
Overcoming the blood-brain barrier in primary central nervous system lymphoma: a review on new strategies to solve an old problem
Introduction
Primary central nervous system (CNS) lymphoma (PCNSL) is an aggressive malignancy confined to the CNS, with rare cases of extra-CNS dissemination, characterized by a diffuse large B-cell lymphoma (DLBCL) morphology in more than 95% of cases and a peculiar molecular profile. For this reason, this disease is classified as a distinct entity named “primary diffuse large B-cell lymphoma of the CNS” in the 2017 WHO classification of haematopoietic and lymphoid tumors (1-4).
PCNSL still represents a challenge from both diagnostic and therapeutic point of views. As for other brain tumors, imaging is based on gadolinium-enhanced MRI scans, on which PCNSL often has a peculiar appearance (5): on unenhanced acquisitions the lesions are hypo- to iso-intense at T1- and T2-weighted imaging, while they show an intense and homogeneous contrast enhancement after contrast injection with restricted diffusion. Usually, the vasogenic edema is small since the mass effect is little, independently of tumor dimensions. These features are related to multiple factors, such as presence of densely packed cells, high nuclear/cytoplasmic ratio, and an altered BBTB.
However, the results of histopathology studies and tumor imaging at relapse suggest that PCNSL is a whole brain disease. Indeed, while autopsy studies reveal that most PCNSL extensively infiltrate the brain, no correlation occurs between autopsy findings and neuroimaging assessment of tumor burden (5-7). This is due to the fact that the current neuroimaging techniques are inadequate to identify the presence of microscopic tumor foci because of the presence of an intact blood brain barrier (BBB) in these tumor areas that impairs the passage of contrast agents.
The BBB is not an immutable entity, experiencing significant changes in response to pathologies affecting the CNS. In the presence of a primary or secondary brain tumor, for example, it is now customary to refer to a blood-brain tumor barrier (BBTB) rather than to a BBB, to evidence differences between the two (8). Some of these differences are the consequence of the abnormal angiogenesis that characterizes tumor formation (9), of inflammatory changes that are closely intertwined with neoangiogenic changes (10) and of the compression of existing vessels by the growing tumor, thereby impairing blood flow (11). These alterations lead to increased leakiness of the BBTB, which, however, is very heterogeneous even within individual malignant foci (12) and, therefore, inappropriate to be exploited for a homogeneous drug delivery within affected brain areas.
Accordingly, the BBTB represents a major obstacle also for chemotherapy regimens considered standard treatment for extra-CNS DLBCL, as demonstrated by the negative results obtained with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) (13) or similar regimen (14) as part of the first-line treatment for PCNSL patients. Indeed, these drugs, which represent in combination with rituximab the preferred choice for patients with systemic DLBCL, cannot cross the BBB and achieve efficacious concentrations in tumor tissues (15). Because of this limitation, clinical studies in this setting are taking advantage of the use of high-dose drug combinations, with methotrexate (MTX) as the key drug (16). However, these combinations require hospitalization and extensive clinical experience for the management of side effects (17). Thus, the development of alternative strategies for PCNSL treatment are highly desirable. Here, we describe new strategies aimed at overcoming the BBB for more efficient drug delivery in patients with PCNSL, with a special focus on approaches based on tumor vascular targeting with TNF.
Literature search: methods
Published studies were identified through a search of PubMed using the reported keywords alone or combined. No filters have been added regarding publication dates, article types (i.e., review, meta-analysis, clinical trial) and text availability (abstract or full text). However, the reviewed literature was limited to studies published in English language in peer-reviewed, high-quality international journals.
The impact of BBB on neuro-imaging in PCNSL
The most commonly used response criteria for PCNSL were published by the International PCNSL Collaborative Group (IPCG) (18). The gold-standard imaging of the brain parenchyma and tumor responses is represented by gadolinium-enhanced MRI: in this setting, the complete disappearance of all enhancing abnormalities is required to define a complete response (CR). However, some observations related to the pattern of relapses in PCNSL patients support the idea that other neuro-imaging features could represent potential disease foci. Indeed, a study conducted on 85 patients with PCNSL enrolled in a prospective trial showed that in >50% of patients the tumor relapse in the brain could involve a site distant from that of the initial tumor (19). In particular, the authors noted that some relapses occurred where non-enhancing T2-FLAIR hypersignal lesions were located at baseline, which markedly decreased (>50%) after chemotherapy (supporting their neoplastic nature). Another study evaluated the images of 37 patients relapsed after treatment with intra-arterial high-dose metotrexate (HD-MTX) with or without rituximab combined with osmotic disruption of the BBTB. At relapse, the new enhancement occurred in a spatially distinct site in 81% of patients suggesting a possible reactivation of occult lymphoma cell reservoirs behind an intact BBTB (20). The results of these studies suggest that BBTB alterations in tumors may occur at different degrees in different tumor regions, with certain areas in which the BBTB is unaltered at all. This is an important observation, since the penetration and distribution of low-molecular weight MRI contrast agents are limited by an intact BBB; consequently, the tumor burden could be underestimated when the current methodologies and criteria are used. The need of improving the current imaging techniques is, therefore, a key point in PCNSL management, because of the potential impact on patients’ outcome. However, to date, advanced MRI techniques, such as dynamic contrast-enhanced MRI (DCE-MRI) and PET imaging using different tracer (21-23) are limited to clinical trials.
