
HIV-associated lymphoma—advances in clinical management
Historical context
The association between human immunodeficiency virus (HIV) and lymphoma was observed early in the HIV epidemic. In 1982, a year after the first reports of young men succumbing to Pneumocystis jirovecii pneumonia (1), a series of four patients with “Burkitt-like” lymphoma was published (2). This was followed by a number of case series in the context of an emerging underlying acquired immune deficiency syndrome (AIDS) (3,4). Over time it was established that those with HIV have a much higher risk of developing lymphoma. The World Health Organization classification of lymphoid malignancies formally recognises these separate entities with differing presentation, morphological and clinical characteristics to their normal subtypes (5).
Burkitt lymphoma (BL), diffuse large B-cell lymphoma (DLBCL) and primary central nervous system lymphoma (PCNSL) were first embedded into the surveillance case definition of AIDS in 1987 as an AIDS defining illness, even in the absence of a low CD4+ T-cell count <200 cells/μL (6). This occurred during the first 15 years of the epidemic (the pre-treatment era), and the value of this definition was primarily limited to public health surveillance. Subsequently the Kaposi sarcoma-associated herpesvirus [human herpesvirus 8 (HHV8)] was identified in 1994, followed by descriptions of primary effusion lymphoma (PEL) and plasmablastic lymphoma (PBL) of the oral cavity in 1995 and 1997 respectively (7,8). The introduction of combination antiretroviral therapy (ART) produced significant improvements in the prognosis of patients. However, lymphoma remains the most common cause of cancer related death and the leading cause of death in individuals with AIDS at 25% (9,10).
Over the following decade, the HIV-1 retrovirus was identified and significant insights into the modes of transmission, testing and treatment strategies described. It is now known that HIV cannot be transmitted when on ART with undetectable levels (<50 copies/mL) in the blood (‘Undetectable = Untransmittable’) (11-13). Results can be delivered within minutes and over 30 drugs are available to suppress HIV. These include single tablets of multi-drug formulations and two-drug therapy options increasingly used alongside the original standard triple therapy. This has transformed HIV into a manageable chronic disease. Prognosis is near comparable to HIV-negative patients, a welcome turn-around from an AIDS diagnosis in the 1980s being almost synonymous with death.
Despite the advances made within HIV medicine, low to middle income countries continue to be disproportionally impacted, driven by socio-economic inequality and limited healthcare access. Global collaboration is essential. Continued support for locally delivered healthcare development, poverty relief, and HIV-specific prevention and treatment strategies will be key to maintaining progress towards ending the HIV epidemic.
As well as our understanding, the language has evolved to emphasise a destigmatising approach that focuses on the individual as opposed to the disease (14). For example, the term advanced or late-stage HIV is often used instead of AIDS and people living with HIV (PLWH) is a preferred term in the HIV community.
HIV-associated lymphomas
Non-Hodgkin lymphoma (NHL) is the commonest haematological malignancy in PLWH with a cumulative lifetime incidence of around 5% (15,16). Compared to the general population, NHL and classical Hodgkin lymphoma (cHL) incidence are estimated to be 10- (17) and 15-fold higher, respectively (15). DLBCL comprises around 47%, BL 15% and PCNSL 9% of NHL (18-20), although estimates have changed over time with improving ART access. With increasing uptake of ART, PCNSL is less commonly observed. Beyond the AIDS-defining lymphomas, less than 5% are accounted for by PEL, PBL, HHV8 positive DLBCL and HHV8 positive multicentric Castleman disease (MCD), conditions seen almost exclusively in HIV.
These diseases often present with advanced stage and have a propensity for extranodal sites (21,22). Unusual sites include the gastrointestinal tract, oral cavity and central nervous system (CNS). Frequent plasmacytoid morphology is reported (23). Bone marrow, liver and involvement of other extranodal sites are more commonly seen in cHL (24). Constitutional B symptoms are more commonly reported.
Markers of the immune state
CD4+ T-lymphocyte count testing is central to stratifying immune status, determining antimicrobial prophylaxis and estimating prognosis for PLWH (24). HIV-associated lymphoma commonly develops in the context of long standing severe immune suppression with high viral loads and low CD4+ T-cell counts. PCNSL is most prevalent with a CD4+ T-cell count <50 cells/μL. Alongside DLBCL, PBL and PEL it is associated with a trend towards higher rates of opportunistic infections (OIs). BL and cHL however tend to occur in less immunodeficient states (CD4+ T-cell >200 cells/μL) and thus functional immunity may be involved in the pathogenesis (25,26). These patterns of presentation were more common prior to access to ART and since its introduction are less commonly observed.
Beyond absolute CD4+ T-cell count, measures of immunosuppression include the dynamics of recent, peak and nadir counts alongside HIV viral loads. In a North American study of 712 PLWH with NHL, average viral loads (>500 copies/mL, between 3.5 years and 6 months ago) and a recent low CD4+ T-cell count (<500 cells/μL, 6 months ago) were the strongest independent predictors of NHL risk, with differential risk amongst subtypes of NHL (27).
Lymphoma is believed to develop in PLWH through multiple mechanisms (28). Reduced immune surveillance by loss of T-cell function and enhanced virus driven oncogenesis have been postulated as pathogenic pathways (29,30). Both Epstein-Barr virus (EBV) and HHV8 are central oncogenic γ-herpes viruses observed in PLWH (31). PEL and MCD are almost always associated with HHV8 while EBV is found in almost all of PCNSL and cHL, with variable prevalence in PBL, PEL, DLBCL and BL (32). Suppression of T cell immunity is associated with viral reactivation. Furthermore, chronic B cell activation may cause DNA-modifying events that contribute to oncogene mutation acquisition. Immune suppression alone may not, however, be solely responsible, as the incidence of these lymphomas is greater than that observed in the immunosuppressed transplant population (26). More recently, it has been suggested that direct HIV proteins may contribute to disease via influence on the microenvironment. HIV-1 gp120 and matrix protein p17 accumulate and persist in lymph nodes even in PLWH compliant with ART and absent HIV-1 replication (33). These may be important for local lymphomagenesis both within lymph nodes and extranodally.
Management principles
The key tenants of management of HIV-lymphoma involves the use of ART, choice and intensity of anti-lymphoma treatment and supportive care (34).
Antiretroviral treatment
Prior to ART, BL and PCNSL were around 100–1,000 times more prevalent in PLWH than the general population. These patients often had advanced immune suppression, organ dysfunction and consequently poor PS. Achieving sustained viral suppression with lifelong ART is now the cornerstone of HIV management and substantially improves outcomes. Whilst conventional ART regimens constitute three-drugs, non-inferiority of two-drug combinations is now established (35-38). They are increasingly utilised to simplify treatment, avoid interactions, or prevent potential side effects.
The SMART and START trials were two landmark studies that established updated principles in utilising ART (39,40). SMART revealed that ART interruptions significantly increased the risk of OIs, HIV and non-HIV related co-morbidities, AIDS defining malignancy, or death from any cause compared to continuous treatment [hazard ratio (HR) 2.6; P<0.001] (39). START showed initiating ART irrespective of baseline CD4+ T-cell count was superior to deferred initiation once CD4+ T-cell counts were <350 cells/μL, crucially, for both serious HIV-related (HR 0.28, P<0.001) and non-HIV-related (HR 0.61; P=0.04) outcomes (40). This was replicated in an African cohort (41). As such, commencing ART as close to diagnosis as possible, irrespective of CD4+ T-cell count, is standard practice (11,12). ART use prevents transmission of HIV (13), thus maintaining patients on an effective regimen with sustained compliance is advocated.
Since the advent of ART, the incidence of PCNSL and DLBCL has fallen precipitously. However epidemiological data shows stable rates of cHL BL, PEL and PBL. cHL incidence appears to be unaffected by the relative improvements in CD4+ T-cell count (42,43), consistent with presentation in the absence of severe immune deficiency and on improved reconstitution after initiation of ART.
ART was historically considered to be a potential risk factor for additional toxicity. Concerns over drug interactions reducing chemotherapy concentrations and ART adverse effects compromising patient adherence were often cited (44,45). Previously, opinions have varied over suspending ART during lymphoma treatment. However more recently it is standard practice to continue or commence ART during chemotherapy (46) as the concerns regarding significantly increased toxicity have not been realised with modern ART agents. It is relevant that ART regimens are often undefined (47-49), outlined only by class (50-53), or include drugs now avoided e.g. zidovudine, dideoxynucleoside analogues (didanosine, stavudine), and older protease inhibitors (PIs) (47,54). This may underestimate the positive impact of modern ART which has better tolerability, with greater ART options to manage drug interactions and single-tablet formulations reducing pill burden. Hence, current evidence supports continuing or initiating ART wherever possible. Figure 1 outlines the potential interactions between key medications and their clinical impact. Changes to HIV treatment should be made by an HIV specialist, although broadly, a combination utilising two nucleos(t)ide reverse transcriptase inhibitors with an integrase inhibitor is the most compatible and typically preferred. Low pill burden has been associated with higher adherence (55). General enquiry at routine appointments to pill boxes and phone reminders to counselling and psychological support are options to consider should concerns arise. Close collaboration with an HIV specialist is paramount.
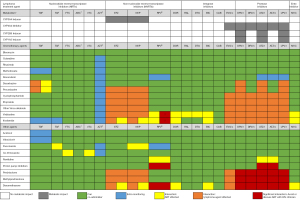
Following ART initiation, immune reconstitution inflammatory syndrome (IRIS) may result from exaggerated inflammation in response to immune system recovery. Although well-known, diagnosis and treatment can be challenging. IRIS is well described for unmasking OIs and Kaposi’s sarcoma with little reported on lymphoma IRIS other than one cohort of 56 PLWH (56) and more recent case reports (57).
