabine and filgrastim; IVE/MTX, ifosfamide, epirubicin, etoposide, methotrexate.
γδ lymphoma, non-classifiable
One patient reported in our case series was a 47-year-old male who presented with nodal and marrow involvement of disease phenotypical for γδ lymphoma. His prior history was relevant for X-linked lymphoproliferative disorder associated with common variable immunodeficiency disorder. He was treated with CHOEP and consolidated with alloSCT in CR. He remains in remission 74 months post-transplant. Although not yet recognized by the WHO, there are numerous case reports of patients with γδ histology that do not fit within the existing classifications describing patients with primarily nodal or marrow involvement typically treated with aggressive induction chemotherapy (3,4).
An overview of the γδ lymphomas
HSPTCL
HSPTCL is an aggressive peripheral T-cell lymphoma (PTCL) subtype involving the liver, spleen, and bone marrow. Diagnoses in all age groups have been reported but it occurs predominately in younger men with a median age of 34. Constitutional symptoms are common and often accompanied by splenomegaly. As seen in our cohort, typical laboratory findings include cytopenia, elevated LDH and derangement of liver enzymes (5). There is an association with hemophagocytic lymphohistiocytosis (HLH) which portends a worse prognosis (6).
First described in 1981 and recognized as a distinct entity in 1990, the hallmark of HSTCLs is expression of γδ TCRs however cases of αβ TCR expression have been reported. The malignant cells infiltrate extranodal sites in a marked sinusoidal pattern. Recurrent isochromosome 7q and trisomy 8 is noted on cytogenetics (7). STAT5B is mutated in up to 31% of cases (8). There is a reported association with prior immunosuppression in up to 20% of cases and the combination of thiopurine and tumor necrosis factor inhibitor treatment for Crohn disease as a risk factor for HSPTCL has been particularly well described (9-11).
Standard induction regimens have not been defined but several retrospective case series support non-CHOP (Cyclophosphamide, doxorubicin, vincristine, prednisolone) regimens due to disappointing results observed with frontline induction with CHOP or CHOP-like treatment. Despite achieving satisfactory early response rates, they are usually of short duration with early relapse common (12,13). This was well demonstrated by Belhadj et al. who reported a single centre retrospective series of 21 patients with HSPTCL where all except two were treated with CHOP or CHOP-like therapy with a median survival of 16 months. All patients treated with CHOP/CHOP-like regimens died of disease, three patients within 2 months of starting therapy. The two patients treated with platinum and cytarabine based regimens were alive and in remission at 62 and 42 months at time of reporting (13). The addition of cytarabine and methotrexate to a CHOP-like backbone (fractionated cyclophosphamide, liposomal doxorubicin, vincristine, and dexamethasone) or Hyper-CVIDD was reported by Falchook et al. from the MD Anderson Cancer Centre to obtain a higher rate of CR with durable responses in patients after SCT when compared to patients treated with CHOP/CHOP-like regimens (12). The prospective registry T Cell Project (http://www.tcellproject.org) reported one of the largest cohorts of HSPTCL so far with 31 patients. Non-Anthracycline-based regimens were well represented (40% vs. 60%) and they reported a CR rate of 40% with a 3-year OS of 40% (14). Smaller retrospective cohorts have also demonstrated durable responses with non-CHOP based regimens such as ICE (ifosfamide, carboplatin, etoposide) or IVAC (ifosfamide, etoposide, high dose cytarabine). A retrospective series reported 14 pts with HSPTCL of which seven remained alive with median follow-up of 65.6 months: 6 of the 7 long-term survivors had received alternative non-CHOP induction chemotherapy regimens followed by consolidation with AutoSCT or AlloSCT (15).
Expression of CD52 is common in HSTCL and there is anecdotal evidence of treatment with alemtuzumab in combination with a purine analogue (16-18). The patient in our cohort treated with this combination obtained a PR with progression after 3 months. The role of the novel “T-cell lymphoma agents” such as romidepsin and pralatrexate are less well defined but have demonstrated activity in HSPTCL (19). The combination of both was able to achieve a CR in one patient in our cohort for over 6 months.
Allogeneic SCT (AlloSCT) is performed where feasible and is supported by retrospective registry data however many patients do not proceed to transplantation due to early relapse or refractory disease despite encouraging initial response rates (13). A retrospective registry review by the European Society for Bone and Marrow Transplantation of 25 patients with HSTCL revealed a 3-year PFS and OS of 48% and 54%, respectively following a median follow-up of 36 months in the 18 patients that underwent an alloSCT. Disease status at time of transplant was CR (39%), PR (44%) or refractory disease (17%). All patients with data available were in CR prior to autograft. In the seven patients who underwent an autologous SCT only one was alive and progression-free 58 months after transplantation (20). A retrospective systematic review of outcomes in alloSCT reported similar findings; the estimated 3-year relapse free survival and OS were 42% and 56%, respectively in 44 patients (21).