The BBB and therapy with anti-CD20 monoclonal antibodies (mAb)
PCNSL as other B-cell neoplasms expresses CD20. Thus, Rituximab, an anti-CD20 mAb largely used in the treatment of non-Hodgkin lymphoma (NHL), was evaluated also in this setting (24). However, because of its large size, the penetration of this mAb into the brain is restricted to areas of bulky tumor with a very leaky BBTB. Furthermore, also in these areas the barrier tends to reconstitute after therapy-induced tumor shrinkage (25). In PCNSL patients, the levels of rituximab in the cerebral spinal fluid (CSF) were shown to reach only 0.1% of the serum concentration after intravenous administration, while in patients with active leptomeningeal disease, it increases to 3–4% suggesting that BBTB alteration, as it occurs in active leptomeningeal disease, allows antibody penetration to some extent (26). Because of this limitation a phase I dose-escalation study has been performed in 10 patients with recurrent CNS NHL using intrathecal rituximab monotherapy (27). Rituximab was administered through an Ommaya reservoir at 3 different doses (10, 25 or 50 mg) in the first week and twice per week thereafter (day 1 and day 4) for 4 weeks, for a maximum of nine doses. The maximum tolerated dose (MTD) was 25 mg and a significant, rapid distribution from the site of rituximab administration to the brain ventricles and throughout the cranio-spinal axis was demonstrated. An encouraging anti-CNS lymphoma activity and clinical benefit were registered, with cytologic responses in 6 patients, intraocular improvement in two, and resolution of brain parenchymal lymphoma in one. However, the duration of response was very short, with meningeal or parenchymal recurrence occurring in almost all cases within a few weeks. The authors suggested that this study could represent the basis for a combined intra-CSF injection of rituximab plus methotrexate therapy in the treatment of recurrent CNS and ocular lymphomas.
MAb penetration across the BBB in PCNSL has also been evaluated using a murine anti-CD20 mAb (ibritumomab) coupled to a linker-chelator (tiuxetan), a conjugate that allows the incorporation of radioisotopes for both imaging studies and radioimmunotherapy. In particular in a single center prospective study this conjugate was labelled with both 111In, a γ-emitting radioisotope, for biodistribution and dosimetry study, and 90Y, a pure γ-emitter of ionizing radiation, for therapeutic activity (28). The median absorbed dose delivered to the brain lesions was 701 cGy, which is lower compared to prior studies on systemic lymphomas (1,484 cGy), but higher than that observed in normal brain in the same study (70 cGy). However, only 2 out of 6 enrolled patients responded with short-lasting, non-complete responses. There were no infusion-related reactions. Toxicities were mainly hematological with no cases of neurotoxicity, myelodysplasia or secondary malignancies, but the follow-up and survival periods were short. MAb uptake in PCNSL after systemic intravenous administration was demonstrated also in another similar study where SPECT imaging after 111In-ibritumomab tiuxetan provided evidence that the mAb is able to cross the BBTB and to accumulate within the target area 48 h and more after injection (6). In this study, nine patients received treatment with 90Y-ibritumomab tiuxetan and 4 responded (2 CR and 2 PR), and almost all were taking concomitant steroids. Moreover, also in this case responses were of short duration with only one CR lasting 30+ months, while all the others were <4 weeks. Of note, relapses occurred distant to target lesions, confirming the incapability of this large molecular weight molecule to cross the BBTB in those areas and the presence of tumor foci “invisible” to current neuroimaging. In addition, a delayed hemato-toxicity was observed, causing infectious complications and interfering with salvage treatment. Again, no acute neurotoxicity was registered.
Based on these results, despite the evidence of tumor penetration, the use of monotherapy with 90Y-ibritumomab tiuxetan did not prove to be sufficiently efficacious for the treatment of recurrent PCNSL and was also burdened by undesirable side effects. Thus, this drug was abandoned as a therapeutic option.
Targeted therapies
During the last years, following the acquisition of knowledge regarding the role of the B-cell receptor (BCR) pathway as a key mechanism in the pathogenesis of PCNSL (4,29,30), several clinical trials investigated the activity of novel agents in the R/R setting with encouraging results (31-38). Besides the targeted mechanism, these molecules have the advantage of being small and thus able to cross the BBB without the need of disrupting it, as demonstrated both for ibrutinib and lenalidomide.
CSF ibrutinib concentrations were evaluated two hours post-dose at two different time points (day 1 and day 29) that had been chosen based on the reported median time of ibrutinib peak plasma concentrations. A trend to increased CSF concentrations was observed in patients receiving a higher ibrutinib dose (840 vs. 560 mg) and after one month of therapy (33).
CSF penetration of lenalidomide at 10-, 15-, and 20-mg dose levels has been measured on a total of 34 time-matched plasma-CSF sample pairs collected at through time points (∼16 hours after lenalidomide dose). As for ibrutinib, a trend linked to a dose-dependency has been recorded: the estimated CSF/plasma partition coefficient was 10%, 20.4% and 25.5% at 10-, 15-, and 20-mg, respectively. Of note, lenalidomide was detected in the ventricular CSF of 2 patients treated at 10- and 20-mg dose levels with no radiographic or cytologic evidence of brain or leptomeningeal disease. Moreover, both of them had normal CSF protein concentration, supporting the idea that CSF penetration of lenalidomide does not require a disrupted BBB (39).