Clinical trials
Despite improvements in the outcomes of PLWH and life-expectancy matching the general population with well controlled disease, representation in clinical trials remains lacking. A positive serostatus remains an exclusion criterion for the majority of trials open to patients with lymphoma (58). This has previously been due to concerns about profound immune dysregulation or potential pharmacokinetic interactions with ART. However, from a review of 32 UK National Institute for Health Research interventional lymphoma trials that excluded PLWH, only one provided an explicit biologically valid justification (59). Furthermore, a review of 107 trials of immune checkpoint inhibitors including haematological malignancy identified enrolment of only five studies with PLWH, all of which were academic studies (60). As such, the American Society of Clinical Oncology have devised recommendations for eligibility criteria more inclusive of modern, virally suppressed, immune reconstituted HIV cohorts (58). Expanding access to trials for PLWH would yield valuable data for treatment options and are paramount for this patient group.
DLBCL
Historically DLBCL had poor outcomes with HIV, with time-to-progression of 35 weeks, in the pre-rituximab era (61). In the HIV-negative setting, the addition of rituximab to the CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) backbone exhibited significant improvements in overall survival (OS), but no PLWH were included in this seminal trial (62). Benefit was not reproduced in AMC010, a phase 3 trial which randomised 150 PLWH to CHOP ± rituximab including a rituximab maintenance regimen. Although there were improved tumour response rates, excess infectious deaths were reported, almost entirely with CD4+ T-cell <100 cells/μL (60% of infectious deaths with CD4+ T-cell <50 cells/μL) (63). However this level of toxicity has not been replicated elsewhere. Subsequent pooled analysis from 19 prospective trials including 1,546 PLWH demonstrated the addition of rituximab to CHOP was indeed beneficial with a near threefold increase in the complete response (CR) rate and did not lead to higher mortality from infectious complications (48). A CD4+ T-cell count of less than 50 cells/μL did not preclude treatment with concomitant ART use, in a trial of 40 patients administered rituximab, liposomal doxorubicin, cyclophosphamide, vincristine and prednisolone [2-year progression-free survival (PFS) 52%, OS 62%] (64).
The International Prognostic Index (IPI) is predictive of outcome in DLBCL (65) and enhanced IPI (NCCN-IPI) for patients with DLBCL treated in the rituximab era (66). The French lymphoma research groups reported on outcomes with RCHOP. In a sub-analysis of 52 PLWH with DLBCL, 85% received RCHOP with similar outcomes to HIV-negative patients on the Lymphoma Study Association trials (25). Furthermore, HIV was not an independent prognostic indicator of outcome after controlling for rituximab use and IPI in a retrospective Italian series including 465 PLWH (67).
Infusion of EPOCH-R (etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin, rituximab) has been employed as potential escalation of therapy of RCHOP in high-risk lymphoma (52). Dose adjustment was developed to reduce haematopoietic toxicity while maintaining maximum dose intensity titrated to cytopenias. Prolonged continuous exposure may enhance tumour cell death in vitro (68). This has been trialled successfully for PLWH with superior outcomes in those receiving concurrent rather than sequential rituximab (69-71).
No randomised trial comparing R-CHOP and dose-adjusted EPOCH-R (DA-EPOCH-R) has been performed in PLWH. Retrospective analysis of DA-EPOCH-R showed favourable performance compared to RCHOP in OS though failed to achieve statistical significance (P=0.087) (49). The ALLIANCE study randomised HIV-negative patients between these regimens with no benefit to DA-R-EPOCH and greater toxicity with the latter (72). This did not target high-risk patients and subset analysis is still pending.
There have been attempts to reduce treatment duration due to potential associated toxicity. In HIV-negative patients with low IPI DLBC, the FLYER study presented non-inferiority of four cycles of RCHOP plus two rituximab compared to six cycles of RCHOP (73). In a post-hoc analysis of PLWH and low risk DLBCL, similar 2-year event-free survival (EFS) was seen for those with four or fewer cycles of EPOCH-R achieving CR on computed tomography (CT) after two cycles, compared with five to six cycles (78% vs. 85%), with 2-year OS 78% vs. 90% (74).
Fluorodeoxyglucose (FDG)-positron emission tomography (PET) has been utilised in a response-adapted regimen to permit shorter duration of therapy. In a phase II trial of 33 PLWH and DLBCL, interim PET CT allowed 79% of patients to undertake only three cycles of short-course EPOCH with double-dose rituximab (SC-EPOCH-RR), with an overall 5-year OS of 68% (75). FDG-PET has high negative predictive value but may confer poor positive predictive value. HIV-associated nodal reactive hyperplasia and infections may confound FDG-PET interpretations so caution should be employed (as expanded upon in the cHL discussion).
Several trials have attempted to improve first-line therapy for DLBCL in PLWH. The histone deacetylase inhibitor, vorinostat, proposed to induce lytic reactivation of EBV and HHV8, showed no benefit when added to EPOCH±R in a study including 71% PLWH and DLBCL (76). A phase I trial of ibrutinib with DA-EPOCH-R for PLWH and DLBCL has been registered (NCT03220022).
In the relapsed setting, there had been initial hesitancy in considering anti-CD19 chimeric antigen receptor T-cell (CAR-T) therapy for PLWH due to their exclusion from pivotal studies from which the license was based (77,78). However, as evidence for its successful manufacture and efficacy has grown (79-81), this has provided better equity in their potential use for PLWH. The immune checkpoint inhibitor, pembrolizumab, has been trialled in a phase I study including five PLWH with relapsed NHL (3 DLBCL, 2 PEL). There was an acceptable safety profile similar to those without HIV and clinical benefit was described (82).
Preventing CNS relapse
PLWH have an increased risk of aggressive disease with extranodal involvement more commonly involving the CNS. A systematic review of 886 newly diagnosed AIDS-related lymphomas (DLBCL and BL) revealed de novo CNS involvement at baseline was 13% and 5–30% in trials. CNS relapse is an early event, at a median of 4.2 months after diagnosis with dismal median survival of 1.6 months. Despite intrathecal (IT) CNS prophylaxis in over 90%, 5% experienced CNS relapse (83). The prevalence of de novo presentation did not differ between the pre- and post-ART eras. The optimal regimen, dose intensity and CNS prophylaxis strategy (doses of intravenous CNS-penetrating agents vs. IT therapy) has not been well established in this setting. The US National Comprehensive Cancer Network guidelines suggest IT methotrexate for CNS prophylaxis in all PLWH with lymphoma independent of CNS risk score (84) but other guidelines suggest a similar approach to those without HIV (85,86).
Primary CNS lymphoma
PCNSL is an extranodal lymphoma affecting the brain, spinal cord, cranial nerves, eyes or meninges, in the absence of systemic disease. For PLWH, PCNSL develops in the context of severe immune suppression and is associated with EBV, unlike in the immunocompetent (87). PCNSL correlates with loss of EBV-specific CD4+ T-cells rather than overall CD4+T-cell loss (88). HIV-associated cases had more frequent mutations compared to immunocompetent EBV+ PCNSL in one study of 91 PCNSL tissue samples (89). This suggests HIV itself may contribute to the burden of DNA mutations in this disease and indirectly lead to this lymphoproliferative disorder.
Prior to ART survival was less than four months with complications arising from OIs and refractory disease (90). Data from ten Surveillance, Epidemiology and End Results cancer registries from 1992–2011 show the steepest decline in rates (30% annually) from 1995–1998 for immunocompromised men (91). Encouragingly, the proportion of PLWH receiving chemotherapy for PCNSL has increased over the last few decades (from 29% to 47%, 2004 to 2012) (18). Prospective trials are difficult to recruit to as patients often have poor PS, compounded by the increasing rarity of this condition. Diagnosis ideally requires neurosurgical intervention with brain biopsy confirming histology. EBV PCR in the cerebrospinal fluid is almost always detectable but is presently not sufficient to confirm a diagnosis.
Challenges in treatment include optimal dose delivery of agents to penetrate the blood brain barrier. Whole-brain radiotherapy (WBRT) or high dose methotrexate (HD MTX) have historically been treatment approaches (92) with WBRT dose independently prognostic (93), although data regarding neurocognitive sequalae is scant. In a retrospective series of 23 patients, WBRT in PLWH demonstrated leukoencephalopathy (grade ≥2) in a fifth of those surviving more than a year after radiation (94). This delayed complication impairs cognition considerably given cerebral small vessel disease and overall cardiovascular events are already more common amongst PLWH (95).
Consolidation in first remission with high-dose chemotherapy with stem cell rescue has been shown to be feasible and efficacious (96), however, this approach has been superseded by less intensive approaches. The addition of other agents that cross the blood-brain barrier (such as cytarabine and thiotepa) to HD MTX have been shown to improve outcomes in HIV-negative patients (97) but recent reports suggest less intensive approaches are effective in PLWH with the introduction of ART. This is one of the only situations where PLWH with lymphoproliferative disorders are treated differently and reflects that this EBV+ PCNSL is a distinct immunobiological entity (89). A retrospective study of 51 PLWH treated with a median of six infusions of HD MTX (3 g/m2) monotherapy and ART demonstrated a median OS of 5.7 years, 2-year OS 51% and time to progression 69% (98).
The only prospective trial conducted in this setting enrolled 12 patients treated with HD MTX and rituximab (99). Data showed improvements in mini-mental state examination and all surviving patients were free of severe general neurocognitive dysfunction after treatment. A phase I trial including PLWH has commenced (ACTRN12618001541291) examining the use of ibrutinib with EBV-specific T-cells in EBV+ cerebral lymphoma with or without systemic involvement.
BL
A higher incidence of BL persists despite improvements with ART and typically occurs in those with CD4+ T-cell counts >200 cells/μL (100). The incidence of BL does not increase as CD4+ T-cell count decreases (17,101), however, ART is associated with improved tolerance to chemotherapy. CNS involvement is seen in up to 30% (102) and is associated with worse outcomes (103). Improved therapeutic strategies for these patients are needed.
Most patients with BL are now treated with multiagent regimens utilised in the HIV-negative setting. These include R-CODOX-M/R-IVAC (rituximab, cyclophosphamide, vincristine, doxorubicin, methotrexate, ifosfamide, etoposide, cytarabine), HyperCVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone) and the less intensive regimen DA-EPOCH-R.
