
Narrative review: biomarkers, a hope towards early diagnosis in primary CNS lymphoma
Introduction
Early diagnosis is crucial in the cure of primary central nervous system lymphoma (PCNSL) patients. Prolonged exposure of brain parenchyma to tumor infiltration generates severe neurological impairments, that can be irreversible with possible consequent loss of autonomy and poor performance status (PS). Moreover, management of symptoms related to the central nervous system (CNS) lesions usually requires the use of steroids, which produce confounding effects on neuroimaging, delays of diagnostic biopsy, as well as complications like diabetes and infections. All these circumstances compromise patients’ general conditions and negatively influence both their accrual in clinical trials and tolerance to treatment with consequent delay of therapy, reduction of intensity of care and, overall, their outcome.
After the onset of neurological symptoms, radiological features may suggest the diagnosis of PCNSL, but in order to verify the suspicion, neurosurgical procedures are essential and represent, along with the subsequent histopathological assessment, the current gold standard of diagnosis in this setting. In particular, stereotactic biopsy is the most frequent way used to collect tumor tissue. Thus, in cases of deep lesions or after steroids therapy the risk of ‘misleading diagnosis’ is increased due to a surgical geographical failure or risk of morbidity. Indeed, 8% of the patients experience biopsy-related complications, sometimes with irreversible sequelae (1). Cytology or flow cytometry assessment on cerebrospinal fluid (CSF) or on vitreous humor represent possible diagnostic alternatives to stereotactic biopsy in cases of leptomeningeal or vitreous involvement, respectively.
However, the differential diagnosis with other neurological conditions (such as other CNS primary malignancies, infectious/inflammatory neuro-disorders and neurodegenerative diseases) should be considered. Clinical onset is rarely specific for PCNSL, and sometimes graved of an irreversible diagnostic delay (2-4). Thus, in the last years, many efforts have been made to identify less invasive diagnostic approaches, like the assessment of several biomarkers with the help of more or less modern techniques such as flow cytometry, next generation sequencing (including whole genome/whole-exome sequencing), enzyme-linked immunosorbent assay (ELISA) or polymerase chain reaction (PCR) on biological fluids (blood, CSF and vitreous humor) with the aim to extend the liquid biopsy concept to PCNSL field. In particular, chemokines, genomic fragments such as cell free DNA and microRNA, and transmembrane receptors have demonstrated to be highly sensitive and specific in identifying PCNSL presence especially when detected into the CSF and/or in the vitreous humor instead of blood samples, applying them as promising biomarkers for an early diagnosis of this disease.
Based on this background, the development of alternative strategies to stereotactic biopsy in order to improve early diagnosis of PCNSL could be really beneficial and desirable. Here, we describe the current knowledge and the developed proposals based on the genomic and molecular markers discovered in the PCNSL's research.
We present the following article in accordance with the Narrative Review Reporting Checklist (available at http://dx.doi.org/10.21037/aol-20-56).
Liquid biopsy in PCNSL
MicroRNA (miRNAs)
MicroRNAs (miRNAs) are noncoding RNA molecules that are not translated into a protein. They are composed by small regulatory RNA molecules of 16–22 nucleotides, that bind the 3’-untranslated regions of messenger RNA (mRNA) transcripts and inhibit gene expression at a post-transcriptional level interfering with translational initiation or degradation of mRNA (5,6). MiRNAs have been shown to play key regulatory roles in a wide range of genetic pathways that control cellular differentiation, proliferation and apoptosis in physiologic as well as in several pathological conditions. Particularly, the dysfunctional expression of miRNAs demonstrated to play a direct role in the pathogenesis of tumors, including hematological malignancies, functioning as oncogenes (promotors of gene transcription) and/or tumor suppressors (inhibitors of gene transcription) (5-7). Detection of miRNAs can be performed by quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) both on tumor samples and on biological fluids in the form of circulating miRNAs, that are included in cell membrane–derived particles, such as apoptotic bodies (8).
Fischer et al. investigated a large miRNAs panel on paraffin-embedded biopsy specimens obtained from PCNSL and systemic Diffuse Large B-Cell lymphoma (DLBCL). Eighteen miRNAs were found to be differentially expressed between the two subgroups suggesting a different molecular mechanism in their pathogenesis. MiRNAs upregulated in PCNSL were associated with the Myc pathway (miR-17-5p, miR-20a, miR-9), with blocking of terminal B-cell differentiation (miR-9, miR-30b/c), or with upregulation by inflammatory cytokines (miR-155). Moreover, some tumor-suppressor miRNAs (such as miR-199a, miR-214, miR-193b and miR-145) resulted to be downregulated in PCNSL (9).
Few studies investigated also the diagnostic value of circulating miRNAs in extracellular fluids such as CSF and blood in PCNSLs. Baraniskin et al. collected CSF from patients with histologically confirmed PCNSL (n=23) and from control subjects (n=30) affected by inflammatory CNS disorders or other neurological conditions. A CSF miRNA level diagnostic tree, firstly testing miR-21, followed by testing for miR-19 or miR-92a elevation, demonstrated to have a significant diagnostic value in PCNSLs. This diagnostic proposal demonstrated a sensitivity of 95% and a specificity of 97% in PCNSL diagnosis (10). A subsequent study with a larger cohort of PCNSL patients (n=39), confirmed the previous findings on the proposed diagnostic tree reporting a sensitivity of 97.4% in diagnosing PCNSL. Of note, MiR-21, miR-19b, and miR-92 were detected also on 14 and 8 serum samples belonging to PCNSL and control group, respectively. However, no significant difference on the expression levels on serum samples between two groups was found (11).
Two more recent study reported a positive correlation between miRNA-21 expression (a key oncogene in B-lymphomagenesis) in paired CSF and serum/plasma samples in PCNSL (12,13). Both studies reported a higher expression levels of miR21 on serum/plasma of PCNSL patients compared with those of control groups composed by glioblastoma, healthy people and, in one of the studies, also by subjects with CNS inflammation and brain metastases. Of note, miR21 expression was higher than the other component in the control group, but it resulted relatively lower than those observed in PCNSL patients. Thus, the authors suggested that serum/plasma miR-21 could discriminate PCNSL from other neurologic conditions and in particular glioblastoma. However, further studies demonstrated altered expression of miR-21 on serum was both in glioblastoma and in brain metastatic carcinoma patients (e.g., lung and breast), suggesting the need of more appropriate controls before validating the diagnostic accuracy of serum miR-21 as biomarker for PCNSL (14-17).