Strategies for opening the BBB
Failure to efficiently deliver therapeutics to cancer cells behind a functional BBTB is one of the major causes for disease recurrence in PCNSL and other brain tumors. Strategies aimed at improving drug penetration in these settings are, therefore, of great interest (Table 1). Many alternatives, both invasive and noninvasive, have been already explored in preclinical (43) and clinical studies of PCNSL (44). In particular the use of focused ultrasounds and hyperosmotic solutions have been largely evaluated for this purpose.
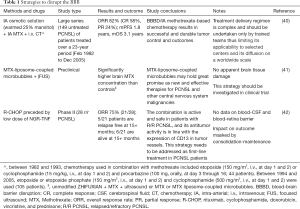
Full table
A transient BBTB disruption can be achieved using focused ultrasounds (FUS), which can induce a reversible BBTB disruption in a targeted region-of-interest (ROI) by opening capillary endothelial cell tight junctions (45,46). Preclinical studies in murine models demonstrated that FUS, preceded by intravenous injection of microbubbles, can open the BBB without causing apparent tissue damages (47). Moreover, acoustically active microbubbles have been investigated as drug carriers across the BBB (48). In particular, MTX-liposome-coupled microbubbles have shown higher cytotoxic effects against cancer cells and a more efficient delivery of MTX to the brain via targeted disruption of the BBB when coupled with FUS. The authors concluded that these strategies could represent interesting alternatives for the treatment of CNS lymphoma and other CNS malignancies (41). However, for this purpose the use of microbubbles and FUS is still limited to pre-clinical trials.
More than ten years ago, Angelov et al. published results on transient reversible BBB disruption by intra-arterial infusion (BBBD/IA) of an osmotic solution before MTX administration using the same route (40). Patients (n=149) with newly diagnosed PCNSL were treated. Overall response rate was 82% (57.8% complete; 24.2% partial) with a median PFS of 1.8 years (5-year PFS of 31% and 7-year PFS of 25%); median OS was 3.1 years. Focal seizures (9.2%) were the most frequent side effects, but lacked long-term sequelae. Of note, 9 (9.4%) of the 96 deaths occurred within 30 days after BBBD/IA because of pulmonary embolism, infections, complications related to carotid dissection, myocardial infarction, heart failure, and unknown cause (one each). Using this approach, BBBD/IA enhanced drug delivery by as much as 50- to 100-fold not only to diseased brain areas, but also to other areas of the brain and cerebrospinal fluid (CSF) (49). However, the same authors underscored the fact that this treatment regimen is complex and should be undertaken only by trained personnel at hospitals where neuro-oncology, interventional neurosurgery/neuroradiology, neuro-anesthesia, and experienced oncology nursing are present.
NGR-TNF: an agent targeting the tumor vasculature
Recent studies suggested that TNF, an inflammatory cytokine capable of altering the endothelial barrier function, might be used for enhancing the permeability of the BBB (50). Indeed, these studies have shown that intravenous delivery of TNF to mice bearing brain metastases of breast cancer can induce BBTB permeabilization and enhance tumor penetration of a therapeutic agent (50). Unfortunately, systemic administration of TNF in patients with CNS or extra-CNS tumors is limited by prohibitive systemic toxicity (51). For this reason, high TNF doses have been used only for the locoregional treatment of sarcomas confined to the extremities through the use of isolated limb perfusion in combination with melphalan (52,53). In this setting, TNF can promote the penetration of melphalan in tumor tissues and exert significant therapeutic effects, suggesting that this cytokine can indeed be exploited as an anticancer agent if its systemic toxicity is kept under control.