Only four prospective trials have addressed the optimal intensive treatment in the rituximab era. These have included CODOX-M/IVAC or DA-EPOCH regimens with less than 35 PLWH recruited (19,104-106). Two-year OS is approximately 70% with treatment-related mortality (TRM) reported at less than 5% for modified R-CODOXM/R-IVAC (104) and DA-EPOCH-R (105,106). The EPOCH-R regimen does not include HD MTX, cytarabine or ifosfamide. Whilst they increase the toxicity of treatment, they are considered important for disease control, particularly in the CNS, as they cross the blood brain barrier. A retrospective series including 142 PLWH with BL demonstrated 19% with CNS involvement. This was independently associated with HIV infection on multivariate analysis. Of 641 patients, DA-EPOCH-R had the highest CNS relapse rate compared to other regimens, although only 45% adhered to the strict IT schedule from the original protocol (107). In the largest international retrospective analysis of 249 PLWH with BL, 11% had CNS relapse. The highest rates were seen with DA-EPOCH when compared to HyperCVAD and CODOX-M/IVAC (16% vs. 9% vs. 8%, P=0.032) (103). Here, prognostic factors were associated with lymphoma characteristics rather than HIV control. Similarly, the recently proposed BL-IPI includes prognostic factors of age ≥40 years, LDH >3× upper limit of normal, Eastern Cooperative Oncology Group PS ≥2, and CNS involvement. HIV status did not affect patient outcomes on univariate analysis in the rituximab era (108).
Newer regimens have been proposed. The CARMEN phase II study trialled a dose-dense and short-term regimen, with autologous stem cell transplantation (ASCT) as first-line consolidation for those not achieving CR post-induction. To limit toxicity, methotrexate dose reduction and restriction to two doses of cyclophosphamide and a single dose of doxorubicin were employed. Twenty PLWH were enrolled with a 5-year PFS and OS of 70% and 75% respectively, although TRM was 10% (109).
Plasmablastic lymphoma
PBL was first described in a series of 16 cases of oral cavity DLBCL with distinct plasmacytic differentiation, 15 of which had HIV without ART use (8). Extraoral disease has been reported in 43% of cases in a review of 248 patients (110) including the gastrointestinal tract, bone and skin. It is now seen outside of HIV-positive cohorts (111). Malignant cells have plasmablastic morphology and express a plasma cell-like immunophenotype (expression of CD38, CD138, MUM1, the antigen detected by the VS38c antibody, frequently CD79a, variably CD30, with a loss of CD20) and high Ki-67, usually above 90% (112). EBV is often associated with type I latency pattern expression; EBV latent membrane protein 1 (LMP1) is usually negative. PBL is enriched with recurrent genetic events in MYC (113).
There is no validated standard of care. In the absence of prospective data, evidence is based on case reports and retrospective series. Retrospective review of 197 patients, including 37% PLWH, across Australia, UK and Canada, demonstrated HIV status did not affect outcomes in PBL (111).
PBL regression after ART alone has been reported rarely (114,115). CHOP-based regimens are typically utilised but have poor outcomes with high rates of relapse or progression. A systematic review of 127 HIV-PBL cases found a median OS of 12 months (116). Fifty patients in a multi-centre study across 13 US sites had poor prognosis irrespective of the therapy received, with no survival benefit from more intense regimens (including EPOCH, hyperCVAD, CODOXM/IVAC, VDT-PACE: bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide). PS ≥2, advanced stage and MYC gene rearrangements were significantly associated adverse prognosticators (117).
A small, single-centre experience of 19 PLWH showed improved survival associated with DA-EPOCH compared to CHOP (17 vs. 7 months, P=0.014) (118). Use of DA-EPOCH is widely adopted and some propose routine IT CNS prophylaxis given the high proliferation rate, degree of extranodal involvement and presence of MYC translocations, although there are sparse data to support this approach (112,119).
Targeted treatments appear promising. Drugs with antimyeloma activity such as the proteasome inhibitor bortezomib, which blocks NF-κB, and the immunomodulatory agent lenalidomide have been used in case reports (120,121). In 16 patients, of which 38% were PLWH, the addition of bortezomib to frontline EPOCH resulted in a 5-year OS of 63% and median OS of 62 months at 48 months follow up (122). The anti-CD30 antibody-drug conjugate, brentuximab vedotin (BV) and daratumumab have been used for a small number of HIV-negative patients but outcomes were disappointing in this refractory setting (123-125). A feasibility trial of daratumumab in addition to DA-EPOCH in newly diagnosed PBL is active (NCT04139304). CAR-T was used in a patient after multiple prior lines of therapy including DA-EPOCH, ICE, carfilzomib, lenalidomide and daratumumab with a one month response (126). Given CD19 is rarely expressed on these tumours, this is not a strategy commonly pursued.
Primary effusion lymphoma
HHV8 DNA sequences were first found in a distinct category of eight cases of AIDS-related B-cell lymphoma in body-cavity effusions, establishing this HHV8 driven neoplasm (7). The following year this was termed “primary effusion lymphoma” (127). PEL is normally confined to body cavities (pleural, pericardial, peritoneal) in the absence of a tumour mass, although the less common extracavitary subtype is described. This rare disorder develops in the context of immune suppression. Neoplastic cells have immunoblastic to plasmablastic features, and the presence of HHV8 is required. The diagnosis is typically made based on an immunophenotype of serous fluid expressing activation and plasma-cell associated antigens [CD38, CD138, MUM1, VS38c and HHV8 latency-associated nuclear antigen 1 (LANA1)]. As with PBL, pan-B-cell markers are absent whilst most express CD30 (128). Coinfection with EBV is seen in 80% of cases but EBV LMP1 is not expressed, EBV early RNA (EBER) may be detected.
There is a paucity of data and no standard of care. Due to the low incidence, management has been extrapolated from trials examining aggressive lymphomas. OS is dismal. Even in the ART era, 2-year OS in one recent series of 13 patients was 15.4% with median survival of less than one year (129). Over a third are primary refractory and responses to chemotherapy are often short lived (117). Non-chemotherapy approaches have been reported in limited cases where PS precludes chemotherapy with generally poor responses. Combinations of ART, intracavity or intravenous antiviral cidofovir, and interferon have been utilised (130,131). Little information is available regarding the effect of ART on HHV8 viral load in peripheral blood or effusions.
There is a need to improve current treatment. Poor prognostic factors in CHOP-treated patients included PS ≥2 and an absence of ART prior to diagnosis on multivariate analysis (132) and >1 involved cavity on univariate analysis (117). The addition of HD MTX to CHOP was utilised in seven PLWH with some promise (133), however, this is limited by concerns of toxicity from accumulation in effusions causing delayed drug clearance.
A number of drugs may provide a targeted treatment approach. More intense regimens (EPOCH or hyperCVAD) and rituximab in rare CD20-positive cases have been suggested for use in those with adequate PS (131). Amongst 19 patients treated for PEL with EPOCH with or without rituximab, bevacizumab or HD MTX, there was a CR rate of 54%. EBER positivity was associated with improved survival and elevated interleukin-6 (IL-6) level associated with greater mortality (134). Bortezomib has been shown to inhibit cell survival and induce apoptosis via NF-kB in PEL cell lines (135), though this has unverified clinical benefit (131,136). Mouse models have shown reduced effusions with lenalidomide and arsenic trioxide (137) but clinical data is scant (138). A phase I/II trial is recruiting to investigate the addition of lenalidomide to DA-EPOCH-R and includes PLWH (NCT02911142). Rituximab has been added to target the HHV8-infected B-cell reservoir with encouraging preliminary data in six patients (99). BV may also be useful for those expressing CD30 (139) and daratumumab has also been used (140).
HHV8 positive MCD
MCD is a polyclonal lymphoproliferative disorder of benign lymphocytes, plasma cells and vessels. In PLWH it is almost exclusively associated with HHV8, in the subset HHV8-associated MCD. It occurs in the context of relatively preserved CD4+ T-cell counts (141). Although not a neoplasm, B-cells have plasmacytic differentiation with high levels of IgM lambda light chain restriction, HHV8 LANA1, CD38, MUM1, and may express CD30 and CD20. There is an excess production of dysregulated cytokines, most prominently IL-6. Viral and human IL-6 contribute to remitting and relapsing inflammatory features (142). These include fever, lymphadenopathy, hepatosplenomegaly, effusions, skin rash, cytopenias, raised C-reactive protein, hypoalbuminaemia, direct antiglobulin test positivity, and hypergammaglobulinaemia. Concurrent Kaposi’s sarcoma is common (143). A single institution cohort study of 60 PLWH with MCD showed a 15-times risk of developing NHL thought to be related to HHV8 (PEL, PBL, PCNSL) (144). Hence, repeat biopsy is appropriate in the relapse or refractory setting.
Outcomes have been poor in this aggressive disease. Cytokine storm may result in fatal multiorgan failure with haemophagocytic lymphohistiocytosis (HLH) seen in one series of 140 PLWH (145). Prospective trials demonstrate more favourable outcomes with rituximab, which is considered the standard of care (146,147). A single arm phase II trial of 21 PLWH receiving four infusions of weekly rituximab offered good responses with 2-year OS and disease-free survival of 95% and 79% respectively (146), a considerable improvement compared to the pre-rituximab era (2 year OS 94% vs. 42%) (148). There may however be a worsening of Kaposi’s sarcoma in those with concomitant lesions or failure to control disease (149,150). The addition of liposomal doxorubicin to rituximab has been used in combination with interferon-α consolidation or antiviral therapy to reduce Kaposi’s sarcoma progression with some efficacy (n=17) (151). In vitro studies showed etoposide can inhibit HHV8 replication (152). Forty-nine PLWH treated in a non-controlled study received either weekly rituximab monotherapy (n=35) or for those at high risk, in combination with weekly intravenous etoposide (n=14) for four weeks. High risk patients had either a PS >2, end organ involvement, lung involvement, HLH or severe haemolytic anaemia. The 2- and 5-year relapse-free survival rates were 85% and 61%, respectively. CD4+ T-cell count and ART use were not independently prognostic, although a lower HIV viral load was associated with longer OS (148).