PCGDTCL
PCGDTCL accounts for <1% of cutaneous T-cell lymphomas and has been characterized by an aggressive course with rapidly progressive skin plaques and early ulceration although indolent cases have also been reported. There is typically an absence of lymph and bone marrow involvement, while patients with subcutaneous involvement are reported to have a worse prognosis. The histological appearance can include both epidermotrophism and subcutaneous involvement and the classic immunophenotype is typically lacking expression of both CD4 and CD8 and positive for CD2 and CD3. BF1 is typically negative (22). The reported median survival is approximately 15 months, although this can be considerably less in the presence of hemophagocytic syndrome with which there is an association (22,23). To date, the largest published retrospective series included 48 patients that met diagnostic criteria for PCGDTCL: in this study, elevated LDH and ECOG >1 were identified as poor prognostic markers, while alloSCT in first remission was associated with improved OS (24).
There is no standardized treatment consensus with most series reporting heterogenous frontline therapies and a poor response to multi-agent chemotherapy (23). European Society Medical Oncology (ESMO) guidelines suggest treatment as per PTCL with aggressive upfront chemotherapy (25). Variations of CHOP have been typically employed and responses have also been observed with bendamustine, bexarotene and the fusion toxin denileukin diftitox. Furthermore, a complete response to pralatrexate has been demonstrated in relapsed disease (26-29). Indolent cases have also been treated effectively with methotrexate and narrowband ultraviolet therapy (30). CD30 expression is uncommon in PCGDL but brentuximab vedotin has demonstrated activity in these patients anecdotally (31).
Despite most patients presenting with wide-spread lesions necessitating systemic therapy, PCGDTCL has been shown to respond to radiation therapy with excellent responses and acceptable infield disease control demonstrated following localized radiation therapy (32). Radiation therapy is particularly important in the management of patients with localized disease, patients who are not fit for intensive systemic chemotherapy or in the palliation of symptomatic sites of disease. Previous reports have utilized fractionated courses of radiation therapy to 30 Gy, although the optimal radiation dose is not known (32-34).
A recent case series of seven patients demonstrated the efficacy of allogenic stem cell transplant; all patients received multi-agent chemotherapy prior to transplant with median number of 3 (1-5) prior therapies, and 71% were in CR at time of transplant) (35). With median follow-up of 5 years (1.7–14 years), the authors report 29% 100-day mortality with three surviving patients who are currently disease-free at time of reporting [one patient remaining in remission 12 years post-transplant, and two achieving ongoing remission with brentuximab vedotin after relapse post allograft (35)].
MEITL
Monomorphic epitheliotropic intestinal T-cell lymphomas are derived from intestinal intraepithelial lymphocytes. Lacking the association with coeliac disease typical of enteropathy associated T-cell lymphoma (EATL) it was previously known as EATL type 2 before being recognized as a separate entity by the WHO in 2016. Accounting for less than 5% of all gastrointestinal lymphomas and more common in Asian populations compared with EATL, survival is generally poor with a median survival of less than 12 months (36). The most common sites of involvement are the jejunum and ileum, but involvement may be found anywhere along the gastrointestinal tract as well as extraintestinal sites. MEITL is generally positive for CD3, CD8, and CD56 and negative for CD5 and most cases are positive for the γδ TCR with expression of the mucosal homing receptor CD103. Monomorphic cell shapes, epitheliotropic patterns and expression of CD8 and CD56 help distinguish it from other types of T-cell lymphoma. Gains in chromosome 8q24 involving MYC are common and mutation in STAT5B is seen in around a third of cases, similar to HSPTCL (2,37).
No frontline treatment has demonstrated superiority with evidence limited to case reports and registry reviews. The Asia lymphoma study reported 38 patients with a 1-year OS of 36%. Consolidation with AutoSCT or AlloSCT were associated with improved survival while poor performance status was associated with poor outcomes (38). Anthracycline-based chemotherapy was the most commonly utilized regimen (53%) and there was no difference in OS between regimens that did or did not contain an anthracycline. Supporting the use of AutoSCT in the management of MEITL was another retrospective review of 42 patients with MEITL by a South Korean group who demonstrated that patients who did not receive AutoSCT had poorer OS (36). A recent pooled analysis of 116 patients was able to demonstrate that SCT was associated with an OS benefit compared with chemotherapy alone (9 vs. 34 months) and that surgical resection was also associated with improved survival (39).