A growing body of evidence suggests that the therapeutic index of TNF can be improved by targeted delivery strategies (51). Among the various approaches so far developed for this purpose, TNF fusion with a peptide containing the NGR motif is one of the most deeply investigated to date. NGR is a tumor-homing tripeptide sequence originally discovered by panning peptide-phage libraries in tumor-bearing mice, which can specifically recognize a membrane-bound form of aminopeptidase N (CD13) up-regulated in angiogenic vessels, including angiogenic tumor vessels (54-59). Because of this property, NGR peptides have been exploited as vehicles for ligand-directed delivery to tumor vessels of a large variety of therapeutic and diagnostic compounds, including chemotherapeutic drugs, liposomes, anti-angiogenic compounds, DNA complexes, viral particles, imaging compounds and cytokines (51,60-72). A recombinant CNGRCG-TNF fusion protein (for brevity called NGR-TNF) represents the prototypic example of this class of peptide-cytokine conjugates (51,72,73). Various studies have shown that the CNGRCG ligand improves the tumor vasculature-homing properties and the anti-tumor activity of TNF in various animal models of extra-CNS solid tumors, including lymphomas (72). Notably, administration of ultra-low doses of NGR-TNF to tumor-bearing mice (100 pg, 105–106 times lower that the LD50) is sufficient to enhance the antitumor effects of various chemotherapeutic drugs, such as melphalan, doxorubicin, cisplatin, gemcitabine and paclitaxel (74,75). Studies on the mechanism of action have shown that NGR-TNF, administered 2 h before chemotherapy, can increase drug delivery to tumor cells (including lymphoma cells), while at later time points it causes vascular damage. In animal models of solid tumors, low-dose NGR-TNF can also up-regulate leukocyte adhesion molecules on tumor vessels, promote lymphocyte extravasation in tumors, exert synergistic effects with active and adoptive immunotherapy, and enhance the therapeutic efficacy of immune checkpoint blockers in combination with adoptive cell therapy (76-78). Given that NGR-TNF is a recombinant homotrimeric protein made by subunits consisting of two functional domains (CNGRCG peptide and TNF), the receptor system of this fusion protein includes the receptors of both domains, i.e., CD13 and TNF membrane receptors (TNF-R1 and TNF-R2). Notably, the CNGRCG domain does not prevent folding, oligomerization, and binding of the TNF domain to TNF-R1 and TNF-R2. On the other hand, the TNF domain does not impair the interaction of CNGRCG with CD13 (72). Consequently, NGR-TNF may undertake high-avidity multivalent interactions with both CD13 and TNF receptors on cells that express both receptor types. While CD13 is expressed by angiogenic vessels, little or no expression of CD13 occurs in quiescent vessels. In tumors, CD13 is expressed on endothelial cells and pericytes, and, in some cases, also by fibroblasts and tumor cells (79). CD13 is also expressed by many cell types in normal tissues, including epithelial cells of the small intestine, prostate, and proximal renal tubules, by bile duct canaliculi, mast cells, myeloid cells, keratinocytes, and antigen-presenting cells (79-82). However, despite this broad distribution, NGR-peptides bind CD13-positive tumor blood vessels, but not other CD13-rich tissues, as observed by immunohistochemical and biodistribution studies (54). The structural basis of this selectivity is unknown. Thus, low-dose NGR-TNF can engage high-avidity interactions with the tumor vasculature, which express both CD13 and TNF receptors, and less with the vasculature of normal tissues that lack CD13.
Based on the results of preclinical studies, NGR-TNF has been tested in >1,000 patients with different types of solid-tumors, such as non-small cell lung cancer, mesothelioma, colorectal cancer, hepato-cellular carcinoma and ovarian cancer, alone or in combination with chemotherapy or immunotherapy, in some cases with evidence of efficacy and good tolerability (2). For example, in a randomized phase III trial on 400 patients with relapsed or refractory mesothelioma, the addition of NGR-TNF to the best investigator choice was associated with significantly improved survival in the subgroup of patients with short treatment-free interval (i.e., in patients with more aggressive disease, accounting for 50% of total patients) (83).
Use of NGR-TNF in PCNSL
Patients with systemic DLBCL exhibit high cure rates when treated with standard R-CHOP combination, but R-CHOP drugs are unable to cross the BBTB and achieve sufficient tumor concentrations (15). This pharmacokinetic limitation and the negative results of a randomized trial led to abandon CHOP as part of first-line treatment of PCNSL patients (13). The observation that TNF can induce selective BBTB permeabilization and enhance tumor penetration of chemotherapeutic agents in animal models of brain metastasis, and the above-mentioned results of studies performed with NGR-TNF in combination with chemotherapy in solid tumors, provided the rationale for testing the NGR-TNF/R-CHOP combination in PCNSL. The final results of a prospective single-arm phase II study with this combination (the INGRID trial, EUDRACT, 2014-001532-11; clinicaltrials.gov, NCT03536039) have been published recently. In this study, the treatment consisted of 6 courses of standard R-CHOP 21 (repeated every 21 days) preceded by the administration of low doses of NGR-TNF (to permeabilize the BBTB) in HIV-negative adults with relapsed/refractory (R/R) PCNSL (84). Of note, any type of consolidation/maintenance therapy was allowed in responding patients.
The INGRID study consisted of two distinct phases: a proof-of-principle/exploratory phase involving the first 10 enrolled patients, which assessed the effects of NGR-TNF on vascular permeability and the feasibility of NGR-TNF/R-CHOP combination (84), and an expansion phase on the whole patients’ cohort (28 patients) focused on the activity of the same combination (42). Dynamic contrast-enhanced MRI (DCE-MRI) and single-photon emission computed tomography (SPECT) were used to evaluate the BBTB permeability in the lymphomatous lesions and in the normal-appearing brain parenchyma. After the first NGR-TNF infusion, both techniques showed a selective enhancement of the vascular permeability in tumor and peritumoral areas, which was more evident in the latter. Moreover, studies on the R-CHOP concentrations in plasma and CSF and histopathological analysis on CD13 expression, confirmed the specificity of the NGR-TNF targeting to the tumor vasculature. Indeed, NGR-TNF did not interfere with the pharmacokinetics of the investigated drugs: doxorubicin and rituximab, as expected, were not detected in CSF samples, whereas CSF levels and CSF/plasma ratio of cyclophosphamide did not change after NGR-TNF administration. Immunohistochemical and confocal immunofluorescence analysis of tissue sections revealed the presence of CD13 on the luminal side of tumor vessels in diagnostic brain biopsy specimens, confirming the accessibility of the target by the NGR-TNF delivered by the intravenous route.