Non-chemotherapy approaches include antiviral valganciclovir plus zidovudine to target the HHV8 viraemia seen during MCD flares (with a reported median PFS of 6 months and 1 year OS of 86% (142,153). Splenectomy and steroids have also been described without durable responses (143).
Tocilizumab, a monoclonal antibody targeting the IL-6 receptor, has led to transient responses with a median PFS of 3.2 months seen in eight PLWH, five of whom had tocilizumab alone (154). Siltuximab, an anti-IL-6 antibody, used in idiopathic MCD, does not bind viral IL-6.
Hodgkin lymphoma
Unlike NHL, the incidence of cHL has not significantly decreased since the introduction of ART and after an initial increase has now stabilised from 2004–2020 (155-157). ART has improved the patient survival with this highly curable lymphoma (51). PLWH do however present with more widespread disease. Historically, a relatively increased proportion of mixed cellularity subtype, strongly associated with EBV, was reported (158). The incidence increased with higher CD4+ T-cell counts from US cancer registry data (159). Data from 17 French centres between 2010–2015 showed around a third of those with cHL had a CD4 >500 cells/μL (160). Interactions between EBV, the tumour microenvironment and Reed Sternberg cells have been proposed as pathogenic drivers (161).
Prior to ART, median survival was less than two years (162,163). Outcomes of 66 PLWH were compared in the pre-ART to post-ART era in Germany (50). ART had a significant impact on improved OS, independent of remission status achieved. A retrospective UK study compared 93 PLWH with 131 HIV-negative, newly diagnosed patients deemed fit for ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) treatment. Eighty percent presented with advanced stage disease and two thirds had an IPI ≥3, compared to 35% and 26% of the HIV-negative patients. As is now standard, almost all had concomitant ART. There were no significant differences in survival with 5-year OS 88% vs. 81% and EFS 66% vs. 59% in PLWH and HIV-negative, respectively (164). This has been replicated in the US study of over 2000 PLWH diagnosed between 2004–2012 where OS was similar regardless of HIV status after adjustment for stage and sociodemographic factors (20). CD4+ T-cell count at diagnosis may be prognostic. A retrospective study of 229 patients with advanced stage cHL treated with ABVD showed CD4+ T-cells <200 cells/µL was independently associated with both PFS and OS, although not with death from cHL-related causes (119).
In the immunocompetent, cHL management is increasingly focussed on identifying risk groups and adapted response assessments with improvements in OS and reduced potential for long-term treatment toxicities. A prospective series of 108 PLWH highlighted that a stage-adapted approach is feasible (165). Evidence of use of escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) is limited to small studies and has a high TRM (166).
As previously alluded to, PET has a low positive predictive value and in the setting of HIV positivity, interim results should be interpreted with caution due to inflammatory or infective changes. A negative interim PET was prognostic amongst a retrospective series of 23 ABVD-treated PLWH with advanced stage cHL. The 2-year PFS for interim PET-positive and PET-negative patients was 50% and 100% respectively (167). Twelve PLWH out of 336 were prospectively enrolled on a phase II trial of advanced stage cHL utilising treatment adapted to interim PET after 2 cycles ABVD (SWOGS0816). PET-negative responses were similar to the HIV-negative population (168).
Prospective trials in this area are limited. A phase II study (NCT01771107) is actively examining BV with combination chemotherapy in treating stage II-IV HIV associated cHL after promising results of six patients in the phase I trial who achieved CR with minimal toxicity (169). An update of 41 patients showed a 2-year PFS estimate of 86% and OS 92% (170).
In the relapse setting, novel agents including nivolumab, an immune checkpoint inhibitor, are active and feasible in case reports (171,172). The AMC-095 (NCT02408861) trial is recruiting for the use of nivolumab and ipilimumab in the management of HIV-associated relapsed or refractory cHL or solid tumours.
Haemopoietic stem cell transplantation
For those who are chemo-immunotherapy responsive, ASCT consolidation is standard of care in the relapsed setting. Three series from the European Society for Blood and Marrow Transplantation registry evaluating PLWH showed comparable outcomes to HIV-negative patients. Disease status rather than CD4+ T-cell count at time of ASCT was independently prognostic in the era of ART (173-175). The largest prospective multicentre study examined 40 PLWH who underwent BEAM (carmustine, etoposide, cytarabine, and melphalan) conditioned ASCT. Pretransplant HIV viral load was undetectable in 80% with a median CD4+ T-cell count of 249 cells/μL. ART interruption was limited to administration of BEAM and a minimum of 7 days after completion. At a median follow-up of 25 months, 2-year OS was 82% and PFS 80%, with a 1-year TRM of 5%. Outcomes were not statistically different to matched controls (176).
Case series support improving survival rates for PLWH after allogeneic stem cell transplant and this should be considered for those with an appropriate donor. One retrospective series reported 81 cases with no difference in inpatient mortality rate (11.5% vs. 10.2%) based on HIV status although OIs increased (cytomegalovirus and nontuberculous mycobacterium) (177). The only prospective multicentre study showed feasibility in 17 PLWH with at least 7/8 HLA-matched donors. The 100-day TRM was 0% and HIV viral load undetectable in patients achieving complete chimerism (178). Encouraging data from seven PLWH showed post-transplant cyclophosphamide for graft-versus-host-disease prophylaxis allowed expansion of donor options by less restrictive HLA-matching. ART interruption was minimised by use of subcutaneous enfuvirtide when oral ART was not tolerated (179).
Cases of HIV ‘cure’ through allogeneic transplant have garnered interest. First described in the ‘Berlin patient’, total body irradiation was employed in the setting of acute myeloid leukaemia (180). Later the ‘London patient’ underwent reduced intensity-conditioned transplantation for relapsed cHL (181). Donors expressing the HIV co-receptor CCR5 polymorphism (homozygous CCR5∆32) conferred natural resistance through prevention of HIV entry into CD4+ T-cells. This variant occurs in 1% of Caucasian donors and less frequently in other ethnicities so is not a viable option for the majority of patients. Viral entry may alternatively occur via CXCR4. Viral escape has been described following shift from CCR5-tropic to CXCR4-tropic HIV after transplantation leading to loss of HIV ‘cure’ (182). The risk of HIV relapse from sequestered virus in sanctuary sites is unknown. Gene editing of CCR5 into CAR-T cells has been proposed as a future functional cure for HIV and lymphoma (183).
Antimicrobial prophylaxis
OIs are a major cause of morbidity and mortality in immunocompromised patients. Comparative studies for OI prophylaxis in HIV-lymphoma do not exist. Two guidelines recommend co-trimoxazole prophylaxis against Pneumocystis jirovecii pneumonia and toxoplasmosis. Although one still focuses on those with a CD4+ T-cell count <200 cells/μL (85), the other reflects current practice by recommending prophylaxis for everyone commencing chemotherapy (184). Systemic antifungal azoles are similarly recommended regardless of CD4+ T-cell count, particularly with associated prolonged neutropenia (184), although consideration is required with coadministration of vinca alkaloids and practice varies (84,184). Antivirals are routinely used for all commencing chemotherapy. Prophylaxis for Mycobacterium avium complex is recommended when CD4+ T-cell counts <50 cells/μL and if hepatitis B core antigen positive. Vaccinations against influenza, pneumococcus, and hepatitis B virus are also stipulated (85). Cytomegalovirus remains a contentious issue, both in prophylaxis and management, with some adopting for management dictated by end-organ disease as opposed to viral thresholds (184). Tuberculosis (TB) screening should be considered in everyone with key factors including local incidence, country of origin, TB exposure, and additional TB risk factors, e.g., diabetes. Strongyloides infection should be excluded if from high-prevalence areas before using immunosuppression because of the risk of hyperinfection (185).
Future directions
Gene therapy for HIV when treating HIV-associated lymphomas has gained interest. Lentivector transfused pre-selected CD34+ stem cells with an anti-HIV gene transfer construct is being studied in those receiving ASCT for relapsed lymphoma to confer resistance to HIV-1 entry and prevent new infection (NCT02797470 and NCT03593187).
Since the widespread incorporation of ART within HIV-associated lymphoma management, there has been less focus on HIV-related factors in favour of lymphoma characteristics. An anticipated paradigm shift in HIV management is the availability of long-acting, intramuscular ART as an alternative to daily oral treatment. Phase II/III data is supportive of monthly dual injections (186,187) and randomised trials are ongoing (NCT03299049). Further understanding of the pathogenesis and prognostic factors in HIV-associated lymphoma may inform future management strategies. Tissue samples from PLWH are being collected to correlate clinical, genetic, and immunologic parameters including complete genome sequences of HIV-associated DLBCL (NCT01567722).
The more recent permissive clinical trial inclusion criteria for PLWH without concurrent OIs and adequate CD4+ T-cell counts is a welcome step. The key to future advances will be the inclusion of PLWH into large, prospective general lymphoma trials in order to facilitate evidenced-based practice. Data is scant for the rarer subtypes and international collaboration may aid defining optimal treatment strategies.
As previously highlighted, phase I/II trials including PLWH have been registered including ibrutinib with DA-EPOCH-R for DLBCL (NCT03220022), lenalidomide with DA-EPOCH-R for PEL (NCT02911142), pembrolizumab for relapsed or refractory NHL or cHL (NCT02595866) and daratumumab with DA-EPOCH for PBL (NCT04139304). A phase I trial examining dose and side effects of nivolumab and ipilimumab for HIV-associated relapsed or refractory cHL is recruiting (NCT02408861) and pomalidomide and nivolumab in patients with virus-associated malignancies is due to recruit (NCT04902443). Immunotherapy trial and safety data will be essential in understanding toxicity and efficacy of this promising targeted treatment. Overall, broadening access will provide better equity for PLWH.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Christopher P. Fox, Claire Shannon-Lowe) for the series “Lymphoma and Viruses” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/aol-21-16). The series “Lymphoma and Viruses” was commissioned by the editorial office without any funding or sponsorship. KC reports that she received payments or honoraria from Roche, Takeda, KITE and Gilead, and financial support from Roche, Takeda, KITE, Janssen and BMS, and has participated on Board for Roche, Takeda, Gelgene/BMS, Atara, Gilead, KITE and Janssen. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gottlieb MS, Schroff R, Schanker HM, et al. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med 1981;305:1425-31. [Crossref] [PubMed]
- Ziegler JL, Drew WL, Miner RC, et al. Outbreak of Burkitt's-like lymphoma in homosexual men. Lancet 1982;2:631-3. [Crossref] [PubMed]
- Ziegler JL, Beckstead JA, Volberding PA, et al. Non-Hodgkin's lymphoma in 90 homosexual men. Relation to generalized lymphadenopathy and the acquired immunodeficiency syndrome. N Engl J Med 1984;311:565-70. [Crossref] [PubMed]
- Centers for Disease Control (CDC). Diffuse, undifferentiated non-Hodgkins lymphoma among homosexual males--United States. MMWR Morb Mortal Wkly Rep 1982;31:277-9. [PubMed]
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- CDC. AIDS: 1987 revision of CDC/WHO case definition. Bull World Health Organ 1988;66:259-63, 69-73.