MiR-30c seems to support lymphoma cells engraftment into CNS by interaction with CELSR2 (Cadherin EFG LAG seven-pass G-type receptor 2) gene, that controls the development and function of ependymal cilia and, thus, facilitates the circulation of CSF. Decrease of CELSR2 expression leads to impairment of CSF circulation, facilitating CNS engraftment of lymphoma cells after their migration into the CSF from blood circulation (18,19). MiR-30c levels on CSF are different in PCNSL (n=61) and secondary CNS lymphoma (SCNSL) (n=14), showing significantly increased CSF levels in SCNSL patients. These data suggest that CSF miR-30c can be used as a biomarker to distinguish between PCNSL and SCNSL with a sensitivity of 91% and a specificity of 85%. However, a validation in a larger cohort is needed. Interestingly, no significant differences in expression levels of miR-30c in serum between PCNSL patients (n=15) and SCNSL patients (n=4) were found (20).
Baraniskin et al. had reported that high levels of U2 small nuclear RNA fragments (RNU2-1f) on CSF enabled the differentiation of patients with PCNSL (n=72) from the controls, represented by subjects with neurological disorders (n=47), with a sensitivity of 68% and a specificity of 91%. The diagnostic accuracy was further improved by combined determination of RNU2-1f and miR-21 with a sensitivity of 92% and a specificity of 96%. Of note, no significant differences in the expression levels of RNU-1f on serum was found between PCNSL (n=14) and control patients (n=8) (21).
MiRNA detection on biological fluids was performed by means of real-time PCR, an easy reproducible and commercially available technique potentially applicable in clinical practice.
Circulating tumor DNA (ctDNA)
Studies investigating DNA profile on formalin-fixed, Paraffin-embedded (FFPE) or fresh frozen samples of PCNSL have identified multiple recurrent gene mutations, that code members of B-cell receptor (BCR) signaling (i.e., MYD88, CD79B, CARD11, PIM1, CD79A), epigenetic regulators (KMT2D, CREBBP, MEF2B, Blimp-1/PRDM1) and members of cycle/apoptotic signaling pathways (GNA13, MYC, TP53, CCND3, CDKN2A, ATM) (22-26). The most frequent mutated genes result to be MYD88, PIM1, KMT2D and PRDM1 that occurred in a range of 30–66% of PCNSL (27).
In the last years, there has been growing interest in cancer diagnosis using ctDNA as a source for tumor biomarkers investigating their diagnostic accuracy. CtDNA is the result of DNA fragmentation after tumor cells disruption with passive and active mechanisms of cellular lysis (apoptosis/necrosis or phagocytosis by macrophages) with subsequent its release in biological fluids. CtDNAs are composed by 150–180 bp nucleosomes’ fragments containing somatic alterations of tumor cells. They can be exploited to analyze the tumor genotype and could represent a non-invasive and «real-time» access to tumor genomic heterogeneity. Thus, ctDNA appears as a promising tool in early diagnosis, monitoring response during therapy and assessment of relapse in tumoral diseases (28). CtDNA becomes recognizable and detectable when it harbors somatic mutations. Recently, highly sensitive techniques, such as the droplet digital PCR (ddPCR) and next-generation sequencing (NGS), have been employed as investigational strategies to analyze targeted mutations on CSF samples (29-31).
Initially, the detection of somatic alterations with NGS on CSF have showed to have a high accuracy in patients with brain metastatic solid tumors (sensitivity of 63%) and with primary brain tumors (sensitivity of 50%). Contrary, in the control group, represented by subjects without CNS involvement by tumor, no somatic alterations were observed on CSF. Furthermore, ctDNA detectable on CSF resulted to be more sensitive than cytomorphology analysis in identifying leptomeningeal involvement in metastatic CNS solid tumors. Somatic mutations were found in 100% of patients with positive cytology and in 25% of patients with negative cytology (32). Furthermore, a higher sensitivity in detecting ctDNA on CSF than on serum (58% vs. 0%) in patients with tumor limited to CNS was described (33).
CtDNA detectable on CSF has demonstrated to be a more sensitive tool than standard procedures [flow cytometry and cerebral Magnetic resonance imaging (MRI)] to monitor relapse, to identify residual disease and to predict CNS relapse also in cases of CNS lymphomas (primary and secondary) or with DLBCL at high risk of CNS relapse (34).
In PCNSL patients, ctDNAs detectable on serum samples resulted to have a low sensitivity (24%) in predicting the presence of lymphoma. The release of ctDNA in peripheral blood seemed to be independent of tumor volume or cerebral location. It is yet unclear whether the corticosteroids administered before fluid samples collection can influence ctDNA levels (35).
The detection of ctDNA on biological fluids was performed with actually not-commercially available techniques that require a large expertise for interpretation of the results, and this might limit its applicability in clinical practice.
MYD88 mutations and interleukins (ILs)
The L265P mutation of the MYD88 gene is already used in the diagnosis of Waldenstrom macroglobulinemia. Moreover, the MYD88 mutations are more prevalent in the non-germinal center B-cell (non GCB)-type DLBCL and seem to play an important supportive role in the diagnosis of primary vitreoretinal lymphoma (PVRL) (36-38). This genetic alteration has demonstrated to characterize also DLBCL arising in immune-privileged sites (testicular and CNS), occurring in 75% of PCNSL and in 78% of primary testicular lymphoma, while it is much rarer in systemic DLBCL (12%) (23,25,39) (see Table 1).