An encouraging overall response rate (ORR) of 75% was reported in the first exploratory phase of the study (84), that was confirmed in the expansion phase, with a tumor response recorded in 21 out of 28 patients, 11 of which were complete responses (27). Treatment was well tolerated: sixteen serious adverse events were reported in 12 patients, but none of them resulted in treatment discontinuation. No cases of iatrogenic neurotoxicity nor treatment-related mortality were registered. Patients that did not respond, or responded poorly, to NGR-TNF/R-CHOP tended to have abnormal levels of plasma chromogranin A (CgA), a protein known to enhance the endothelial barrier function and inhibit the synergism between NGR-TNF and chemotherapy (27). Notably, these patients were treated for gastroprotection with proton pump inhibitors (PPIs), i.e., with drugs known to increase the circulating levels of CgA. Replacement with other gastroprotective agents before therapy with NGR-TNF/R-CHOP and CgA monitoring until its levels reach the baseline might further increase the overall response to this treatment.
As highlighted by the authors, the results of the INGRID trial showed that the NGR-TNF/R-CHOP combination is active and safe in patients with R/R PCNSL (42). However, the low number of patients with concomitant ocular involvement (3/28, 10%) and the absence of meningeal dissemination do not allow to draw conclusions on the effect of NGR-TNF on the blood–retina barrier and the blood-CSF barrier. Moreover, the absence of modifications on drug concentrations in the subarachnoid space suggests a lack of impact of NGR-TNF on the blood–CSF barrier, highlighting the limitation of this approach in case of concomitant involvement of the CSF and, possibly, of the eyes (85).
Another important point is represented by the duration of the response: due to a potential effect of consolidation, it cannot be compared with those reported in most of the previous trials in the same setting (31-38). One of the strengths of this approach is represented by the specificity of the target, which is conducive to an increased permeability only in tumor or peri-tumoral areas. However, this could represent a weakness if we look at this concept from another point of view: as discussed above, PCNSL is a whole brain disease, thus the microscopic foci may not be reached by R-CHOP, thereby representing a potentially dangerous reservoir for relapsing disease. Of course, this is just a speculation, but the relative short duration of response reported in the other trials suggest that this still remain a key point in the treatment of these patients.
Conclusions
The BBTB still represents a major issue for both diagnosis and treatment of brain tumors, including PCNSL. It must be kept in mind that PCNSL is a whole brain disease and that, currently, we are probably focusing our diagnostic attention just on tumor regions with an altered BBTB. Although strategies aimed at breaching the BBTB and improving immune-chemotherapy efficacy and tolerability have shown promising results, these approaches are still burdened by several caveats. Nevertheless, the development of new strategies that overcome the BBTB, an old problem, still appears to be the right way to a modern and more comprehensive approach of PCNSL and to improve patient outcomes.
Acknowledgments
Funding: This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC, grant IG 2019 - ID. 23470 project - P.I. Angelo Corti).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Andrés J. M. Ferreri, Maurilio Ponzoni) for the series “Central Nervous System Lymphomas” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-54). The series “Central Nervous System Lymphomas” was commissioned by the editorial office without any funding or sponsorship. AC is inventor of a patent on the use of NGR-TNF in PCNSL, and was also consultant for Molmed S.p.A. during NGR-TNF development. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fukumura K, Kawazu M, Kojima S, et al. Genomic characterization of primary central nervous system lymphoma. Acta Neuropathol 2016;131:865-75. [Crossref] [PubMed]
- Braggio E, Van Wier S, Ojha J, et al. Genome-Wide Analysis Uncovers Novel Recurrent Alterations in Primary Central Nervous System Lymphomas. Clin Cancer Res 2015;21:3986-94. [Crossref] [PubMed]
- Vater I, Montesinos-Rongen M, Schlesner M, et al. The mutational pattern of primary lymphoma of the central nervous system determined by whole-exome sequencing. Leukemia 2015;29:677-85. [Crossref] [PubMed]
- Chapuy B, Roemer MG, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood 2016;127:869-81. [Crossref] [PubMed]
- Lai R, Rosenblum MK, DeAngelis LM. Primary CNS lymphoma: a whole-brain disease? Neurology 2002;59:1557-62. [Crossref] [PubMed]