- Cesarman E, Chang Y, Moore PS, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 1995;332:1186-91. [Crossref] [PubMed]
- Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood 1997;89:1413-20. [Crossref] [PubMed]
- Morlat P, Roussillon C, Henard S, et al. Causes of death among HIV-infected patients in France in 2010 (national survey): trends since 2000. AIDS 2014;28:1181-91. [Crossref] [PubMed]
- Simard EP, Engels EA. Cancer as a cause of death among people with AIDS in the United States. Clin Infect Dis 2010;51:957-62. [Crossref] [PubMed]
- Rodger AJ, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet 2019;393:2428-38. [Crossref] [PubMed]
- Rodger AJ, Cambiano V, Bruun T, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA 2016;316:171-81. [Crossref] [PubMed]
- Safren SA, Mayer KH, Ou SS, et al. Adherence to Early Antiretroviral Therapy: Results From HPTN 052, a Phase III, Multinational Randomized Trial of ART to Prevent HIV-1 Sexual Transmission in Serodiscordant Couples. J Acquir Immune Defic Syndr 2015;69:234-40. [Crossref] [PubMed]
- Dancy-Scott N, Dutcher GA, Keselman A, et al. Trends in HIV Terminology: Text Mining and Data Visualization Assessment of International AIDS Conference Abstracts Over 25 Years. JMIR Public Health Surveill 2018;4:e50 [Crossref] [PubMed]
- Silverberg MJ, Lau B, Achenbach CJ, et al. Cumulative Incidence of Cancer Among Persons With HIV in North America: A Cohort Study. Ann Intern Med 2015;163:507-18. [Crossref] [PubMed]
- Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med 2008;148:728-36. [Crossref] [PubMed]
- Hernández-Ramírez RU, Shiels MS, Dubrow R, et al. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV 2017;4:e495-504. [Crossref] [PubMed]
- Olszewski AJ, Fallah J, Castillo JJ. Human immunodeficiency virus-associated lymphomas in the antiretroviral therapy era: Analysis of the National Cancer Data Base. Cancer 2016;122:2689-97. [Crossref] [PubMed]
- Oriol A, Ribera JM, Bergua J, et al. High-dose chemotherapy and immunotherapy in adult Burkitt lymphoma: comparison of results in human immunodeficiency virus-infected and noninfected patients. Cancer 2008;113:117-25. [Crossref] [PubMed]
- Olszewski AJ, Castillo JJ. Outcomes of HIV-associated Hodgkin lymphoma in the era of antiretroviral therapy. AIDS 2016;30:787-96. [Crossref] [PubMed]
- Levine AM. AIDS-related malignancies: the emerging epidemic. J Natl Cancer Inst 1993;85:1382-97. [Crossref] [PubMed]
- Desai J, Mitnick RJ, Henry DH, et al. Patterns of central nervous system recurrence in patients with systemic human immunodeficiency virus-associated non-hodgkin lymphoma. Cancer 1999;86:1840-7. [Crossref] [PubMed]
- Mahe E, Ross C, Sur M. Lymphoproliferative Lesions in the Setting of HIV Infection: A Five-Year Retrospective Case Series and Review. Patholog Res Int 2011;2011:618760 [Crossref] [PubMed]
- Ford N, Meintjes G, Pozniak A, et al. The future role of CD4 cell count for monitoring antiretroviral therapy. Lancet Infect Dis 2015;15:241-7. [Crossref] [PubMed]
- Besson C, Lancar R, Prevot S, et al. High Risk Features Contrast With Favorable Outcomes in HIV-associated Hodgkin Lymphoma in the Modern cART Era, ANRS CO16 LYMPHOVIR Cohort. Clin Infect Dis 2015;61:1469-75. [Crossref] [PubMed]
- Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59-67. [Crossref] [PubMed]
- Hernández-Ramírez RU, Qin L, Lin H, et al. Association of immunosuppression and HIV viraemia with non-Hodgkin lymphoma risk overall and by subtype in people living with HIV in Canada and the USA: a multicentre cohort study. Lancet HIV 2019;6:e240-9. [Crossref] [PubMed]
- Dolcetti R, Gloghini A, Caruso A, et al. A lymphomagenic role for HIV beyond immune suppression? Blood 2016;127:1403-9. [Crossref] [PubMed]
- Arvey A, Ojesina AI, Pedamallu CS, et al. The tumor virus landscape of AIDS-related lymphomas. Blood 2015;125:e14-22. [Crossref] [PubMed]
- Chadburn A, Abdul-Nabi AM, Teruya BS, et al. Lymphoid proliferations associated with human immunodeficiency virus infection. Arch Pathol Lab Med 2013;137:360-70. [Crossref] [PubMed]
- Carbone A, Vaccher E, Gloghini A, et al. Diagnosis and management of lymphomas and other cancers in HIV-infected patients. Nat Rev Clin Oncol 2014;11:223-38. [Crossref] [PubMed]
- Carbone A, Cesarman E, Spina M, et al. HIV-associated lymphomas and gamma-herpesviruses. Blood 2009;113:1213-24. [Crossref] [PubMed]
- Popovic M, Tenner-Racz K, Pelser C, et al. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc Natl Acad Sci U S A 2005;102:14807-12. [Crossref] [PubMed]
- Noy A. Optimizing treatment of HIV-associated lymphoma. Blood 2019;134:1385-94. [Crossref] [PubMed]
- Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019;393:143-55. [Crossref] [PubMed]
- Cahn P, Madero JS, Arribas JR, et al. Durable Efficacy of Dolutegravir Plus Lamivudine in Antiretroviral Treatment-Naive Adults With HIV-1 Infection: 96-Week Results From the GEMINI-1 and GEMINI-2 Randomized Clinical Trials. J Acquir Immune Defic Syndr 2020;83:310-8. [Crossref] [PubMed]
- van Wyk J, Ajana F, Bisshop F, et al. Efficacy and Safety of Switching to Dolutegravir/Lamivudine Fixed-Dose 2-Drug Regimen vs Continuing a Tenofovir Alafenamide-Based 3- or 4-Drug Regimen for Maintenance of Virologic Suppression in Adults Living With Human Immunodeficiency Virus Type 1: Phase 3, Randomized, Noninferiority TANGO Study. Clin Infect Dis 2020;71:1920-9. [Crossref] [PubMed]
- Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 2018;391:839-49. [Crossref] [PubMed]
- Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283-96. [Crossref] [PubMed]
- INSIGHT START Study Group. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med 2015;373:795-807. [Crossref] [PubMed]
- TEMPRANO ANRS 12136 Study Group, Danel C, Moh R, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med 2015;373:808-22.
- Shiels MS, Engels EA, Linet MS, et al. The epidemic of non-Hodgkin lymphoma in the United States: disentangling the effect of HIV, 1992-2009. Cancer Epidemiol Biomarkers Prev 2013;22:1069-78. [Crossref] [PubMed]
- Gibson TM, Morton LM, Shiels MS, et al. Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: a population-based study. AIDS 2014;28:2313-8. [Crossref] [PubMed]
- Dunleavy K, Wilson WH. How I treat HIV-associated lymphoma. Blood 2012;119:3245-55. [Crossref] [PubMed]
- Uldrick TS, Little RF. How I treat classical Hodgkin lymphoma in patients infected with human immunodeficiency virus. Blood 2015;125:1226-35; quiz 1355. [Crossref] [PubMed]
- Lawn SD, Török ME, Wood R. Optimum time to start antiretroviral therapy during HIV-associated opportunistic infections. Curr Opin Infect Dis 2011;24:34-42. [Crossref] [PubMed]
- Lim ST, Karim R, Nathwani BN, et al. AIDS-related Burkitt's lymphoma versus diffuse large-cell lymphoma in the pre-highly active antiretroviral therapy (HAART) and HAART eras: significant differences in survival with standard chemotherapy. J Clin Oncol 2005;23:4430-8. [Crossref] [PubMed]
- Barta SK, Xue X, Wang D, et al. Treatment factors affecting outcomes in HIV-associated non-Hodgkin lymphomas: a pooled analysis of 1546 patients. Blood 2013;122:3251-62. [Crossref] [PubMed]
- Tan CRC, Barta SK, Lee J, et al. Combination antiretroviral therapy accelerates immune recovery in patients with HIV-related lymphoma treated with EPOCH: a comparison within one prospective trial AMC034. Leuk Lymphoma 2018;59:1851-60. [Crossref] [PubMed]
- Hentrich M, Maretta L, Chow KU, et al. Highly active antiretroviral therapy (HAART) improves survival in HIV-associated Hodgkin's disease: results of a multicenter study. Ann Oncol 2006;17:914-9. [Crossref] [PubMed]
- Gérard L, Galicier L, Boulanger E, et al. Improved survival in HIV-related Hodgkin's lymphoma since the introduction of highly active antiretroviral therapy. AIDS 2003;17:81-7. [Crossref] [PubMed]
- Sparano JA, Lee S, Chen MG, et al. Phase II trial of infusional cyclophosphamide, doxorubicin, and etoposide in patients with HIV-associated non-Hodgkin's lymphoma: an Eastern Cooperative Oncology Group Trial (E1494). J Clin Oncol 2004;22:1491-500. [Crossref] [PubMed]
- Xicoy B, Ribera JM, Miralles P, et al. Results of treatment with doxorubicin, bleomycin, vinblastine and dacarbazine and highly active antiretroviral therapy in advanced stage, human immunodeficiency virus-related Hodgkin's lymphoma. Haematologica 2007;92:191-8. [Crossref] [PubMed]
- Vaccher E, Spina M, di Gennaro G, et al. Concomitant cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy plus highly active antiretroviral therapy in patients with human immunodeficiency virus-related, non-Hodgkin lymphoma. Cancer 2001;91:155-63. [Crossref] [PubMed]
- Nachega JB, Parienti JJ, Uthman OA, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials. Clin Infect Dis 2014;58:1297-307. [Crossref] [PubMed]
- Gopal S, Patel MR, Achenbach CJ, et al. Lymphoma immune reconstitution inflammatory syndrome in the center for AIDS research network of integrated clinical systems cohort. Clin Infect Dis 2014;59:279-86. [Crossref] [PubMed]
- Cheema A, Shakeel M, Mannan A, et al. Primary effusion lymphoma (PEL) and disseminated Kaposi Sarcoma (KS) in an HIV-infected patient: A case of Immune Reconstitution Inflammatory Syndrome (IRIS). Journal of Hospital Medicine 2017;12. Abstract 385.
