
Primary central nervous system lymphoma: epidemiology and clinical presentation
Introduction
Primary central nervous system lymphoma (PCNSL) is a non-Hodgkin lymphoma that manifests in the nervous system. It is a rare, but highly aggressive neoplasm associated with high morbidity and mortality. However, it is potentially curable, which warrants rapid diagnosis and prompt treatment. Since it can present in any part of the central nervous system (CNS) and may commonly manifest with non-specific symptoms, diagnosis is often delayed. Additionally, the incidence of PCNSL has been increasing among the elderly population and thus a high degree of clinical suspicion is important. We will review the epidemiology of the PCNSL as well as provide an overview of the highly variable clinical presentations of PCNSL.
Epidemiology
PCNSL affects both immunocompromised and immunocompetent patient populations. In immunocompetent patients, it is a rare tumor comprising 2–4% of newly diagnosed intracranial tumors (1,2). Histologically, most PCNSL are of diffuse large B-cell lymphoma (DLBCL-NOS) subtype, but a minority is comprised of T-cell lymphomas, low-grade lymphomas, and very rarely Hodgkin lymphoma (3). In immunocompromised patients, such as in patients with human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS), CNS lymphoma is postulated to be a separate clinical entity and on imaging often appears as ring-enhancing and multifocal lesions compared with immunocompetent patients (4). Organ transplant recipients are another immunocompromised population in which CNS lymphoma can be among the most common secondary malignancies, which fall the spectrum of primary central nervous system post-transplant lymphoproliferative disorder (PCNS-PTLD). Higher risk of developing CNS lymphoma may be associated with more intensive immunosuppressive regimens (5,6). There might also be an increase of PCNSL risk in diseases that lead to immunosuppression such as systemic lupus erythematosus and vasculitis and possibly, to a lesser extent, in patients with autoimmune disease that requires immunosuppression such as multiple sclerosis (5).
In general, PCNSL is more prevalent in men compared to women (7), there is a higher incidence in white versus the black population (2) and in the elderly population (7). The median age of diagnosis is 66 years in immunocompetent adults (2). PCNSL is rare in childhood, but the median age is approximately 14 years in the pediatric population (8).
Temporal trends
PCNSL incidence has undergone fluctuations over the past six decades with a general increase in incidence from the 1960s to 1990s, followed by a period of stabilization and increased incidence in the elderly in the last two decades. In the 1960s to 1990s, an increase in incidence was noted in immunocompetent populations based on improved diagnostic capabilities (9) as well as overall increased incidence (5). Given improved imaging techniques through computed tomography (CT) and magnetic resonance imaging (MRI) scans at the time, the incidence was initially thought to be increasing due to better diagnostic tools. However, despite global improvements in imaging techniques in the 1973–1977 period, the incidence of CNS lymphoma appeared to be increasing compared to systemic lymphomas and is not completely attributable to improved diagnostic methods and availability (10).
In the 1990s, PCNSL did experience a spike in incidence largely attributed to the HIV pandemic (1). Patients with AIDS had a 3,600-fold higher rate of developing CNS lymphoma compared with the general population (11). Thus, CNS lymphoma peaked in the mid-1990s, but then decreased in the late 1990s corresponding with a decreasing incidence of AIDS, and better treatment with of highly active anti-retroviral therapy (HAART) (1,12). The prevalence of PCNSL cases occurring in HIV positive individuals declined from 64.1% in 1992 to 12.7% in 2011 (13). Afterwards, the incidence of PCNSL stabilized after the late 1990s in the general population (7,13). However, the rates continued to increase in women as well as in the elderly (13,14). In the past two decades, there is again some evidence of increasing incidence (15,16). However, a study in an Irish population found stabilization of incidence from 2007 to 2017 (17). Despite possible stabilization in the general population (13), the rates in the elderly continue to increase (18).
Survival
Survival in PCNSL has significantly improved since the 1960s but continues to lag behind systemic lymphoma. The median survival is estimated to be 14 months with relative 5-year survival estimated at 31.2% (7). For those younger than 49 years of age, survival is improved in whites versus blacks. However, in those older than 50 years of age, blacks have a trend of improved survival versus whites, but this difference is not statistically significant (7). In the younger population, white females have improved survival, whereas in older populations, black females have improved survival.