- Maza S, Kiewe P, Munz DL, et al. First report on a prospective trial with yttrium-90-labeled ibritumomab tiuxetan (Zevalin) in primary CNS lymphoma. Neuro Oncol 2009;11:423-9. [Crossref] [PubMed]
- Schulte-Altedorneburg G, Heuser L, Pels H. MRI patterns in recurrence of primary CNS lymphoma in immunocompetent patients. Eur J Radiol 2012;81:2380-5. [Crossref] [PubMed]
- Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer 2020;20:26-41. [Crossref] [PubMed]
- Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci 2007;8:610-22. [Crossref] [PubMed]
- Sowers JL, Johnson KM, Conrad C, et al. The role of inflammation in brain cancer. Adv Exp Med Biol 2014;816:75-105. [Crossref] [PubMed]
- Seano G, Nia HT, Emblem KE, et al. Solid stress in brain tumours causes neuronal loss and neurological dysfunction and can be reversed by lithium. Nat Biomed Eng 2019;3:230-45. [Crossref] [PubMed]
- Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 2010;16:5664-78. [Crossref] [PubMed]
- Mead GM, Bleehen NM, Gregor A, et al. A medical research council randomized trial in patients with primary cerebral non-Hodgkin lymphoma: cerebral radiotherapy with and without cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy. Cancer 2000;89:1359-70. [Crossref] [PubMed]
- Schultz C, Scott C, Sherman W, et al. Preirradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone for primary CNS lymphomas: initial report of radiation therapy oncology group protocol 88-06. J Clin Oncol 1996;14:556-64. [Crossref] [PubMed]
- Ferreri AJ. How I treat primary CNS lymphoma. Blood 2011;118:510-22. [Crossref] [PubMed]
- Ferreri AJM, Holdhoff M, Nayak L, et al. Evolving Treatments for Primary Central Nervous System Lymphoma. Am Soc Clin Oncol Educ Book 2019;39:454-66. [Crossref] [PubMed]
- Ferreri AJ, Cwynarski K, Pulczynski E, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 2016;3:e217-27. [Crossref] [PubMed]
- Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 2005;23:5034-43. [Crossref] [PubMed]
- Tabouret E, Houillier C, Martin-Duverneuil N, et al. Patterns of response and relapse in primary CNS lymphomas after first-line chemotherapy: imaging analysis of the ANOCEF-GOELAMS prospective randomized trial. Neuro Oncol 2017;19:422-9. [PubMed]
- Ambady P, Fu R, Netto JP, et al. Patterns of relapse in primary central nervous system lymphoma: inferences regarding the role of the neuro-vascular unit and monoclonal antibodies in treating occult CNS disease. Fluids Barriers CNS 2017;14:16. [Crossref] [PubMed]
- Maza S, Buchert R, Brenner W, et al. Brain and whole-body FDG-PET in diagnosis, treatment monitoring and long-term follow-up of primary CNS lymphoma. Radiol Oncol 2013;47:103-10. [Crossref] [PubMed]
- Jang SJ, Lee KH, Lee JY, et al. (11)C-methionine PET/CT and MRI of primary central nervous system diffuse large B-cell lymphoma before and after high-dose methotrexate. Clin Nucl Med 2012;37:e241-4. [Crossref] [PubMed]
- Herhaus P, Lipkova J, Lammer F, et al. CXCR4-Targeted PET Imaging of Central Nervous System B-Cell Lymphoma. J Nucl Med 2020;61:1765-71. [Crossref] [PubMed]
- Bromberg JEC, van der Meulen M, Doorduijn JK. The Role of Rituximab in Primary Central Nervous System Lymphoma. Curr Oncol Rep 2020;22:78. [Crossref] [PubMed]
- Ott RJ, Brada M, Flower MA, et al. Measurements of blood-brain barrier permeability in patients undergoing radiotherapy and chemotherapy for primary cerebral lymphoma. Eur J Cancer 1991;27:1356-61. [Crossref] [PubMed]
- Rubenstein JL, Combs D, Rosenberg J, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood 2003;101:466-8. [Crossref] [PubMed]
- Rubenstein JL, Fridlyand J, Abrey L, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 2007;25:1350-6. [Crossref] [PubMed]
- Iwamoto FM, Schwartz J, Pandit-Taskar N, et al. Study of radiolabeled indium-111 and yttrium-90 ibritumomab tiuxetan in primary central nervous system lymphoma. Cancer 2007;110:2528-34. [Crossref] [PubMed]
- Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 2018;24:679-90. [Crossref] [PubMed]
- Schmitz R, Wright GW, Huang DW, et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med 2018;378:1396-407. [Crossref] [PubMed]
- Korfel A, Schlegel U, Herrlinger U, et al. Phase II Trial of Temsirolimus for Relapsed/Refractory Primary CNS Lymphoma. J Clin Oncol 2016;34:1757-63. [Crossref] [PubMed]
- Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II 'proof-of-concept' iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer 2019;117:121-30. [Crossref] [PubMed]
- Grommes C, Pastore A, Palaskas N, et al. Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov 2017;7:1018-29. [Crossref] [PubMed]
- Grommes C, Tang SS, Wolfe J, et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood 2019;133:436-45. [Crossref] [PubMed]
- Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell 2017;31:833-43.e5. [Crossref] [PubMed]