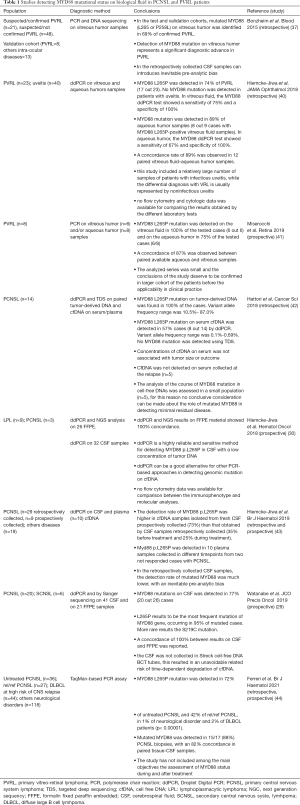
Full table
MYD88 mutations and ILs in PVRL
The detection of MYD88 mutations on vitreous humor has demonstrated to have a high accuracy to identify the presence of DLBCL cells in the vitreous-retina. MYD88 gene mutations were detected in vitreous humor with both PCR and DNA sequencing techniques.
The MYD88 mutations were investigated retrospectively in 75 samples of vitrectomy belonging to 69 patients with suspicious diagnosis of vitreoretinal DLBCL. MYD88 mutation was evaluated in the vitreous humor firstly with an allele-specific PCR technique and subsequently validated by sequencing technics in a separate cohort (n=21) (30,37). Fourteen patients had a confirmed diagnosis of PVRL on cytological findings and/or on clonal rearrangement of immunoglobulin heavy chain (IgH) by PCR, the other 55 patients had final diagnosis of inflammatory disease or suspected PVRL due to presence of clonal IgH rearrangement and of low/small lymphocytes. With allele-specific PCR technique, MYD88 L265P mutation has been detected in 50% of the patients (7 out of 14) with diagnosis of PVRL, and in 11% of the patients (6 out 55) with no confirmed PVRL (3 cases classified as inflammation and 3 as suspected PVRL). DNA sequencing analysis confirmed the presence of MYD88 L265P mutation in all positive cases reported by PCR and additionally identified a rare mutation (p.P258L) in a case resulted MYD88 L265 wild-type (wt). Review of clinical data has confirmed the diagnosis of PVRL in 21 of 69 (30%) patients, including 13 out of the 14 with an initial PVRL diagnosis and in all the 6 carriers of MYD88 L265P mutation originally considered no-diagnostic or suspicious for PVRL. The MYD88 wt patient, initially diagnosed as PVRL based on numerous atypical cells and B-cell clonality, was reclassified as having viral retinitis. Likewise, in the validation cohort of 21 filed DNA samples derived from vitrectomy specimens, MYD88 L265P mutation was identified in 75% of PVRL patients (6 out 8) and in none of 13 reactive samples. Considering the entire population (test and validation cohorts), mutated MYD88 (L265 or P258L) were identified in 69% of clinically, histologically, and molecularly confirmed PVRL. The authors concluded that the presence of MYD88 mutation represents a significant diagnostic advance in this rare entity in which often diagnosis is missing due to the limited material available for analysis (37).
Two studies demonstrated that aqueous humor could represent an alternative sample to the vitreous humor for detecting MYD88 mutation (40,41). Both studies have found a good sensitivity in the aqueous humor (67–75%), with a high concordance between paired available aqueous and vitreous samples (87–89%). In both studies, MYD88 mutation has not been found in the control cohort, represented by patients with uveitis. The aqueous humor, that required a less invasive aspiration procedure than vitreous humor, could be used for MYD88 testing in patients with suspicious of PVRL. However, these conclusions need to be validated in larger cohort.
Beside MYD88, an increased level of interleukin IL10 was found in the vitreous humor. Elevated IL10 and an IL10/IL6 ratio >1.0 resulted to be a useful tool for differentiating between PVRL and intraocular infectious diseases (such as uveitis) with a sensitivity of 74–90% and a specificity of 75–85% (45-48). Moreover, an interleukin score for intra-ocular lymphoma diagnosis (ISOLD) with the aim to predict the probability of PVRL diagnosis based on the levels of IL10 and IL6 on vitreous or aqueous humor was proposed. Subjects with suspicious PVRL were classified into 4 ordinal groups ranging from “certainly not PVRL diagnosis” to “certainly PVRL diagnosis” with an estimated sensitivity and specificity of 93% and 95%, respectively (49).
A prospective study carried out on 56 patients with PVRL suggested that besides the IL10 level and the IL10/IL6 ratio on vitreous humor, also increased levels of IL1 receptor antagonist (IL1RA), chemokines monocyte chemotactic protein (MCP) and of macrophage inflammatory protein (MIP)-1b can contribute in differentiating PVRL from non-PVRL uveitis (50).
MYD88 mutations and ILs in PCNSL
MYD88 mutation assessment on CSF was performed by different researchers’ groups in patients with CNS involvement by large B-cell lymphoma and Waldenstrom macroglobulinaemia (Bing-Neel syndrome) (29,30,35,44,51,52).
MYD88 mutations gene on CSF resulted be retrospectively detectable in 77% (20 out 26) of CNS lymphoma patients (20 with PCNSL and 6 with CNS relapse of systemic lymphoma) (29). The L265P mutation occurred in most of the cases (95%), rarely it was detected the S219C mutation. In 21 cases with available FFPE tissue of primary lymphoma, DNA sequencing of MYD88 was performed also on biopsy tissue, showing a concordance of 100% between CSF and biopsy specimens.
To improve the diagnostic value of MYD88 mutation in PCNSL patients, a recent retrospective/prospective study evaluated the sensitivity and specificity of MYD88 mutational status in combination with high levels of IL6 and IL10, detected on CSF by ELISA (44). A total of 198 HIV-negative adults were enrolled. The study cohort included 63 cases with confirmed PCNSL or at presentation (n=36) or at relapse (n=27), while the control cohort was represented by subjects with neurological disorders currently considered in differential diagnosis with PCNSL (n=118: degenerative and inflammatory disorders, toxic or infective encephalitis, gliomas and n=44: systemic DLBCL at high-risk of CNS dissemination). The concomitant presence of MYD88 mutation and high level of IL10 on the CSF demonstrates to have a sensitivity and specificity to detect PCNSL of 94% and 98%, respectively. The IL10 level and mutational status of MYD88 not result significantly associated with PCNSL features (such as tumor size, the site of involvement and number of lesions). The authors concluded that these results support the routine use of the evaluation of MYD88 mutational status along with the level of IL10 on CSF to diagnose PCNSL, particularly in patients with lesions localized in areas unsuitable for biopsy (i.e., brainstem, spinal cord).