- Uldrick TS, Ison G, Rudek MA, et al. Modernizing Clinical Trial Eligibility Criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research HIV Working Group. J Clin Oncol 2017;35:3774-80. [Crossref] [PubMed]
- Venturelli S, Dalla Pria A, Stegmann K, et al. The exclusion of people living with HIV (PLWH) from clinical trials in lymphoma. Br J Cancer 2015;113:861-3. [Crossref] [PubMed]
- Sorotsky H, Hogg D, Amir E, et al. Characteristics of Immune Checkpoint Inhibitors Trials Associated With Inclusion of Patients With HIV: A Systematic Review and Meta-analysis. JAMA Netw Open 2019;2:e1914816 [Crossref] [PubMed]
- Kaplan LD, Straus DJ, Testa MA, et al. Low-dose compared with standard-dose m-BACOD chemotherapy for non-Hodgkin's lymphoma associated with human immunodeficiency virus infection. National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group. N Engl J Med 1997;336:1641-8. [Crossref] [PubMed]
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235-42. [Crossref] [PubMed]
- Kaplan LD, Lee JY, Ambinder RF, et al. Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lymphoma: AIDS-Malignancies Consortium Trial 010. Blood 2005;106:1538-43. [Crossref] [PubMed]
- Levine AM, Noy A, Lee JY, et al. Pegylated liposomal doxorubicin, rituximab, cyclophosphamide, vincristine, and prednisone in AIDS-related lymphoma: AIDS Malignancy Consortium Study 047. J Clin Oncol 2013;31:58-64. [Crossref] [PubMed]
- Ziepert M, Hasenclever D, Kuhnt E, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol 2010;28:2373-80. [Crossref] [PubMed]
- Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014;123:837-42. [Crossref] [PubMed]
- Cingolani A, Cozzi Lepri A, Teofili L, et al. Survival and predictors of death in people with HIV-associated lymphoma compared to those with a diagnosis of lymphoma in general population. PLoS One 2017;12:e0186549 [Crossref] [PubMed]
- Wilson WH, Grossbard ML, Pittaluga S, et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood 2002;99:2685-93. [Crossref] [PubMed]
- Little RF, Pittaluga S, Grant N, et al. Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: impact of antiretroviral therapy suspension and tumor biology. Blood 2003;101:4653-9. [Crossref] [PubMed]
- Spina M, Jaeger U, Sparano JA, et al. Rituximab plus infusional cyclophosphamide, doxorubicin, and etoposide in HIV-associated non-Hodgkin lymphoma: pooled results from 3 phase 2 trials. Blood 2005;105:1891-7. [Crossref] [PubMed]
- Sparano JA, Lee JY, Kaplan LD, et al. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood 2010;115:3008-16. [Crossref] [PubMed]
- Bartlett NL, Wilson WH, Jung SH, et al. Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303. J Clin Oncol 2019;37:1790-9. [Crossref] [PubMed]
- Poeschel V, Held G, Ziepert M, et al. Four versus six cycles of CHOP chemotherapy in combination with six applications of rituximab in patients with aggressive B-cell lymphoma with favourable prognosis (FLYER): a randomised, phase 3, non-inferiority trial. Lancet 2019;394:2271-81. [Crossref] [PubMed]
- Sparano JA, Lee JY, Kaplan LD, et al. Response-adapted therapy with infusional EPOCH chemotherapy plus rituximab in HIV-associated, B-cell non-Hodgkin's lymphoma. Haematologica 2021;106:730-5. [Crossref] [PubMed]
- Dunleavy K, Little RF, Pittaluga S, et al. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood 2010;115:3017-24. [Crossref] [PubMed]
- Ramos JC, Sparano JA, Chadburn A, et al. Impact of Myc in HIV-associated non-Hodgkin lymphomas treated with EPOCH and outcomes with vorinostat (AMC-075 trial). Blood 2020;136:1284-97. [Crossref] [PubMed]
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017;377:2531-44. [Crossref] [PubMed]
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380:45-56. [Crossref] [PubMed]
- Allred J, Bharucha K, Özütemiz C, He F, Janakiram M, Maakaron J, et al. Chimeric antigen receptor T-cell therapy for HIV-associated diffuse large B-cell lymphoma: case report and management recommendations. Bone Marrow Transplant 2021;56:679-82. [Crossref] [PubMed]
- Abramson JS, Irwin KE, Frigault MJ, et al. Successful anti-CD19 CAR T-cell therapy in HIV-infected patients with refractory high-grade B-cell lymphoma. Cancer 2019;125:3692-8. [Crossref] [PubMed]
- Abbasi A, Peeke S, Shah N, et al. Axicabtagene ciloleucel CD19 CAR-T cell therapy results in high rates of systemic and neurologic remissions in ten patients with refractory large B cell lymphoma including two with HIV and viral hepatitis. J Hematol Oncol 2020;13:1. [Crossref] [PubMed]
- Uldrick TS, Gonçalves PH, Abdul-Hay M, et al. Assessment of the Safety of Pembrolizumab in Patients With HIV and Advanced Cancer-A Phase 1 Study. JAMA Oncol 2019;5:1332-9. [Crossref] [PubMed]
- Barta SK, Joshi J, Mounier N, et al. Central nervous system involvement in AIDS-related lymphomas. Br J Haematol 2016;173:857-66. [Crossref] [PubMed]
- NCCN. NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx#site (Accessed on 5th May, 2021).
- Bower M, Palfreeman A, Alfa-Wali M, et al. British HIV Association guidelines for HIV-associated malignancies 2014. HIV Med 2014;15:1-92. [Crossref] [PubMed]
- McKay P, Wilson MR, Chaganti S, et al. The prevention of central nervous system relapse in diffuse large B-cell lymphoma: a British Society for Haematology good practice paper. Br J Haematol 2020;190:708-14. [Crossref] [PubMed]
- Chimienti E, Spina M, Vaccher E, et al. Management of immunocompetent patients with primary central nervous system lymphoma. Clin Lymphoma Myeloma 2009;9:353-64. [Crossref] [PubMed]
- Gasser O, Bihl FK, Wolbers M, et al. HIV patients developing primary CNS lymphoma lack EBV-specific CD4+ T cell function irrespective of absolute CD4+ T cell counts. PLoS Med 2007;4:e96 [Crossref] [PubMed]
- Gandhi MK, Hoang T, Law SC, et al. EBV-associated primary CNS lymphoma occurring after immunosuppression is a distinct immunobiological entity. Blood 2021;137:1468-77. [Crossref] [PubMed]
- Formenti SC, Gill PS, Lean E, et al. Primary central nervous system lymphoma in AIDS. Results of radiation therapy. Cancer 1989;63:1101-7. [Crossref] [PubMed]
- Shiels MS, Pfeiffer RM, Besson C, et al. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol 2016;174:417-24. [Crossref] [PubMed]
- Nelson DF. Radiotherapy in the treatment of primary central nervous system lymphoma (PCNSL). J Neurooncol 1999;43:241-7. [Crossref] [PubMed]
- Reni M, Ferreri AJ, Garancini MP, et al. Therapeutic management of primary central nervous system lymphoma in immunocompetent patients: results of a critical review of the literature. Ann Oncol 1997;8:227-34. [Crossref] [PubMed]
- Nagai H, Odawara T, Ajisawa A, et al. Whole brain radiation alone produces favourable outcomes for AIDS-related primary central nervous system lymphoma in the HAART era. Eur J Haematol 2010;84:499-505. [Crossref] [PubMed]
- Grand M, Bia D, Diaz A. Cardiovascular Risk Assessment in People Living With HIV: A Systematic Review and Meta-Analysis of Real-Life Data. Curr HIV Res 2020;18:5-18. [Crossref] [PubMed]
- O'Neill A, Mikesch K, Fritsch K, et al. Outcomes for HIV-positive patients with primary central nervous system lymphoma after high-dose chemotherapy and auto-SCT. Bone Marrow Transplant 2015;50:999-1000. [Crossref] [PubMed]
- Ferreri AJ, Cwynarski K, Pulczynski E, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 2016;3:e217-27. [Crossref] [PubMed]
- Moulignier A, Lamirel C, Picard H, et al. Long-term AIDS-related PCNSL outcomes with HD-MTX and combined antiretroviral therapy. Neurology 2017;89:796-804. [Crossref] [PubMed]
- Lurain K, Uldrick TS, Ramaswami R, et al. Treatment of HIV-associated primary CNS lymphoma with antiretroviral therapy, rituximab, and high-dose methotrexate. Blood 2020;136:2229-32. [Crossref] [PubMed]
- Guech-Ongey M, Simard EP, Anderson WF, et al. AIDS-related Burkitt lymphoma in the United States: what do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood 2010;116:5600-4. [Crossref] [PubMed]
- Biggar RJ, Chaturvedi AK, Goedert JJ, et al. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst 2007;99:962-72. [Crossref] [PubMed]
- Bernstein SH, Unger JM, Leblanc M, et al. Natural history of CNS relapse in patients with aggressive non-Hodgkin's lymphoma: a 20-year follow-up analysis of SWOG 8516 -- the Southwest Oncology Group. J Clin Oncol 2009;27:114-9. [Crossref] [PubMed]