HIV positive individuals and immunocompromised individuals have worse survival rates compared to immunocompetent patients (4). The 5-year survival in HIV positive patients was 9% compared to 26% in non-HIV positive patients. However, 5-year survival did mildly improve to 15.8% in HIV positive individuals with the advent HAART therapy (13). In immunocompetent individuals, the 5-year survival also mildly improved from 19% to 30% from the 1990s to the 2000s (13). However, survival is worse in the elderly with median survival of 6–7 months (18). Survival is especially poor in the over 85 years old population with women generally living longer than men (19).
Clinical presentation
PCNSL has a variable clinical presentation depending on which part of the CNS is initially involved such as brain, meninges, spinal cord, eyes or peripheral nerves. As a result, initial symptoms can be wide-ranging and requires a high degree of suspicion and prompt investigation.
Given the heterogeneity of clinical presentations in PCNSL, there are five clinical entities based on the initial CNS compartment involved: (I) intracranial lesions; (II) leptomeningeal lesions; (III) ocular/vitreoretinal lesions; (IV) spinal cord lesions; (V) peripheral nerve lymphoma [also called neurolymphomatosis (NL)] (20,21). While primary CNS lymphoma is predominantly of B-cell origin, primary CNS lymphoma can also be of T-cells subtype. Clinical presentation of T-cell PCNSL lymphoma is similar to B-cell PCNSL with possible increase in B-symptoms and a decrease in ocular involvement (22). Rarely, intravascular lymphoma can present with isolated CNS manifestations (23,24).
Intracranial lesions
Intracranial lesions in the brain parenchyma are the most common manifestation of PCNSL (25). The most common presentation of intracranial PCNSL is a focal neurologic deficit in 70% of patients, followed by neuropsychiatric or behavior changes in 43% of patients (25) (Table 1). Focal neurologic symptoms were most commonly motor or sensory, with headache as the most common non-focal symptom, and hemiparesis and papilledema as the most common signs (27). Focal neurologic symptoms at presentation can prompt neurologic investigation and imaging leading to a faster diagnosis. Psychiatric manifestations include depression, personality change, apathy, slowness of thought, impulsive behavior, psychosis, or hallucinations (20,28). While a focal neurologic manifestation was the most common manifestation, followed by neuropsychiatric symptoms (25), personality change was the most common symptom noted to subsequently affect approximately 60% of patients (20). Personality changes were thought to be due to infiltration of the brain parenchyma and visual hallucinations and symptoms were due to involvement of the occipital lobe or direct eye involvement (20). With psychiatric or nonspecific presentations, diagnosis is often delayed with a mean of 80 days from symptom onset to admission in one study (25). Intracranial meningeal involvement can later develop leading to cranial neuropathies or new onset of headache (20). Primary CNS lymphoma can also present with increased intracranial pressure (ICP) (33% of patients), seizures (14%) and vitreous involvement leading to impaired vision and symptoms of floaters (4%). Rarely, in approximately 7% of patients, systemic symptoms such as fever, gastrointestinal, or respiratory symptoms will occur prior to neurologic symptoms. B symptoms such as fever, night sweats and weight loss, that are observed in systemic lymphoma, are rare in PCNSL (29).

Full table
PCNSL can present as either single or multiple intracranial masses. Approximately 45–50% of intracranial lesions are multifocal (20,27,30) [Figure 1 (20)]. In PCNSL, most lesions are supratentorial rather than infratentorial in approximately a 3:1 ratio (20,27). In terms of the supratentorial lesions, the most common location was in the frontal lobe, while the cerebellum was the most common location among the infratentorial lesions (27). MRI with or without contrast is the preferred diagnostic modality to evaluate for PCNSL. On brain MRI, PCNSL lesions are generally homogenously enhancing with areas of diffusion restriction and edema (31). Solitary lesions in immunocompetent patients compared to immunocompromised patients were found to be larger in size and occurred most commonly in the frontal lobes, corpus callosum or basal ganglia (32). One imaging study examining immunocompetent patients with CNS lymphoma found that 96% had diffusion restriction and 98.5% had enhancement, of which 51.5% was homogenous, 42.6% was heterogeneous and 4.4% was ring. The majority (98.6%) also had a degree of edema with 43.4% showing mild edema and 55.2% with marked edema. Necrosis was seen in a small minority (~7%) of lesions. Approximately 97% of lesions were in contact with a cerebrospinal fluid (CSF) surface (33).