IL10 is an anti-inflammatory cytokine that plays a role in lymphoma development by promoting B-cell lymphoma proliferation and inhibiting apoptosis (53-55). It’s unclear whether CSF IL10 is secreted by tumor cells or by microenvironment. It was observed that the levels of IL10 on CSF in PCNSL patients correlate with infiltration of tumor associated macrophages (TAM) and with TAM cells expressing IL10, suggesting that IL10 may be primarily secreted into the CSF by TAMs (56). However, PCNSL tumor cells express IL10. Furthermore, other brain tumors such as T-cell PCNSL and primary or secondary CNS solid tumors that present TAM infiltration, show negligible IL10 levels into the CSF. To date, the molecular mechanism behind elevated IL10 on CSF of PCNSL remains unclear. However, it has been hypothesized that the excessive activation of the NF-κ B pathway secondary to MYD88 L265P, CD79B and CARD11 mutations can cause an increased secretion of IL-10 with the aim to inhibit tumor microenvironment (38), thus promoting tumorigenesis and progression (57). Of course, further studies are required to confirm this hypothesis.
Whitcup’s group first reported increased level of IL10 on CSF in two cases of PCNSL (58). Subsequently, few studies reported a correlation between CSF IL10 and PCNSL, suggesting that CSF IL10 may act as a biomarker of this disease (59-63). Although, the sensitivity and specificity of CSF IL10 in PCNSL diagnosis varies across the reports. Moreover, to date, some issues remain unanswered and the most important of them looks the optimal cut-off value of IL10 detectable on CSF to distinguish PCNSL from other pathological conditions such as CNS infection, secondary CNS tumors and CNS autoimmune diseases.
Song et al. prospectively investigated the diagnostic value of IL-10, IL-6, IL-8 and tumor necrosis factor α (TNF-α) on CSF of 22 PCNSL patients and 80 patients with other CNS diseases (other brain tumors, neuroinfectious or neurodegenerative diseases). CSF IL10 was significantly higher in PCNSL patients than in the control group (median 74.7 vs. <5.0 pg/mL, P<0.000). Using a CSF IL10 cutoff value of 8.2 pg/mL, the diagnostic sensitivity and specificity for PCNSL were 95% and 96%, respectively. Moreover, using a CSF IL10/IL6 ratio cutoff value of 0.72, the sensitivity was 96%, and the specificity was 100%. Conversely, a low diagnostic value for PCNSL was found with both IL8 and TNF-α. They also investigated the prognostic role of CSF IL10 level observing that an increased value both at diagnosis and post-treatment was predictive for a poor progression free survival (PFS). Based on these results, the authors concluded that increased CSF IL10 was a reliable diagnostic biomarker for PCNSL, and that IL10/IL6 ratio facilitates differentiation from other neurological disorders (63).
MYD88 mutational status and IL6 and IL10 levels on biological fluid were detected by means of RT-PCR and ELISA, respectively. Both these are rapid, easy to assess and commercially-available techniques. For this reason, MYD88 and IL10 represent ideal candidates as detectable biomarkers on biological fluids also in routine clinical practice.
CD79B
CD79B, a gene encoding for the Igβ protein of the BCR, is frequently mutated in PCNSL (64). In particular, mutations of this gene tend to occur in the portion coding the immunoreceptor tyrosine-based activation motif (ITAM) region causing persistently active BCR signaling, and thus NF-κB signaling activation.
Recently, a Japanese group evaluated the mutations of CD79B along with those of MYD88 in the DNA extracted from the vitreous fluid of 17 patients with PVRL upon diagnosis by using direct sequencing and allele-specific PCR (65). MYD88 L265P was detected in 71% of the study population (12/17) consistently with previous report, while the positivity rate of CD79BY196 was 35% (6/17). Among 17 patients, 11 patients (65%) showed CNS progression during the follow-up period of 11–103 months (mean of 38 months). All patients with CD79BY196 mutation developed CNS disease, while by contrast five of the eight patients CD79Bwt did not have CNS disease. Moreover, CNS disease onset was significantly earlier (16.5 months) in patients with mutated CD79B than patients with CD79Bwt (67 months; P=0.0135). Based on these results the authors concluded that, despite the study limitations due to the small sample size and the relatively short observation period, detecting CD79BY196 in vitreous DNA may have a diagnostic and prognostic value in PVRL, thus deserving further evaluation.
Chemokines and soluble ligands
The molecular basis of tropism and selective dissemination of lymphoma within the brain seem to be essential for the pathogenesis of PCNSL. In vitro chemotactic responses by large B-cell lymphoma cells isolated from brain lesions have been demonstrated in response to chemokines ligands 12 (CXCL12) and 13 (CXCL13), that are classified as neurotropic factors (66,67).
Diagnostic sensitivity of IL10 and CXCL13 on CSF samples have been investigated in a retrospective series of 60 PCNSL (43 at presentation and 17 at relapse), 23 SCNSL (10 at the presentation and 13 at relapse) and 137 controls (20 primary or secondary brain tumors, 71 neuro-inflammatory conditions, 46 malignancies and infections originated outside of brain) (60). High concentrations of either CXCL13 (>116 pg/mL) or IL-10 (>23 pg/mL) in the CSF demonstrated to have a sensitivity of 84% and a specificity of 91% in the diagnosis of non-HIV PCNSL. Elevation of both biomarkers into the CSF showed a higher specificity (99%) but at cost of lower sensitivity (50%) in detecting PCNSL. Of note, the authors reported a rate concordance of 89% between the level of IL10 on CSF and the activated Jak-1 expression on the examined biopsy (n=9). This result suggests that intra-tumoral phospho–Jak-1 may be a biomarker of pro-survival cytokine signaling in CNS lymphomas (68). The authors concluded that the assessment of CXCL13 and IL10 on CSF may constitute a complementary diagnostic information in PCNSL cases with sensitivity significantly greater than reference standard CSF analysis (cytology and flow cytometry), and equivalent to the diagnostic sensitivity of stereotactic biopsy. Although a prospective larger cohort and the definition of a more appropriate threshold are needed before translate this conclusion in general practice.