- Alderuccio JP, Olszewski AJ, Evens AM, et al. Prognostication, Survival and Treatment-Related Outcomes in HIV-Associated Burkitt Lymphoma (HIV-BL): A US and UK Collaborative Analysis. Blood 2020;136:49-50. [Crossref]
- Noy A, Lee JY, Cesarman E, et al. AMC 048: modified CODOX-M/IVAC-rituximab is safe and effective for HIV-associated Burkitt lymphoma. Blood 2015;126:160-6. [Crossref] [PubMed]
- Dunleavy K, Pittaluga S, Shovlin M, et al. Low-intensity therapy in adults with Burkitt's lymphoma. N Engl J Med 2013;369:1915-25. [Crossref] [PubMed]
- Roschewski M, Dunleavy K, Abramson JS, et al. Multicenter Study of Risk-Adapted Therapy With Dose-Adjusted EPOCH-R in Adults With Untreated Burkitt Lymphoma. J Clin Oncol 2020;38:2519-29. [Crossref] [PubMed]
- Zayac AS, Evens AM, Danilov A, et al. Outcomes of Burkitt lymphoma with central nervous system involvement: evidence from a large multi-center cohort study. Haematologica 2021;106:1932-42. [Crossref] [PubMed]
- Olszewski AJ, Jakobsen LH, Collins GP, et al. Burkitt Lymphoma International Prognostic Index. J Clin Oncol 2021;39:1129-38. [Crossref] [PubMed]
- Ferreri AJM, Cattaneo C, Lleshi A, et al. A dose-dense short-term therapy for human immunodeficiency virus/acquired immunodeficiency syndrome patients with high-risk Burkitt lymphoma or high-grade B-cell lymphoma: safety and efficacy results of the "CARMEN" phase II trial. Br J Haematol 2021;192:119-28. [Crossref] [PubMed]
- Castillo JJ, Winer ES, Stachurski D, et al. Prognostic factors in chemotherapy-treated patients with HIV-associated Plasmablastic lymphoma. Oncologist 2010;15:293-9. [Crossref] [PubMed]
- Di Ciaccio PR, Polizzotto MN, Cwynarski K, et al. Survival Outcomes for Plasmablastic Lymphoma: An International, Multicentre Study By the Australasian Lymphoma Alliance. Blood 2020;136:1-2. [Crossref]
- Castillo JJ, Bower M, Brühlmann J, et al. Prognostic factors for advanced-stage human immunodeficiency virus-associated classical Hodgkin lymphoma treated with doxorubicin, bleomycin, vinblastine, and dacarbazine plus combined antiretroviral therapy: a multi-institutional retrospective study. Cancer 2015;121:423-31. [Crossref] [PubMed]
- Garcia-Reyero J, Martinez Magunacelaya N, Gonzalez de Villambrosia S, et al. Genetic lesions in MYC and STAT3 drive oncogenic transcription factor overexpression in plasmablastic lymphoma. Haematologica 2021;106:1120-8. [Crossref] [PubMed]
- Armstrong R, Bradrick J, Liu YC. Spontaneous regression of an HIV-associated plasmablastic lymphoma in the oral cavity: a case report. J Oral Maxillofac Surg 2007;65:1361-4. [Crossref] [PubMed]
- Nasta SD, Carrum GM, Shahab I, et al. Regression of a plasmablastic lymphoma in a patient with HIV on highly active antiretroviral therapy. Leuk Lymphoma 2002;43:423-6. [Crossref] [PubMed]
- Morscio J, Dierickx D, Nijs J, et al. Clinicopathologic comparison of plasmablastic lymphoma in HIV-positive, immunocompetent, and posttransplant patients: single-center series of 25 cases and meta-analysis of 277 reported cases. Am J Surg Pathol 2014;38:875-86. [Crossref] [PubMed]
- Castillo JJ, Shum H, Lahijani M, et al. Prognosis in primary effusion lymphoma is associated with the number of body cavities involved. Leuk Lymphoma 2012;53:2378-82. [Crossref] [PubMed]
- Ibrahim IF, Shapiro GA, Naina HVK. Treatment of HIV-associated plasmablastic lymphoma: A single-center experience with 25 patients. J Clin Oncol 2014;32:8583. [Crossref]
- Castillo JJ, Chavez JC, Hernandez-Ilizaliturri FJ, et al. CD20-negative diffuse large B-cell lymphomas: biology and emerging therapeutic options. Expert Rev Hematol 2015;8:343-54. [Crossref] [PubMed]
- Bibas M, Grisetti S, Alba L, et al. Patient with HIV-associated plasmablastic lymphoma responding to bortezomib alone and in combination with dexamethasone, gemcitabine, oxaliplatin, cytarabine, and pegfilgrastim chemotherapy and lenalidomide alone. J Clin Oncol 2010;28:e704-8. [Crossref] [PubMed]
- Bose P, Thompson C, Gandhi D, et al. AIDS-related plasmablastic lymphoma with dramatic, early response to bortezomib. Eur J Haematol 2009;82:490-2. [Crossref] [PubMed]
- Castillo JJ, Guerrero-Garcia T, Baldini F, et al. Bortezomib plus EPOCH is effective as frontline treatment in patients with plasmablastic lymphoma. Br J Haematol 2019;184:679-82. [Crossref] [PubMed]
- Holderness BM, Malhotra S, Levy NB, et al. Brentuximab vedotin demonstrates activity in a patient with plasmablastic lymphoma arising from a background of chronic lymphocytic leukemia. J Clin Oncol 2013;31:e197-9. [Crossref] [PubMed]
- Pretscher D, Kalisch A, Wilhelm M, et al. Refractory plasmablastic lymphoma-a review of treatment options beyond standard therapy. Ann Hematol 2017;96:967-70. [Crossref] [PubMed]
- Marvyin K, Tjønnfjord EB, Breland UM, et al. Transformation to plasmablastic lymphoma in CLL upon ibrutinib treatment. BMJ Case Rep 2020;13:235816 [Crossref] [PubMed]
- Raychaudhuri R, Qualtieri J, Garfall AL. Axicabtagene ciloleucel for CD19+ plasmablastic lymphoma. Am J Hematol 2020;95:E28-30. [Crossref] [PubMed]
- Nador RG, Cesarman E, Chadburn A, et al. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood 1996;88:645-56. [Crossref] [PubMed]
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer 2007;111:224-33. [Crossref] [PubMed]
- Hentrich M, Müller M, Wyen C, et al. Characteristics and outcome of human immunodeficiency virus (HIV)-associated primary effusion lymphoma as observed in the German HIV-related lymphoma cohort study. Br J Haematol 2021;194:642-6. [Crossref] [PubMed]
- Hocqueloux L, Agbalika F, Oksenhendler E, et al. Long-term remission of an AIDS-related primary effusion lymphoma with antiviral therapy. AIDS 2001;15:280-2. [Crossref] [PubMed]
- Gupta A, Sen S, Marley E, et al. Management and Outcomes of HIV-Associated Primary Effusion Lymphoma: A Single Center Experience. Clin Lymphoma Myeloma Leuk 2016;16:S175-80. [Crossref] [PubMed]
- Boulanger E, Gérard L, Gabarre J, et al. Prognostic factors and outcome of human herpesvirus 8-associated primary effusion lymphoma in patients with AIDS. J Clin Oncol 2005;23:4372-80. [Crossref] [PubMed]
- Boulanger E, Daniel MT, Agbalika F, et al. Combined chemotherapy including high-dose methotrexate in KSHV/HHV8-associated primary effusion lymphoma. Am J Hematol 2003;73:143-8. [Crossref] [PubMed]
- Lurain K, Polizzotto MN, Aleman K, et al. Viral, immunologic, and clinical features of primary effusion lymphoma. Blood 2019;133:1753-61. [Crossref] [PubMed]
- An J, Sun Y, Fisher M, et al. Antitumor effects of bortezomib (PS-341) on primary effusion lymphomas. Leukemia 2004;18:1699-704. [Crossref] [PubMed]
- Boulanger E, Meignin V, Oksenhendler E. Bortezomib (PS-341) in patients with human herpesvirus 8-associated primary effusion lymphoma. Br J Haematol 2008;141:559-61. [Crossref] [PubMed]
- Moodad S, El Hajj R, Hleihel R, et al. Lenalidomide in Combination with Arsenic Trioxide: an Effective Therapy for Primary Effusion Lymphoma. Cancers (Basel) 2020;12:2483. [Crossref] [PubMed]
- Antar A, El Hajj H, Jabbour M, et al. Primary effusion lymphoma in an elderly patient effectively treated by lenalidomide: case report and review of literature. Blood Cancer J 2014;4:e190 [Crossref] [PubMed]
- Bhatt S, Ashlock BM, Natkunam Y, et al. CD30 targeting with brentuximab vedotin: a novel therapeutic approach to primary effusion lymphoma. Blood 2013;122:1233-42. [Crossref] [PubMed]
- Shah NN, Singavi AK, Harrington A. Daratumumab in Primary Effusion Lymphoma. N Engl J Med 2018;379:689-90. [Crossref] [PubMed]
- Mylona EE, Baraboutis IG, Lekakis LJ, et al. Multicentric Castleman's disease in HIV infection: a systematic review of the literature. AIDS Rev 2008;10:25-35. [PubMed]
- Oksenhendler E, Carcelain G, Aoki Y, et al. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric castleman disease in HIV-infected patients. Blood 2000;96:2069-73. [Crossref] [PubMed]
- Oksenhendler E, Duarte M, Soulier J, et al. Multicentric Castleman's disease in HIV infection: a clinical and pathological study of 20 patients. AIDS 1996;10:61-7. [Crossref] [PubMed]
- Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood 2002;99:2331-6. [Crossref] [PubMed]
- Oksenhendler E, Boutboul D, Fajgenbaum D, et al. The full spectrum of Castleman disease: 273 patients studied over 20 years. Br J Haematol 2018;180:206-16. [Crossref] [PubMed]