- Ghesquieres H, Chevrier M, Laadhari M, et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective 'proof of concept' phase II study of the French Oculo-Cerebral lymphoma (LOC) Network and the Lymphoma Study Association (LYSA)dagger. Ann Oncol 2019;30:621-8. [Crossref] [PubMed]
- Vu K, Mannis G, Hwang J, et al. Low-dose lenalidomide maintenance after induction therapy in older patients with primary central nervous system lymphoma. Br J Haematol 2019;186:180-3. [Crossref] [PubMed]
- Tun HW, Johnston PB, DeAngelis LM, et al. Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood 2018;132:2240-8. [Crossref] [PubMed]
- Rubenstein JL, Geng H, Fraser EJ, et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv 2018;2:1595-607. [Crossref] [PubMed]
- Angelov L, Doolittle ND, Kraemer DF, et al. Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: a multi-institutional experience. J Clin Oncol 2009;27:3503-9. [Crossref] [PubMed]
- Wang X, Liu P, Yang W, et al. Microbubbles coupled to methotrexate-loaded liposomes for ultrasound-mediated delivery of methotrexate across the blood-brain barrier. Int J Nanomedicine 2014;9:4899-909. [PubMed]
- Ferreri AJM, Calimeri T, Ponzoni M, et al. Improving the antitumor activity of R-CHOP with NGR-hTNF in primary CNS lymphoma: final results of a phase 2 trial. Blood Adv 2020;4:3648-58. [Crossref] [PubMed]
- Roman-Goldstein SM, Barnett PA, McCormick CI, et al. Effects of Gd-DTPA after osmotic BBB disruption in a rodent model: toxicity and MR findings. J Comput Assist Tomogr 1994;18:731-6. [Crossref] [PubMed]
- Jahnke K, Doolittle ND, Muldoon LL, et al. Implications of the blood-brain barrier in primary central nervous system lymphoma. Neurosurg Focus 2006;21:E11 [Crossref] [PubMed]
- Dubinsky TJ, Cuevas C, Dighe MK, et al. High-intensity focused ultrasound: current potential and oncologic applications. AJR Am J Roentgenol 2008;190:191-9. [Crossref] [PubMed]
- Mesiwala AH, Farrell L, Wenzel HJ, et al. High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound Med Biol 2002;28:389-400. [Crossref] [PubMed]
- Hynynen K, McDannold N, Vykhodtseva N, et al. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001;220:640-6. [Crossref] [PubMed]
- Unger EC, Porter T, Culp W, et al. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev 2004;56:1291-314. [Crossref] [PubMed]
- Neuwelt EA, Diehl JT, Vu LH, et al. Monitoring of methotrexate delivery in patients with malignant brain tumors after osmotic blood-brain barrier disruption. Ann Intern Med 1981;94:449-54. [Crossref] [PubMed]
- Connell JJ, Chatain G, Cornelissen B, et al. Selective permeabilization of the blood-brain barrier at sites of metastasis. J Natl Cancer Inst 2013;105:1634-43. [Crossref] [PubMed]
- Corti A, Curnis F, Rossoni G, et al. Peptide-mediated targeting of cytokines to tumor vasculature: the NGR-hTNF example. BioDrugs 2013;27:591-603. [Crossref] [PubMed]
- Lienard D, Ewalenko P, Delmotte JJ, et al. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol 1992;10:52-60. [Crossref] [PubMed]
- Eggermont AM, Schraffordt Koops H, Klausner JM, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg 1996;224:756-64. [Crossref] [PubMed]
- Curnis F, Arrigoni G, Sacchi A, et al. Differential binding of drugs containing the NGR motif to CD13 isoforms in tumor vessels, epithelia, and myeloid cells. Cancer Res 2002;62:867-74. [PubMed]
- Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998;279:377-80. [Crossref] [PubMed]
- Corti A, Curnis F, Arap W, et al. The neovasculature homing motif NGR: more than meets the eye. Blood 2008;112:2628-35. [Crossref] [PubMed]
- Pasqualini R, Koivunen E, Kain R, et al. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res 2000;60:722-7. [PubMed]
- Lahdenranta J, Sidman RL, Pasqualini R, et al. Treatment of hypoxia-induced retinopathy with targeted proapoptotic peptidomimetic in a mouse model of disease. Faseb J 2007;21:3272-8. [Crossref] [PubMed]
- Buehler A, van Zandvoort MA, Stelt BJ, et al. cNGR: a novel homing sequence for CD13/APN targeted molecular imaging of murine cardiac angiogenesis in vivo. Arterioscler Thromb Vasc Biol 2006;26:2681-7. [Crossref] [PubMed]
- Seidi K, Jahanban-Esfahlan R, Monhemi H, et al. NGR (Asn-Gly-Arg)-targeted delivery of coagulase to tumor vasculature arrests cancer cell growth. Oncogene 2018;37:3967-80. [Crossref] [PubMed]
- Satpati D, Sharma R, Kumar C, et al. (68)Ga-Chelation and comparative evaluation of N,N'-bis-[2-hydroxy-5-(carboxyethyl)benzyl]ethylenediamine-N,N'-diacetic acid (HBED-CC) conjugated NGR and RGD peptides as tumor targeted molecular imaging probes. Medchemcomm 2017;8:673-9. [Crossref] [PubMed]
- Zou M, Zhang L, Xie Y, et al. NGR-based strategies for targeting delivery of chemotherapeutics to tumor vasculature. Anticancer Agents Med Chem 2012;12:239-46. [Crossref] [PubMed]