Discussion
The γδ lymphomas are a rare, heterogenous and typically aggressive subtype of T-cell lymphoma. There are no standard treatments and evidence is limited to retrospective case series and database analyses (Table 5). To our knowledge this is the first case series of patients with γδ lymphoma reported in Australia. The characteristics of patients with HSPTCL were consistent with international case series with prior immunosuppression and marrow involvement with cytopenia common. The diverging clinical behavior reported of PCGDTCL was also observed with both rapidly progressive and indolent disease seen.
Table 5
| Author | Subtype | Patients | Median age (years) | SCT | Median OS (months) | OS |
|---|---|---|---|---|---|---|
| Yabe (6) | HSPTCL | 28 | 32.5 | 5 allo, 7 auto | 28.3 | NR |
| Belhadj (13) | HSPTCL | 21 | 34 | 2 allo, 6 auto | 16 | NR |
| Falchook (12) | HSPTCL | 15 | 38 | 2 allo, 5 auto | 11 | NR |
| Foss (14) | HSPTCL | 31 | N/A | 7 allo, 1 auto | 13 | 40% 3-year |
| Voss (15) | HSPTCL | 14 | 34 | 8 allo, 4 auto | 59 | NR |
| David (24) | PCGDTCL | 48 | 62 | 6 allo, 1 auto | NR | 36% 2-year |
| Isufi (35) | PCGDTCL | 7 | 52 | 7 allo | NR | 57% 5-year |
| Yi (36) | MEITL | 42 | 59 | 16 auto | 14.8 | NR |
| Tse (38) | MEITL | 38 | 59 | 1 allo, 5 auto | 7 | 36% 1-year |
| Ishibashi (40) | MEITL | 9 | 63 | 2 SCT | NR | 0% 3-year |
SCT, stem cell transplant; OS, overall survival; HSPTCL, hepatosplenic T-cell lymphoma; allo, allogeneic; auto, autologous; NR, not reported; PCGDTCL, primary cutaneous γδ T-cell lymphoma; MEITL, monomorphic epitheliotropic intestinal T-cell lymphoma.
The prognosis of γδ lymphoma was poor regardless of subtype with the 2-year OS of only 53% in this series (Figure 1). Treatment heterogeneity and the small number of patients limited statistical analysis and no clear recommendations can be made with regards to treatment strategies. Anthracycline or “CHOP” based chemotherapy has been reported to have low rates of response in other series. A “CHOP” backbone was used in three patients with HSTCL (two upfront, one at relapse and three with MEITL (three upfront) with generally disappointing responses.
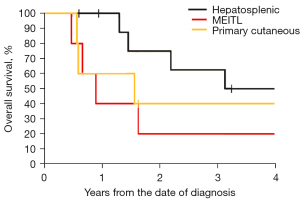
AlloSCT remains controversial but durable responses were seen after AlloSCT in several patients across all subtypes. The patients in this series were all reported to be in CR at time of transplant so perhaps had more favorable disease biology compared to others which had short remissions or refractory disease. The responses seen to novel therapeutic agents active in other T-cell disorders are promising but need further study. Further development of the understanding of the biology of malignant γδ T cells may contribute to much needed novel therapeutic approaches.