- Bower M, Powles T, Williams S, et al. Brief communication: rituximab in HIV-associated multicentric Castleman disease. Ann Intern Med 2007;147:836-9. [Crossref] [PubMed]
- Gérard L, Bérezné A, Galicier L, et al. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus associated multicentric Castleman's disease: ANRS 117 CastlemaB Trial. J Clin Oncol 2007;25:3350-6. [Crossref] [PubMed]
- Bower M, Newsom-Davis T, Naresh K, et al. Clinical Features and Outcome in HIV-Associated Multicentric Castleman's Disease. J Clin Oncol 2011;29:2481-6. [Crossref] [PubMed]
- Neuville S, Agbalika F, Rabian C, et al. Failure of rituximab in human immunodeficiency virus-associated multicentric Castleman disease. Am J Hematol 2005;79:337-9. [Crossref] [PubMed]
- Buchler T, Dubash S, Lee V, et al. Rituximab failure in fulminant multicentric HIV/human herpesvirus 8-associated Castleman's disease with multiorgan failure: report of two cases. AIDS 2008;22:1685-7. [Crossref] [PubMed]
- Uldrick TS, Polizzotto MN, Aleman K, et al. Rituximab plus liposomal doxorubicin in HIV-infected patients with KSHV-associated multicentric Castleman disease. Blood 2014;124:3544-52. [Crossref] [PubMed]
- González-Molleda L, Wang Y, Yuan Y. Potent antiviral activity of topoisomerase I and II inhibitors against Kaposi's sarcoma-associated herpesvirus. Antimicrob Agents Chemother 2012;56:893-902. [Crossref] [PubMed]
- Uldrick TS, Polizzotto MN, Aleman K, et al. High-dose zidovudine plus valganciclovir for Kaposi sarcoma herpesvirus-associated multicentric Castleman disease: a pilot study of virus-activated cytotoxic therapy. Blood 2011;117:6977-86. [Crossref] [PubMed]
- Ramaswami R, Lurain K, Peer CJ, et al. Tocilizumab in patients with symptomatic Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Blood 2020;135:2316-9. [Crossref] [PubMed]
- Hleyhel M, Hleyhel M, Bouvier AM, et al. Risk of non-AIDS-defining cancers among HIV-1-infected individuals in France between 1997 and 2009: results from a French cohort. AIDS 2014;28:2109-18. [Crossref] [PubMed]
- Shiels MS, Islam JY, Rosenberg PS, et al. Projected Cancer Incidence Rates and Burden of Incident Cancer Cases in HIV-Infected Adults in the United States Through 2030. Ann Intern Med 2018;168:866-73. [Crossref] [PubMed]
- Bohlius J, Schmidlin K, Boué F, et al. HIV-1-related Hodgkin lymphoma in the era of combination antiretroviral therapy: incidence and evolution of CD4+ T-cell lymphocytes. Blood 2011;117:6100-8. [Crossref] [PubMed]
- Shiels MS, Koritzinsky EH, Clarke CA, et al. Prevalence of HIV Infection among U.S. Hodgkin lymphoma cases. Cancer Epidemiol Biomarkers Prev 2014;23:274-81. [Crossref] [PubMed]
- Biggar RJ, Jaffe ES, Goedert JJ, et al. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood 2006;108:3786-91. [Crossref] [PubMed]
- Poizot-Martin I, Lions C, Allavena C, et al. Spectrum and Incidence Trends of AIDS- and Non-AIDS-Defining Cancers between 2010 and 2015 in the French Dat'AIDS Cohort. Cancer Epidemiol Biomarkers Prev 2021;30:554-63. [Crossref] [PubMed]
- Bräuninger A, Schmitz R, Bechtel D, et al. Molecular biology of Hodgkin's and Reed/Sternberg cells in Hodgkin's lymphoma. Int J Cancer 2006;118:1853-61. [Crossref] [PubMed]
- Andrieu JM, Roithmann S, Tourani JM, et al. Hodgkin's disease during HIV1 infection: the French registry experience. French Registry of HIV-associated Tumors. Ann Oncol 1993;4:635-41. [Crossref] [PubMed]
- Gold JE, Altarac D, Ree HJ, et al. HIV-associated Hodgkin disease: a clinical study of 18 cases and review of the literature. Am J Hematol 1991;36:93-9. [Crossref] [PubMed]
- Montoto S, Shaw K, Okosun J, et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J Clin Oncol 2012;30:4111-6. [Crossref] [PubMed]
- Hentrich M, Berger M, Wyen C, et al. Stage-adapted treatment of HIV-associated Hodgkin lymphoma: results of a prospective multicenter study. J Clin Oncol 2012;30:4117-23. [Crossref] [PubMed]
- Hartmann P, Rehwald U, Salzberger B, et al. BEACOPP therapeutic regimen for patients with Hodgkin's disease and HIV infection. Ann Oncol 2003;14:1562-9. [Crossref] [PubMed]
- Okosun J, Warbey V, Shaw K, et al. Interim fluoro-2-deoxy-D-glucose-PET predicts response and progression-free survival in patients with Hodgkin lymphoma and HIV infection. AIDS 2012;26:861-5. [Crossref] [PubMed]
- Danilov AV, Li H, Press OW, et al. Feasibility of interim positron emission tomography (PET)-adapted therapy in HIV-positive patients with advanced Hodgkin lymphoma (HL): a sub-analysis of SWOG S0816 Phase 2 trial. Leuk Lymphoma 2017;58:461-5. [Crossref] [PubMed]
- Rubinstein PG, Moore PC, Rudek MA, et al. Brentuximab vedotin with AVD shows safety, in the absence of strong CYP3A4 inhibitors, in newly diagnosed HIV-associated Hodgkin lymphoma. AIDS 2018;32:605-11. [Crossref] [PubMed]
- Rubinstein PG, Moore PC, Bimali M, et al. Safety and Efficacy of Brentuximab Vedotin in Combination with AVD in Stage II-IV HIV-Associated Classical Hodgkin Lymphoma: Results of the Phase 2 Study, AMC 085. Blood 2019;134:130. [Crossref]
- Sandoval-Sus JD, Mogollon-Duffo F, Patel A, et al. Nivolumab as salvage treatment in a patient with HIV-related relapsed/refractory Hodgkin lymphoma and liver failure with encephalopathy. J Immunother Cancer 2017;5:49. [Crossref] [PubMed]
- Serrao A, Canichella M, De Luca ML, et al. Nivolumab as a safe and effective treatment in an HIV patient with refractory Hodgkin lymphoma. Ann Hematol 2019;98:1505-6. [Crossref] [PubMed]
- Hübel K, Re A, Boumendil A, et al. Autologous stem cell transplantation for HIV-associated lymphoma in the antiretroviral and rituximab era: a retrospective study by the EBMT Lymphoma Working Party. Bone Marrow Transplant 2019;54:1625-31. [Crossref] [PubMed]
- Díez-Martín JL, Balsalobre P, Re A, et al. Comparable survival between HIV+ and HIV- non-Hodgkin and Hodgkin lymphoma patients undergoing autologous peripheral blood stem cell transplantation. Blood 2009;113:6011-4. [Crossref] [PubMed]
- Balsalobre P, Díez-Martín JL, Re A, et al. Autologous stem-cell transplantation in patients with HIV-related lymphoma. J Clin Oncol 2009;27:2192-8. [Crossref] [PubMed]
- Alvarnas JC, Le Rademacher J, Wang Y, et al. Autologous hematopoietic cell transplantation for HIV-related lymphoma: results of the BMT CTN 0803/AMC 071 trial. Blood 2016;128:1050-8. [Crossref] [PubMed]
- Mehta K, Im A, Rahman F, et al. Epidemiology and Outcomes of Hematopoietic Stem Cell Transplantation in Human Immunodeficiency Virus-Positive Patients From 1998 to 2012: A Nationwide Analysis. Clin Infect Dis 2018;67:128-33. [Crossref] [PubMed]
- Ambinder RF, Wu J, Logan B, et al. Allogeneic Hematopoietic Cell Transplant for HIV Patients with Hematologic Malignancies: The BMT CTN-0903/AMC-080 Trial. Biol Blood Marrow Transplant 2019;25:2160-6. [Crossref] [PubMed]
- Durand CM, Capoferri AA, Redd AD, et al. Allogeneic bone marrow transplantation with post-transplant cyclophosphamide for patients with HIV and haematological malignancies: a feasibility study. Lancet HIV 2020;7:e602-10. [Crossref] [PubMed]
- Hütter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 2009;360:692-8. [Crossref] [PubMed]
- Gupta RK, Abdul-Jawad S, McCoy LE, et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 2019;568:244-8. [Crossref] [PubMed]
- Kordelas L, Verheyen J, Beelen DW, et al. Shift of HIV tropism in stem-cell transplantation with CCR5 Delta32 mutation. N Engl J Med 2014;371:880-2. [Crossref] [PubMed]
- Rust BJ, Kiem HP, Uldrick TS. CAR T-cell therapy for cancer and HIV through novel approaches to HIV-associated haematological malignancies. Lancet Haematol 2020;7:e690-6. [Crossref] [PubMed]
- EACS. European AIDS Clinical Society Guidelines v10.1, 2020.
- Marcos LA, Terashima A, Canales M, et al. Update on strongyloidiasis in the immunocompromised host. Curr Infect Dis Rep 2011;13:35-46. [Crossref] [PubMed]
- Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-Acting Cabotegravir and Rilpivirine after Oral Induction for HIV-1 Infection. N Engl J Med 2020;382:1124-35. [Crossref] [PubMed]
- Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017;390:1499-510. [Crossref] [PubMed]
Cite this article as: Khwaja J, Burns JE, Ahmed N, Cwynarski K. HIV-associated lymphoma—advances in clinical management. Ann Lymphoma 2021;5:26.