Regulation of B-cell homeostasis involves a complex system that comprises two ligands [B-cell activating factor of the tumor necrosis factor family (BAFF) and a proliferation inducing ligand (APRIL)], and three receptors (B-cell maturation antigen (BCMA), transmembrane activator and CAML interactor (TACI), and BAFF-receptor) (69).
It was demonstrated that soluble TACI (sTACI) and soluble BCMA (sBCMA) levels on CSF reflect the expression of these markers on the cell surface of PCNSL (70,71) and did not correlate necessarily with CSF cell count. A prospective monocentric study investigated the diagnostic potential of sTACI and sBCMA in CSF (n=176) and serum (n=105) as biomarkers of PCNSL. sTACI and sBCMA are significantly increased in patients with PCNSL (sTACI, median: 445 pg/mL; sBCMA, median: 760 pg/mL) compared with controls (subjects with neuro-inflammatory, neuro-infectious, neuro-degenerative disorders or with other brain malignancies). With a cutoff value of 68.4 pg/mL, sTACI shows high sensitivity (88%) and specificity (88%), with a cutoff value of 460 pg/mL sBCMA results less sensitive (73%) and specific (71.8%) for the diagnosis of PCNSL. The combination of both markers increases the specificity (97%), however, at the cost of a lower sensitivity (64%). In serum, both sTACI and sBCMA did not result increased in PCNSL patients. The levels of both markers on CSF were analyzed also during disease course in 24 patients (in 10 at relapse and in 14 at achievement of complete remission). Moreover, long-term longitudinal analysis was performed in two patients. The levels of both sTACI and sBCMA were elevated at the time of diagnosis and during relapses, conversely their levels were low at the time of remission. The authors concluded that sTACI and sBCMA in the CSF are promising new biomarkers for diagnosis and for monitoring therapy response in PCNSL. However, these findings need to be validated in a larger sample size (72).
In a prospective study, levels of APRIL and BAFF were detected on CSF of subjects with suspected focal brain lesions (n=116), including 53 CNS lymphoma (CNSL) patients (PCNSL =46, SCNSL =7). CSF levels of APRIL (above the cut-off value of 6.59 ng/mL) and BAFF (above a cutoff of 299 pg/mL) reliably differentiated CNSL from other focal brain lesions (including primary and metastatic brain tumors, autoimmune-inflammatory lesions, and neuroinfectious lesions) with a specificity of 93.7% and sensitivity of 77% (APRIL, BAFF). Serial CSF analysis of 17 CNSL patients during chemotherapy and at relapse demonstrate a close correlation of APRIL CSF levels and the course of the disease. In this prospective study, the authors illustrated the potential of APRIL and BAFF as reliable diagnostic and therapeutic biomarkers in CNSL. However, they also highlighted that these results must be validated in an independent cohort. Moreover, special considerations should be given for the differential diagnosis with herpes simplex virus encephalitis and multiple sclerosis, conditions where both these ligands play a central role in the inflammatory process (73).
The detection of the above reported cytokines and ligands were performed with ELISA, a laboratory test already widely used in most centers.
Other biomarkers
Other biomarkers, initially considered promising to predict PCNSL diagnosis, proved to be less sensitive in more extended studies. One example is represented by CD27, that is a receptor expressed in the most of the B-cell malignancies (74). Kersten et al. demonstrated that soluble CD27 (sCD27) is increased in the CSF in case of leptomeningeal involvement by acute lymphoblastic leukemia and lymphoid malignancies included PCNSL. A high diagnostic value of CSF sCD27 in detecting leptomeningeal involvement with a sensitivity of 100% and specificity of more than 80% was reported (75-77). However, the inadequate control groups and small numbers of analyzed population may have represented a possible bias in the interpretation of the final results. High levels of sCD27 in the CSF were also reported in CNS infectious and inflammatory diseases. Subsequent studies did not confirm the diagnostic accuracy of CSF sCD27 to predict leptomeningeal involvement by B-cell malignancies showing a low positive predictive value (54%) (78,79).
Pro-inflammatory cytokines detectable on CSF such as neopterin and osteopontin were proposed as potential diagnostic biomarkers for CNS lymphoma demonstrating higher levels in this subgroup of patients respect to a control group composed by CNS inflammatory disorders, brain tumors other than lymphoma or healthy people (80,81). However, these results need to be validated in larger prospective studies.
Conclusions
To date, early diagnosis using a minimally invasive diagnostic procedure represents one of the major challenges for improving the management of patients with neuro-radiological suspicion of PCNSL. Indeed, PCNSL diagnosis is still based on stereotactic biopsy and histopathological analysis. However, in clinical practice, the collection of tumor tissue is a highly invasive procedure hampered by the risk of both severe complications such as cerebral bleeding and “inconclusive biopsy” because of geographical misses in case of deep lesions. The researchers’ efforts in the last years have brought into the light some biomarkers released and detectable in the biological fluids, represented by genomic and molecular material suggestive for the presence of lymphoma cells in the CNS and in the vitreoretinal compartment (see Table 2). All these biomarkers could be potentially used for the so-called “liquid biopsy”, a non-invasive method to detect tumor-related aberrations using biological fluid samples (blood, CSF and vitreous humor), instead of lymphoma tissue. In particular, mutated MYD88 and high levels of IL-10 detected on CSF and/or on vitreous humor represent promising ideal diagnostic biomarkers for PCNSL and PVRL. The use of easy, rapid and commercially available techniques for their detections, makes possible the routine assessment of these two biomarkers. However, potential implications of these diagnostic procedures in patients with suspected PCNSL deserve to be explored in future trials. Indeed, some important questions still remain open and deserve to be investigated in prospective studies. In particular, how the steroid therapy, performed before collecting biological fluid samples for the detection of the reported biomarkers, and how the biopsy of the primary lesion could influence the release of such biomarkers are unknown. The CSF represents the better sample for the detection of circulating biomarkers in PCNSL patients, because of its higher diagnostic accuracy respect to other biological fluids like the peripheral blood. However, some conditions, also if rare, can contraindicate the spinal tap. For this reason, further efforts should be carried out in order to identify alternative fluid samples or other surrogate to CSF analysis. Moreover, as suggested by the several biomarkers already studied, the way to obtain a reliable liquid biopsy in PCNSL is still long and need the collaborative efforts of clinical and preclinical researchers. Indeed, we have to keep in mind that this technique not only has the potential to allow early diagnosis, but could also include the possibility to evaluate minimal residual disease, to study the clonal evolution over time, and to represent a potential prognostic and predictive tool which might help to improve, in a more comprehensive way, the outcome and quality of life of PCNSL patients.
