Daratumumab as first line therapy in primary effusion lymphoma: a case report
Introduction
Primary effusion lymphoma (PEL) is a rare and aggressive form of non-Hodgkin lymphoma (NHL) manifesting as malignant effusions within cavities such as the pleura, pericardium and peritoneum (1). It is seen most commonly in individuals coinfected with the human immunodeficiency virus (HIV) and human herpesvirus-8 (HHV-8) (2-6). It accounts for appropriately <1% of all lymphoma cases in the general population (3-5) and carries a poor prognosis with a median overall survival of approximately six months (2,4,7,8). Treatment options are challenging both from limited evidence in this area, as well as by patient suitability for therapy, as HIV-negative PEL often affects an older male Mediterranean population (4,8,9). Below we describe the only reported case of prolonged clinical remission through the use of daratumumab (an anti-CD38 antibody) as first line of therapy in an 85-year-old gentleman diagnosed with HIV-negative PEL with pericardial involvement. We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/aol-21-26).
Case presentation
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
An 85-year-old Israeli born gentleman presented to the emergency department with acute onset of nausea and vomiting on the background of a four-week history of fatigue and weight loss. His co-morbidities included ischemic heart disease, hypertension, chronic obstructive pulmonary disease, dyslipidaemia and non-insulin dependent diabetes mellitus. Despite his co-morbidities he was independent, from home with his family, with an ECOG performance score of 1.
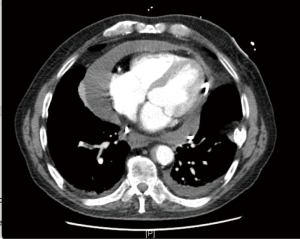
Basic investigations performed included a chest X-ray which showed an enlarged cardiac silhouette, later confirmed on computed tomography (CT) and transthoracic echocardiogram (TTE) to be secondary to a large volume pericardial effusion with associated cardiac tamponade (Figure 1). Pericardiocentesis was performed, draining 700mls, with subsequent temporary resolution of his tamponade. Unfortunately, he suffered reaccumulation of pericardial fluid within 72 hours post his pericardial drain removal, with recurring features of early tamponade, prompting the need for a pericardial window. No further pericardiocentesis was required post pericardial window formation.
Flow cytometry and cytology were performed on the pericardial fluid. Cytology showed numerous large atypical lymphoid cells exhibiting irregular nuclear membranes, vesicular chromatin, multiple, prominent nucleoli and variable amounts of basophilic cytoplasm. Immunohistochemistry (IHC) was positive for CD45, HHV-8 and CD30 as well as a focal positive staining for CD138; whilst being negative for B cell markers (CD20, CD79a and PAX-5), T cell markers (CD3, CD5, ALK-1) and Epstein-Barr virus (EBV) encoded RNA (EBER-ISH). Flow cytometry showed abnormal cells that were positive for CD38 (bright), CD 138, heterogeneous HLA-Dr, moderate CD45 and dim heterogenous CD71; whilst lacking CD19, CD56, surface and cytoplasmic immunoglobulins. Therefore, the diagnosis of PEL was made.
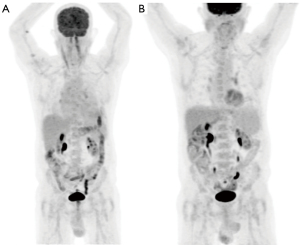
A full blood examination was unremarkable and peripheral blood was negative for Human Immunodeficiency virus serology but positive for HHV-8 DNA detected by PCR. Positron emission tomography (PET) imaging, prior to his pericardial window, showed a large volume pericardial effusion with no evidence of FDG avid lymphoma (Figure 2).
Given this gentleman’s age and co-morbidities he was deemed not to be appropriate for anthracycline containing chemotherapy; instead daratumumab, a mAb to CD138, was explored as a completely novel approach. He received daratumumab monotherapy, at a dose of 16 mg/kg, weekly for eight weeks, followed by a dose every two weeks for four months and remains on monthly maintenance daratumumab, currently undergoing cycle 12 of therapy. After the first dose of daratumumab HHV-8 was no longer detectable in his blood by PCR and has remained so on serial measurements, being tested every two months, throughout treatment. Whilst it is not felt that HHV-8 viral monitoring can be used as a response to chemotherapy (10) both a recent case in New England Journal of Medicine by Shah et al. (2), as well as this case, found decreasing levels of the HHV-8 DNA immediately after commencement of therapy, as well as the NEJM case describing a rise in viral load titre when therapy was delayed (2).