- Huang N, Cheng S, Zhang X, et al. Efficacy of NGR peptide-modified PEGylated quantum dots for crossing the blood-brain barrier and targeted fluorescence imaging of glioma and tumor vasculature. Nanomedicine 2017;13:83-93. [Crossref] [PubMed]
- Hu J, Zhang X, Wen Z, et al. Asn-Gly-Arg-modified polydopamine-coated nanoparticles for dual-targeting therapy of brain glioma in rats. Oncotarget 2016;7:73681-96. [Crossref] [PubMed]
- Huang D, Zhang S, Zhong T, et al. Multi-targeting NGR-modified liposomes recognizing glioma tumor cells and vasculogenic mimicry for improving anti-glioma therapy. Oncotarget 2016;7:43616-28. [Crossref] [PubMed]
- Li W, Hao Q, He L, et al. Recombinant IFN-alpha2a-NGR exhibits higher inhibitory function on tumor neovessels formation compared with IFN-alpha2a in vivo and in vitro. Cytotechnology 2015;67:1039-50. [Crossref] [PubMed]
- Meng J, Yan Z, Wu J, et al. High-yield expression, purification and characterization of tumor-targeted IFN-alpha2a. Cytotherapy 2007;9:60-8. [Crossref] [PubMed]
- Bieker R, Kessler T, Schwoppe C, et al. Infarction of tumor vessels by NGR-peptide-directed targeting of tissue factor: experimental results and first-in-man experience. Blood 2009;113:5019-27. [Crossref] [PubMed]
- Pastorino F, Brignole C, Marimpietri D, et al. Vascular damage and anti-angiogenic effects of tumor vessel-targeted liposomal chemotherapy. Cancer Res 2003;63:7400-9. [PubMed]
- Ellerby HM, Arap W, Ellerby LM, et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nature Medicine 1999;5:1032-8. [Crossref] [PubMed]
- Oostendorp M, Douma K, Hackeng TM, et al. Quantitative molecular magnetic resonance imaging of tumor angiogenesis using cNGR-labeled paramagnetic quantum dots. Cancer Res 2008;68:7676-83. [Crossref] [PubMed]
- Curnis F, Sacchi A, Borgna L, et al. Enhancement of tumor necrosis factor alpha antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13). Nat Biotechnol 2000;18:1185-90. [Crossref] [PubMed]
- Valentinis B, Porcellini S, Asperti C, et al. Mechanism of Action of the Tumor Vessel Targeting Agent NGR-hTNF: Role of Both NGR Peptide and hTNF in Cell Binding and Signaling. Int J Mol Sci 2019;20:4511. [Crossref] [PubMed]
- Curnis F, Sacchi A, Corti A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J Clin Invest 2002;110:475-82. [Crossref] [PubMed]
- Sacchi A, Gasparri A, Gallo-Stampino C, et al. Synergistic antitumor activity of cisplatin, paclitaxel, and gemcitabine with tumor vasculature-targeted tumor necrosis factor-alpha. Clin Cancer Res 2006;12:175-82. [Crossref] [PubMed]
- Calcinotto A, Grioni M, Jachetti E, et al. Targeting TNF-alpha to Neoangiogenic Vessels Enhances Lymphocyte Infiltration in Tumors and Increases the Therapeutic Potential of Immunotherapy. J Immunol 2012;188:2687-94. [Crossref] [PubMed]
- Elia AR, Grioni M, Basso V, et al. Targeting Tumor Vasculature with TNF Leads Effector T Cells to the Tumor and Enhances Therapeutic Efficacy of Immune Checkpoint Blockers in Combination with Adoptive Cell Therapy. Clin Cancer Res 2018;24:2171-81. [Crossref] [PubMed]
- Manzo T, Sturmheit T, Basso V, et al. T Cells Redirected to a Minor Histocompatibility Antigen Instruct Intratumoral TNFalpha Expression and Empower Adoptive Cell Therapy for Solid Tumors. Cancer Res 2017;77:658-71. [Crossref] [PubMed]
- Di Matteo P, Arrigoni GL, Alberici L, et al. Enhanced Expression of CD13 in Vessels of Inflammatory and Neoplastic Tissues. J Histochem Cytochem 2011;59:47-59. [Crossref] [PubMed]
- Taylor A. Aminopeptidases: structure and function. FASEB J 1993;7:290-8. [Crossref] [PubMed]
- Shipp MA, Look AT. Hematopoietic differentiation antigens that are membrane-associated enzymes: cutting is the key. Blood 1993;82:1052-70. [Crossref] [PubMed]
- Dixon J, Kaklamanis L, Turley H, et al. Expression of aminopeptidase-n (CD 13) in normal tissues and malignant neoplasms of epithelial and lymphoid origin. J Clin Pathol 1994;47:43-7. [Crossref] [PubMed]
- Gregorc V, Gaafar RM, Favaretto A, et al. NGR-hTNF in combination with best investigator choice in previously treated malignant pleural mesothelioma (NGR015): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2018;19:799-811. [Crossref] [PubMed]
- Ferreri AJM, Calimeri T, Conte GM, et al. R-CHOP preceded by blood-brain barrier permeabilization with engineered tumor necrosis factor-alpha in primary CNS lymphoma. Blood 2019;134:252-62. [Crossref] [PubMed]
- Nayak L, Batchelor TT. Is it time to revisit R-CHOP for primary CNS lymphoma? Blood 2019;134:221-2. [Crossref] [PubMed]
Cite this article as: Calimeri T, Marcucci F, Corti A. Overcoming the blood-brain barrier in primary central nervous system lymphoma: a review on new strategies to solve an old problem. Ann Lymphoma 2021;5:20.