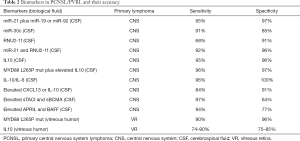
Full table
Acknowledgments
We are grateful to all our colleagues of the Lymphoma Unit of the San Raffaele Scientific Institute in Milan, Italy for the support and collaboration always shown in the care of our patients. Moreover, a special thanks goes to our mentor, Dr. Andrés J. M. Ferreri for guiding us both in clinic and science.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Andrés J. M. Ferreri, Maurilio Ponzoni) for the series “Central Nervous System Lymphomas” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (http://dx.doi.org/10.21037/aol-20-56). The series “Central Nervous System Lymphomas” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khatab S, Spliet W, Woerdeman PA. Frameless image-guided stereotactic brain biopsies: emphasis on diagnostic yield. Acta Neurochir (Wien) 2014;156:1441-50. [Crossref] [PubMed]
- Küker W, Nägele T, Korfel A, et al. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol 2005;72:169-77. [Crossref] [PubMed]
- Zacharia TT, Law M, Naidich TP, et al. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy. J Neuroimaging 2008;18:411-7. [Crossref] [PubMed]
- Ricard D, Idbaih A, Ducray F, et al. Primary brain tumours in adults. Lancet 2012;379:1984-96. [Crossref] [PubMed]
- Lawrie CH. MicroRNAs and haematology: small molecules, big function. Br J Haematol 2007;137:503-12. [Crossref] [PubMed]
- Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 2009;10:704-14. [Crossref] [PubMed]
- Lawrie CH, Gal S, Dunlop HM, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol 2008;141:672-5. [Crossref] [PubMed]
- Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci 2010;101:2087-92. [Crossref] [PubMed]
- Fischer L, Hummel M, Korfel A, et al. Differential microRNA expression in primary CNS and nodal diffuse large B-cell lymphomas. Neuro Oncol 2011;13:1090-8. [Crossref] [PubMed]
- Baraniskin A, Kuhnhenn J, Schlegel U, et al. Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood 2011;117:3140-6. [Crossref] [PubMed]
- Baraniskin A, Kuhnhenn J, Schlegel U, et al. MicroRNAs in cerebrospinal fluid as biomarker for disease course monitoring in primary central nervous system lymphoma. J Neurooncol 2012;109:239-44. [Crossref] [PubMed]
- Mao X, Sun Y, Tang J. Serum miR-21 is a diagnostic and prognostic marker of primary central nervous system lymphoma. Neurol Sci 2014;35:233-8. [Crossref] [PubMed]
- Yang K, Wang S, Cheng Y, et al. Role of miRNA-21 in the diagnosis and prediction of treatment efficacy of primary central nervous system lymphoma. Oncol Lett 2019;17:3475-81. [Crossref] [PubMed]
- Malzkorn B, Wolter M, Liesenberg F, et al. Identification and functional characterization of microRNAs involved in the malignant progression of gliomas. Brain Pathol 2010;20:539-50. [Crossref] [PubMed]
- Hayashita Y, Osada H, Tatematsu Y, et al. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res 2005;65:9628-32. [Crossref] [PubMed]
- Kim K, Chadalapaka G, Lee SO, et al. Identification of oncogenic microRNA-17-92/ZBTB4/specificity protein axis in breast cancer. Oncogene 2012;31:1034-44. [Crossref] [PubMed]
- Baraniskin A, Kuhnhenn J, Schlegel U, et al. Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro Oncol 2012;14:29-33. [Crossref] [PubMed]
- Wang XJ, Zhang DL, Xu ZG, et al. Understanding cadherin EGF LAG seven-pass G-type receptors. J Neurochem 2014;131:699-711. [Crossref] [PubMed]
- Tissir F, Qu Y, Montcouquiol M, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci 2010;13:700-7. [Crossref] [PubMed]
- Baraniskin A, Chomiak M, Ahle G, et al. MicroRNA-30c as a novel diagnostic biomarker for primary and secondary B-cell lymphoma of the CNS. J Neurooncol 2018;137:463-8. [Crossref] [PubMed]
- Baraniskin A, Zaslavska E, Nöpel-Dünnebacke S, et al. Circulating U2 small nuclear RNA fragments as a novel diagnostic biomarker for primary central nervous system lymphoma. Neuro Oncol 2016;18:361-7. [Crossref] [PubMed]
- Fukumura K, Kawazu M, Kojima S, et al. Genomic characterization of primary central nervous system lymphoma. Acta Neuropathol 2016;131:865-75. [Crossref] [PubMed]
- Chapuy B, Roemer MG, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood 2016;127:869-81. [Crossref] [PubMed]
- Zhou Y, Liu W, Xu Z, et al. Analysis of Genomic Alteration in Primary Central Nervous System Lymphoma and the Expression of Some Related Genes. Neoplasia 2018;20:1059-69. [Crossref] [PubMed]
- Kraan W, Horlings HM, van Keimpema M, et al. High prevalence of oncogenic MYD88 and CD79B mutations in diffuse large B-cell lymphomas presenting at immune-privileged sites. Blood Cancer J 2013;3:e139 [Crossref] [PubMed]
- Yamada S, Ishida Y, Matsuno A, et al. Primary diffuse large B-cell lymphomas of central nervous system exhibit remarkably high prevalence of oncogenic MYD88 and CD79B mutations. Leuk Lymphoma 2015;56:2141-5. [Crossref] [PubMed]
- Bödör C, Alpár D, Marosvári D, et al. Molecular Subtypes and Genomic Profile of Primary Central Nervous System Lymphoma. J Neuropathol Exp Neurol 2020;79:176-83. [Crossref] [PubMed]
- Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011;11:426-37. [Crossref] [PubMed]
- Watanabe J, Natsumeda M, Okada M, et al. High Detection Rate of MYD88 Mutations in Cerebrospinal Fluid From Patients With CNS Lymphomas. JCO Precis Oncol 2019;3:1-13. [Crossref]
- Hiemcke-Jiwa LS, Minnema MC, Radersma-van Loon JH, et al. The use of droplet digital PCR in liquid biopsies: A highly sensitive technique for MYD88 p.(L265P) detection in cerebrospinal fluid. Hematol Oncol 2018;36:429-35. [Crossref] [PubMed]
- Kurtz DM, Scherer F, Jin MC, et al. Circulating Tumor DNA Measurements as Early Outcome Predictors in Diffuse Large B-Cell Lymphoma. J Clin Oncol 2018;36:2845-53. [Crossref] [PubMed]
- Pentsova EI, Shah RH, Tang J, et al. Evaluating Cancer of the Central Nervous System Through Next-Generation Sequencing of Cerebrospinal Fluid. J Clin Oncol 2016;34:2404-15. [Crossref] [PubMed]
- De Mattos-Arruda L, Mayor R, Ng CKY, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun 2015;6:8839. [Crossref] [PubMed]
- Bobillo S, Crespo M, Escudero L, et al. Cell free circulating tumor DNA in cerebrospinal fluid detects and monitors central nervous system involvement of B-cell lymphomas. Haematologica 2021;106:513-21. [Crossref] [PubMed]
- Fontanilles M, Marguet F, Bohers É, et al. Non-invasive detection of somatic mutations using next-generation sequencing in primary central nervous system lymphoma. Oncotarget 2017;8:48157-68. [Crossref] [PubMed]
- Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med 2012;367:826-33. [Crossref] [PubMed]
- Bonzheim I, Giese S, Deuter C, et al. High frequency of MYD88 mutations in vitreoretinal B-cell lymphoma: a valuable tool to improve diagnostic yield of vitreous aspirates. Blood 2015;126:76-9. [Crossref] [PubMed]
- Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011;470:115-9. [Crossref] [PubMed]
- King RL, Goodlad JR, Calaminici M, et al. Lymphomas arising in immune-privileged sites: insights into biology, diagnosis, and pathogenesis. Virchows Arch 2020;476:647-65. [Crossref] [PubMed]
- Hiemcke-Jiwa LS, Ten Dam-van Loon NH, Leguit RJ, et al. Potential Diagnosis of Vitreoretinal Lymphoma by Detection of MYD88 Mutation in Aqueous Humor With Ultrasensitive Droplet Digital Polymerase Chain Reaction. JAMA Ophthalmol 2018;136:1098-104. [Crossref] [PubMed]
- Miserocchi E, Ferreri AJM, Giuffrè C, et al. MYD88 L265P Mutation Detection In The Aqueous Humor Of Patients With Vitreoretinal Lymphoma. Retina 2019;39:679-84. [Crossref] [PubMed]
- Hattori K, Sakata-Yanagimoto M, Suehara Y, et al. Clinical significance of disease-specific MYD88 mutations in circulating DNA in primary central nervous system lymphoma. Cancer Sci 2018;109:225-30. [Crossref] [PubMed]
- Hiemcke-Jiwa LS, Leguit RJ, Snijders TJ, et al. MYD88 p.(L265P) detection on cell-free DNA in liquid biopsies of patients with primary central nervous system lymphoma. Br J Haematol 2019;185:974-7. [Crossref] [PubMed]
- Ferreri AJM, Calimeri T, Lopedote P, et al. Myd88 L265P mutation and interleukin-10 detection in cerebrospinal fluid are highly specific discriminating markers in patients with primary CNS lymphoma: results from a prospective study. Br J Haematol 2021;193:497-505. [Crossref] [PubMed]
- Kimura K, Usui Y, Goto H. Clinical features and diagnostic significance of the intraocular fluid of 217 patients with intraocular lymphoma. Jpn J Ophthalmol 2012;56:383-9. [Crossref] [PubMed]
- Usui Y, Wakabayashi Y, Okunuki Y, et al. Immune Mediators in Vitreous Fluids from Patients with Vitreoretinal B-Cell Lymphoma. Invest Ophthalmol Vis Sci 2012;53:5395-402. [Crossref] [PubMed]
- Chan CC. Molecular pathology of primary intraocular lymphoma. Trans Am Ophthalmol Soc 2003;101:275-92. [PubMed]
- Wolf LA, Reed GF, Buggage RR, et al. Vitreous cytokine levels. Ophthalmology 2003;110:1671-2. [Crossref] [PubMed]
- Costopoulos M, Touitou V, Golmard JL, et al. ISOLD: A New Highly Sensitive Interleukin Score for Intraocular Lymphoma Diagnosis. Ophthalmology 2016;123:1626-8. [Crossref] [PubMed]
- de Hoog J, Dik WA, Lu L, et al. Combined cellular and soluble mediator analysis for improved diagnosis of vitreoretinal lymphoma. Acta Ophthalmol 2019;97:626-32. [Crossref] [PubMed]
- Castillo JJ, D'Sa S, Lunn MP, et al. Central nervous system involvement by Waldenstrom macroglobulinaemia (Bing-Neel syndrome): a multi‐institutional retrospective study. Br J Haematol 2016;172:709-15. [Crossref] [PubMed]
- Poulain S, Boyle EM, Roumier C, et al. MYD88 L265P mutation contributes to the diagnosis of Bing Neel syndrome. Br J Haematol 2014;167:506-13. [Crossref] [PubMed]
- Masood R, Zhang Y, Bond MW, et al. Interleukin-10 is an autocrine growth factor for acquired immunodeficiency syndrome-related B-cell lymphoma. Blood 1995;85:3423-30. [Crossref] [PubMed]
- Alas S, Emmanouilides C, Bonavida B. Inhibition of interleukin 10 by rituximab results in down-regulation of bcl-2 and sensitization of B-cell non-Hodgkin's lymphoma to apoptosis. Clin Cancer Res 2001;7:709-23. [PubMed]
- Gupta M, Han JJ, Stenson M, et al. Elevated serum IL-10 levels in diffuse large B-cell lymphoma: a mechanism of aberrant JAK2 activation. Blood 2012;119:2844-53. [Crossref] [PubMed]