Repeat PET imaging four months after commencement of therapy showed a good metabolic response, Deauville Score 3, with reduction in the pericardial effusion and no evidence of FDG lymphoma (Figure 2). Daratumumab therapy has well been tolerated to date with no adverse events to therapy. At the time of this case report the patient has been experiencing an ongoing complete response for approximately twelve months. The only impact to the patient is the half day a month attendance required for therapy and he has a reported excellent quality of life. He will continue on monthly maintenance daratumumab until evidence of disease progression or the development of toxicity to therapy.
Discussion
In relation to therapeutic options for PEL, the most appropriate regimen has not been determined as there are limited large scale studies due to the rarity of HIV-negative PEL, along with its poor prognosis (1,5). Even with therapy, the prognosis remains poor due to a high resistance to chemotherapy (3,5), the median survival being approximately six months (2,4,7,8) with a one-year overall survival rate between 30–40% (6,8). Patients fit for chemotherapy will generally receive a combination regimen, most commonly CHOP (Cyclophosphamide, Doxorubicin, Vincristine, Prednisolone) or EPOCH (Etoposide, Prednisolone, Vincristine, Cyclophosphamide, Doxorubicin), with an estimated complete response ranging from 43–57% (2). Case reports exist for bortezomib, valganciclovir, cidofovir, brentuximab, adjuvant anti-retroviral therapy and autologous stem cell transplantation which all show variable results (4,5).
Given the CD38 positivity in PEL, daratumumab, an IgG1k monoclonal antibody directed against CD38 commonly used in multiple myeloma, was hypothesised as a potential therapy. In 2018, Shah et al., showed their successful use of salvage daratumumab in a 47-year-old gentleman with heavily pre-treated relapsed refractory HIV related PEL (2). Whilst our case also shows a complete response to daratumumab it does so in an elderly gentleman with HIV-negative PEL whom had not received prior lines of therapy. The ongoing duration of remission of twelve months is much longer than anticipated given that the expected median survival for such patients is in the order of less than six months (2,4,7,8). This case provides a robust proof of concept and supports the exploration of daratumumab as an option for first line therapy in individuals with HIV-negative PEL, particularly those who are deemed not fit for chemotherapy.
Acknowledgments
The authors would like to thank Dr. Susan Morgan for her contributions to the pathological aspects of this case.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/aol-21-26
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/aol-21-26). HQ is an advisory board member of Janssen Cilag. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dinmohamed AG, Visser O, Doorduijn JK, et al. Treatment and survival of patients with primary effusion lymphoma in the Netherlands: a population-based analysis. Hemasphere 2018;2:e143. [Crossref] [PubMed]
- Shah NN, Singavi AK, Harrington A. Daratumumab in primary effusion lymphoma. N Engl J Med 2018;379:689-90. [Crossref] [PubMed]
- Fernández-Trujillo L, Bolaños JE, Velásquez M, et al. Primary effusion lymphoma in a human immunodeficiency virus-negative patient with unexpected unusual complications: a case report. J Med Case Rep 2019;13:301. [Crossref] [PubMed]
- Campogiani L, Cerva C, Maffongelli G, et al. Remission of an HHV8-related extracavitary primary effusion lymphoma in an HIV-positive patient during antiretroviral treatment containing dolutegravir. AIDS Res Ther 2019;16:15. [Crossref] [PubMed]
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther 2018;11:3747-54. [Crossref] [PubMed]
- Shimada K, Hayakawa F, Kiyoi H. Biology and management of primary effusion lymphoma. Blood 2018;132:1879-88. [Crossref] [PubMed]
- Re A, Cattaneo C, Rossi G. HIV and lymphoma: from epidemiology to clinical management. Mediterr J Hematol Infect Dis 2019;11:e2019004. [Crossref] [PubMed]
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist 2007;12:569-76. [Crossref] [PubMed]
- Klepfish A, Sarid R, Shtalrid M, et al. Primary effusion lymphoma (PEL) in HIV-negative patients – a distinct clinical entity. Leuk Lymphoma 2001;41:439-43. [Crossref] [PubMed]
- Simonelli C, Tedeschi R, Gloghini A, et al. Characterization of immunologic and virological parameters in HIV-infected patients with primary effusion lymphoma during antiblastic therapy and highly active antiretroviral therapy. Clin Infect Dis 2005;40:1022-7. [Crossref] [PubMed]
Cite this article as: Wiltshire K, Kliman D, Tan J, Quach H, Kalff A, Cameron R, Grigoriadis G, Nandurkar H. Daratumumab as first line therapy in primary effusion lymphoma: a case report. Ann Lymphoma 2021;5:33.