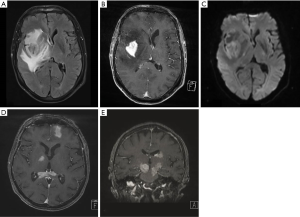
Leptomeningeal
Primary leptomeningeal lymphoma is rare and accounts for 7% of all PCNSL (34). In contrast, leptomeningeal dissemination is more commonly encountered in parenchymal primary CNS lymphoma with a range of 7–42% (35). As a result, diagnosis of leptomeningeal lymphoma should commonly prompt work-up for intracranial or systemic involvement (3). Given the rarity, descriptions of clinical presentations are limited. Patients are generally diagnosed in the 6th decade of life (34,36) with large B-cell lymphoma as the most common entity and a minority (19%) of cases with T-cell (34). Primary CNS T-cell lymphoma is rare, but in one report, it was thought that 23% of primary CNS T-cell lymphoma cases presented with primary leptomeningeal involvement and suggests that isolated leptomeningeal disease may be a more common presentation in T-cell PCNSL (37).
A majority (68%) of patients present with multifocal symptoms (34). The most common symptoms and signs are cranial neuropathies (58%), particularly cranial nerves VI (abducens) and VII (facial), followed by spinal involvement (48%), headache (44%), ataxia (25%) and encephalopathy (25%) (34). A minority of patients present with isolated bowel or bladder symptoms (21%) (34). In another study 33% of patients presented with lumbosacral spinal signs and symptoms such as painful cauda equina syndrome, leg weakness and areflexia without cervical or cranial nerve involvement (36). Rarely (8%), patients present with seizures (34). CSF analysis shows low glucose (median of 47 mg/dL) in 54% of patients, elevated protein (median 235 mg/dL) in 92% of patients and elevated white blood cell count in 92% of patients (median 96/mm3 with lymphocytic predominance). A median of 2 lumbar punctures were required in one report to make the diagnosis (34). Cytology is positive in approximately two-thirds of patients (34,36). Flow cytometry was positive in 80% of patients and molecular rearrangements in immunoglobulin heavy chain (IgH) or T cell receptor (TCR) were positive in 71% of patients (34) (Table 2). A meningeal biopsy may be required if CSF is non-diagnostic. Most patients (81%) had abnormal neuroimaging including 79% with abnormal leptomeningeal enhancement [Figure 2 (38)]. A case report showed meningeal calcification with low-grade marginal zone lymphoma (39).

Full table
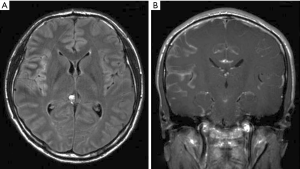
A minority of PCNSL can present with dural-based lesions including DLBCL, marginal zone lymphoma (40-42), small lymphocytic B-cell lymphoma (43) and T-cell lymphoma (40). The radiographic appearance may mimic a meningioma. There is a greater incidence in women (40,41) with the calvarial dura as the most common site of involvement followed by the dura of the skull base (40). Patients commonly present with headaches, cranial nerve deficits, weakness, sensory changes, gait instability or vertigo that are related to mass effect of the tumor (40). CSF studies are reported positive for lymphoma in 46% of cases in one study (40).
Ocular/vitreoretinal
Primary vitreoretinal lymphoma (PVRL) is a subset of PCNSL with initial presentation of ocular involvement that may or may not be followed by brain or CNS involvement. PVRL mainly consists of B-cell lymphoma, with a minority consisting of T-cell lymphomas (44). An estimated 15–25% of patients with PCNSL will have ocular involvement at some point (20,45). Patients with PVRL have a high rate of progression to involvement of other CNS compartments. Approximately half of patients develop other sites of CNS involvement (45,46) and one study demonstrated up to 69% prevalence of non-ocular involvement (45). In one study, 31% of patients had ocular symptoms that preceded the cerebral involvement (47). Additionally, intraocular involvement may be the presenting sign of relapse in PCNSL (48) as well as systemic lymphoma, but less commonly (49).