Conclusions
The γδ lymphomas are an exceedingly rare and aggressive T-cell lymphoma subtype. Front-line treatments utilized in other aggressive lymphomas (such as CHOP) achieve short remissions, but interpretation of all series is hampered by the treatment heterogeneity. Indeed, the array of treatment regimens in this retrospective case series mean no definite conclusions or clear treatment recommendations can be made. Of the patients with γδ lymphomas who obtain a complete response for long enough to proceed to allogeneic transplantation, a minority will achieve durable remissions. The use of novel agents warrants further study with both single-agent and combinations demonstrating efficacy in the relapsed/refractory setting.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://aol.amegroups.com/article/view/10.21037/aol-21-41/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aol.amegroups.com/article/view/10.21037/aol-21-41/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This project was approved by Peter MacCallum Cancer Centre governance with protocol approval by respective local ethics committees. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Waiver of consent was granted by a fully constituted Human Research Ethics Committee.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kabelitz D, Wesch D. Features and functions of gamma delta T lymphocytes: focus on chemokines and their receptors. Crit Rev Immunol 2003;23:339-70. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Kagami Y, Nakamura S, Suzuki R, et al. A nodal gamma/delta T-cell lymphoma with an association of Epstein-Barr virus. Am J Surg Pathol 1997;21:729-36. [Crossref] [PubMed]
- Aoyama Y, Yamane T, Hino M, et al. Nodal gamma/delta T cell lymphoma in complete remission following allogeneic bone marrow transplantation from an HLA-matched unrelated donor. Acta Haematol 2001;105:49-52. [Crossref] [PubMed]
- Pro B, Allen P, Behdad A. Hepatosplenic T-cell lymphoma: a rare but challenging entity. Blood 2020;136:2018-26. [Crossref] [PubMed]
- Yabe M, Medeiros LJ, Tang G, et al. Prognostic factors of hepatosplenic T-cell lymphoma: clinicopathologic study of 28 cases. Am J Surg Pathol 2016;40:676-88. [Crossref] [PubMed]
- Weidmann E. Hepatosplenic T cell lymphoma. A review on 45 cases since the first report describing the disease as a distinct lymphoma entity in 1990. Leukemia 2000;14:991-7. [Crossref] [PubMed]
- Nicolae A, Xi L, Pittaluga S, et al. Frequent STAT5B mutations in γδ hepatosplenic T-cell lymphomas. Leukemia 2014;28:2244-8. [Crossref] [PubMed]
- Selvaraj SA, Chairez E, Wilson LM, et al. Use of case reports and the Adverse Event Reporting System in systematic reviews: overcoming barriers to assess the link between Crohn’s disease medications and hepatosplenic T-cell lymphoma. Syst Rev 2013;2:53. [Crossref] [PubMed]
- Thai A, Prindiville T. Hepatosplenic T-cell lymphoma and inflammatory bowel disease. J Crohns Colitis 2010;4:511-22. [Crossref] [PubMed]
- Yabe M, Medeiros LJ, Daneshbod Y, et al. Hepatosplenic T-cell lymphoma arising in patients with immunodysregulatory disorders: a study of 7 patients who did not receive tumor necrosis factor-α inhibitor therapy and literature review. Ann Diagn Pathol 2017;26:16-22. [Crossref] [PubMed]
- Falchook GS, Vega F, Dang NH, et al. Hepatosplenic gamma-delta T-cell lymphoma: clinicopathological features and treatment. Ann Oncol 2009;20:1080-5. [Crossref] [PubMed]
- Belhadj K, Reyes F, Farcet JP, et al. Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood 2003;102:4261-9. [Crossref] [PubMed]
- Foss FM, Horwitz SM, Civallero M, et al. Incidence and outcomes of rare T cell lymphomas from the T Cell Project: hepatosplenic, enteropathy associated and peripheral gamma delta T cell lymphomas. Am J Hematol 2020;95:151-5. [Crossref] [PubMed]
- Voss MH, Lunning MA, Maragulia JC, et al. Intensive induction chemotherapy followed by early high-dose therapy and hematopoietic stem cell transplantation results in improved outcome for patients with hepatosplenic T-cell lymphoma: a single institution experience. Clin Lymphoma Myeloma Leuk 2013;13:8-14. [Crossref] [PubMed]
- Jiang L, Yuan CM, Hubacheck J, et al. Variable CD52 expression in mature T cell and NK cell malignancies: implications for alemtuzumab therapy. Br J Haematol 2009;145:173-9. [Crossref] [PubMed]
- Jaeger G, Bauer F, Brezinschek R, et al. Hepatosplenic gammadelta T-cell lymphoma successfully treated with a combination of alemtuzumab and cladribine. Ann Oncol 2008;19:1025-6. [Crossref] [PubMed]