- Sasayama T, Tanaka K, Mizowaki T, et al. Tumor-Associated Macrophages Associate with Cerebrospinal Fluid Interleukin-10 and Survival in Primary Central Nervous System Lymphoma (PCNSL). Brain Pathol 2016;26:479-87. [Crossref] [PubMed]
- Sabat R, Grütz G, Warszawska K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev 2010;21:331-44. [Crossref] [PubMed]
- Whitcup SM, Stark-Vancs V, Wittes RE, et al. Association of interleukin 10 in the vitreous and cerebrospinal fluid and primary central nervous system lymphoma. Arch Ophthalmol 1997;115:1157-60. [Crossref] [PubMed]
- Sasayama T, Nakamizo S, Nishihara M, et al. Cerebrospinal fluid interleukin-10 is a potentially useful biomarker in immunocompetent primary central nervous system lymphoma (PCNSL). Neuro Oncol 2012;14:368-80. [Crossref] [PubMed]
- Rubenstein JL, Wong VS, Kadoch C, et al. CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood 2013;121:4740-8. [Crossref] [PubMed]
- Sasagawa Y, Akai T, Tachibana O, et al. Diagnostic value of interleukin-10 in cerebrospinal fluid for diffuse large B-cell lymphoma of the central nervous system. J Neurooncol 2015;121:177-83. [Crossref] [PubMed]
- Nguyen-Them L, Costopoulos M, Tanguy ML, et al. French LOC Network for CNS Lymphoma. The CSF IL-10 concentration is an effective diagnostic marker in immunocompetent primary CNS lymphoma and a potential prognostic biomarker in treatment-responsive patients. Eur J Cancer 2016;61:69-76. [Crossref] [PubMed]
- Song Y, Zhang W, Zhang L, et al. Cerebrospinal Fluid IL-10 and IL-10/IL-6 as Accurate Diagnostic Biomarkers for Primary Central Nervous System Large B-cell Lymphoma. Sci Rep 2016;6:38671. [Crossref] [PubMed]
- Nakamura T, Tateishi K, Niwa T, et al. Recurrent mutations of CD79B and MYD88 are the hallmark of primary central nervous system lymphomas. Neuropathol Appl Neurobiol 2016;42:279-90. [Crossref] [PubMed]
- Yonese I, Takase H, Yoshimori M, et al. CD79B mutations in primary vitreoretinal lymphoma: Diagnostic and prognostic potential. Eur J Haematol 2019;102:191-6. [Crossref] [PubMed]
- Fischer L, Korfel A, Pfeiffer S, et al. CXCL13 and CXCL12 in central nervous system lymphoma patients. Clin Cancer Res 2009;15:5968-73. [Crossref] [PubMed]
- Smith JR, Braziel RM, Paoletti S, et al. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood 2003;101:815-21. [Crossref] [PubMed]
- Smith JR, Falkenhagen KM, Coupland SE, et al. Malignant B cells from patients with primary central nervous system lymphoma express stromal cell-derived factor-1. Am J Clin Pathol 2007;127:633-41. [Crossref] [PubMed]
- Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol 2009;9:491-502. [Crossref] [PubMed]
- Hoffmann FS, Kuhn PH, Laurent SA, et al. The immunoregulator soluble TACI is released by ADAM10 and reflects B cell activation in autoimmunity. J Immunol 2015;194:542-52. [Crossref] [PubMed]
- Laurent SA, Hoffmann FS, Kuhn PH, et al. γ-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun 2015;6:7333. [Crossref] [PubMed]
- Thaler FS, Laurent SA, Huber M, et al. Soluble TACI and soluble BCMA as biomarkers in primary central nervous system lymphoma. Neuro Oncol 2017;19:1618-27. [Crossref] [PubMed]
- Mulazzani M, Huber M, Borchard S, et al. APRIL and BAFF: novel biomarkers for central nervous system lymphoma. J Hematol Oncol 2019;12:102. [Crossref] [PubMed]
- van Oers MH, Pals ST, Evers LM, et al. Expression and release of CD27 in human B-cell malignancies. Blood 1993;82:3430-6. [Crossref] [PubMed]
- Kersten MJ, Evers LM, Dellemijn PL, et al. Elevation of cerebrospinal fluid soluble CD27 levels in patients with meningeal localization of lymphoid malignancies. Blood 1996;87:1985-9. [Crossref] [PubMed]
- Murase S, Saio M, Takenaka K, et al. Increased levels of CSF soluble CD27 in patients with primary central nervous system lymphoma. Cancer Lett 1998;132:181-6. [Crossref] [PubMed]
- Murase S, Saio M, Andoh H, et al. Diagnostic utility of CSF soluble CD27 for primary central nervous system lymphoma in immunocompetent patients. Neurol Res 2000;22:434-42. [Crossref] [PubMed]
- van den Bent MJ, Lamers CH, van ‘t Veer MB, et al. Increased levels of soluble CD27 in the cerebrospinal fluid are not diagnostic for leptomeningeal involvement by lymphoid malignancies. Ann Hematol 2002;81:187-91. [Crossref] [PubMed]
- Hintzen RQ, van Lier RA, Kuijpers KC, et al. Elevated levels of a soluble form of the T cell activation antigen CD27 in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol 1991;35:211-7. [Crossref] [PubMed]
- Viaccoz A, Ducray F, Tholance Y, et al. CSF neopterin level as a diagnostic marker in primary central nervous system lymphoma. Neuro Oncol 2015;17:1497-503. [Crossref] [PubMed]
- Strehlow F, Bauer S, Martus P, et al. Osteopontin in cerebrospinal fluid as diagnostic biomarker for central nervous system lymphoma. J Neurooncol 2016;129:165-71. [Crossref] [PubMed]
Cite this article as: Steffanoni S, Calimeri T. Narrative review: biomarkers, a hope towards early diagnosis in primary CNS lymphoma. Ann Lymphoma 2021;5:19.