- Mittal S, Milner BJ, Johnston PW, et al. A case of hepatosplenic gamma-delta T-cell lymphoma with a transient response to fludarabine and alemtuzumab. Eur J Haematol 2006;76:531-4. [Crossref] [PubMed]
- Amengual JE, Lichtenstein R, Lue J, et al. A phase 1 study of romidepsin and pralatrexate reveals marked activity in relapsed and refractory T-cell lymphoma. Blood 2018;131:397-407. [Crossref] [PubMed]
- Tanase A, Schmitz N, Stein H, et al. Allogeneic and autologous stem cell transplantation for hepatosplenic T-cell lymphoma: a retrospective study of the EBMT Lymphoma Working Party. Leukemia 2015;29:686-8. [Crossref] [PubMed]
- Rashidi A, Cashen AF. Outcomes of allogeneic stem cell transplantation in hepatosplenic T-cell lymphoma. Blood Cancer J 2015;5:e318. [Crossref] [PubMed]
- Guitart J, Weisenburger DD, Subtil A, et al. Cutaneous γδ T-cell lymphomas: a spectrum of presentations with overlap with other cytotoxic lymphomas. Am J Surg Pathol 2012;36:1656-65. [Crossref] [PubMed]
- Toro JR, Liewehr DJ, Pabby N, et al. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood 2003;101:3407-12. [Crossref] [PubMed]
- David KA, Pulitzer M, Guitart J, et al. Characteristics, treatment patterns, and outcomes in primary cutaneous gamma delta T cell lymphoma (PCGDTCL): a real world multi-institutional analysis of a rare malignancy. Blood 2019;134:4028. [Crossref]
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv30-40. [Crossref] [PubMed]
- Kreuter A, Koushk-Jalali B, Mitrakos G, et al. Bendamustine monotherapy for primary cutaneous gamma-delta T-cell lymphoma. JAMA Dermatol 2020;156:1029-30. [Crossref] [PubMed]
- Vidulich K, Jones D, Duvic M. Cutaneous gamma/delta T-cell lymphoma treated with radiation and denileukin diftitox. Clin Lymphoma Myeloma 2008;8:55-8. [Crossref] [PubMed]
- Mehta N, Wayne AS, Kim YH, et al. Bexarotene is active against subcutaneous panniculitis-like T-cell lymphoma in adult and pediatric populations. Clin Lymphoma Myeloma Leuk 2012;12:20-5. [Crossref] [PubMed]
- Imataki O, Uchida S, Yokokura S, et al. Small primary cutaneous γδT-cell lymphoma lesions successfully treated with pralatrexate. Clin Nucl Med 2019;44:e85-6. [Crossref] [PubMed]
- Fujii M, Uehara J, Honma M, et al. Primary cutaneous γδ-T-cell lymphoma treated with low-dose methotrexate and narrowband ultraviolet B irradiation: report of a case with testicular involvement. J Dermatol 2011;38:368-72. [Crossref] [PubMed]
- Rubio-Gonzalez B, Zain J, Garcia L, et al. Cutaneous gamma-delta T-cell lymphoma successfully treated with brentuximab vedotin. JAMA Dermatol 2016;152:1388-90. [Crossref] [PubMed]
- Kelsey CR, Wang E, Stefanovic A, et al. Radiation therapy for primary cutaneous γδ T-cell lymphoma: Case report and literature review. JAAD Case Rep 2019;5:582-6. [Crossref] [PubMed]
- Wu YF, Skinner L, Lewis J, et al. Radiation therapy for primary cutaneous gamma delta lymphoma prior to stem cell transplantation. Cancer Invest 2021; Epub ahead of print. [Crossref] [PubMed]
- Pedretti S, Urpis M, Leali C, et al. Primary cutaneous non-Hodgkin lymphoma: results of a retrospective analysis in the light of the recent ILROG guidelines. Tumori 2018;104:394-400. [Crossref] [PubMed]
- Isufi I, Seropian S, Gowda L, et al. Outcomes for allogeneic stem cell transplantation in refractory mycosis fungoides and primary cutaneous gamma Delta T cell lymphomas. Leuk Lymphoma 2020;61:2955-61. [Crossref] [PubMed]
- Yi JH, Lee GW, Do YR, et al. Multicenter retrospective analysis of the clinicopathologic features of monomorphic epitheliotropic intestinal T-cell lymphoma. Ann Hematol 2019;98:2541-50. [Crossref] [PubMed]
- de Araujo ED, Erdogan F, Neubauer HA, et al. Structural and functional consequences of the STAT5BN642H driver mutation. Nat Commun 2019;10:2517. [Crossref] [PubMed]
- Tse E, Gill H, Loong F, et al. Type II enteropathy-associated T-cell lymphoma: a multicenter analysis from the Asia lymphoma study group. Am J Hematol 2012;87:663-8. [Crossref] [PubMed]
- Haddad PA, Dadi N. Clinicopathologic determinants of survival in monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL): analysis of a pooled database. Blood 2020;136:28. [Crossref]
- Ishibashi H, Nimura S, Hirai F, et al. Endoscopic and clinicopathological characteristics of colorectal T/NK cell lymphoma. Diagn Pathol 2020;15:128. [Crossref] [PubMed]
Cite this article as: Harrop S, Di Ciaccio P, Doo NW, Cochrane T, Campbell BA, Hamad N, Dickinson M, Van Der Weyden C, Prince HM. The gamma delta lymphomas: an Australian multi-centre case series. Ann Lymphoma 2022;6:3.