PVRL affects women slightly more than men (44,50). Monocular or binocular involvement can be the initial presentation. While monocular symptoms may be more common initially (51), binocular involvement eventually develops in 64–83% of patients despite initial monocular symptoms (26,46). The most common presentations of PVRL are blurred vision (52%), decreased visual acuity (37%) and floaters (30%) (48). A minority of patients (17–38%) are asymptomatic (46,48).
Given nonspecific symptoms, patients can be misdiagnosed with corticosteroid refractory chronic uveitis (52). However, the posterior segment of the eye is preferentially involved in PVRL (53), which rarely involves the anterior segment of the eye. Additionally, PVRL does not demonstrate the anterior inflammation encountered in uveitis (54). Diagnosis is often made with vitreal biopsy, vitrectomy, or vitreal fluid examination by cytology, cell markers studies, PCR or other molecular testing (50,55). CSF cytology is positive in 35% of patients (50). Recently, IL-10 and IL-10/IL-6 level in the vitreal fluid has been used to screen for PVRL (55). Given the high rate of concomitant CNS involvement, patients with PVRL should have a contrast-enhanced brain MRI to screen for brain involvement.
Spinal cord
Primary spinal cord lymphoma is rare and presents initially as a myelopathy. While patients with systemic lymphoma can develop leptomeningeal disease or cord compression from epidural lesions, intramedullary spinal lesions may also occur (56). Pathologically, most patients have B-cell morphology, but a minority are T-cell lymphomas (57).
In a retrospective review of 14 patients with primary spinal cord lymphoma most were male and presented in the 7th decade of life. All patients presented with a progressive myelopathy, that was predominantly subacute (56). Sixty-four percent of patients were found to have associated constitutional symptoms. Back pain was the presenting symptom in a minority of patients (29%), but was an associated symptom in 64% of patients. Forty-three percent of patients had lower motor neuron signs with areflexia or flaccid paralysis.
Neuroimaging demonstrates abnormal spinal MRI (Figure 3) in all patients with 64% showing multifocal involvement. Concomitant brain MRI lesions are also observed in 64% of patients. CSF cytology is positive in 27% of patients after 3 lumbar punctures, but non-diagnostic in a majority of patients. CSF demonstrates elevated protein, lymphocytic pleocytosis, but low glucose in a minority of patients (25%). Given the diagnostic limitations of spinal cord biopsy, brain biopsy of CNS lesions when present is preferred. However, spinal cord biopsy may be necessary in these rare primary spinal lymphoma cases to make an accurate diagnosis.
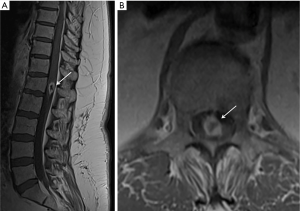
Peripheral nerves
NL is a descriptive term that identifies lymphoma patients with predominant involvement of peripheral nerves, nerve roots, plexuses or cranial nerves and is another rare presentation of PCNSL (58). In a retrospective review of leukemia and lymphoma patients who were diagnosed with NL, 26% of patients had NL as the initial presenting symptom (21). Primary NL typically presents with a painful neuropathy without CNS or systemic lesions. Initial presentation involves multiple peripheral nervous system components in 58% of patients most commonly in the peripheral nerves (60%), spinal nerve roots (48%), cranial nerves (46%) and nerve plexuses (40%). A painful neuropathy occurs in 76% of patients with sensorimotor neuropathy as the most common, but patients can also present with pure motor and, less commonly, pure sensory neuropathy (21). In one study, NL commonly presented in 1 of 4 patterns: (I) painful involvement of the nerves or nerve roots in 31% of patients; (II) cranial neuropathy with or without pain in 21% of patients; (III) painless peripheral polyneuropathy in 28% of patients; (IV) mononeuropathy with or without pain in 15% of patients (58). Approximately 5% of patients had mixed syndromes. Of the cranial nerves involved, the most common are oculomotor (III), abducens (VI), and facial (VII) (58).
CSF analysis demonstrates elevated protein (61% of patients), low glucose (11% of patients) and elevated cell count (44% of patients). Malignant cells are detected in the CSF in 40% of patients and 33% of CSF specimens were positive for monoclonal gene rearrangements (21). MRI scans are abnormal in 77% of patients and fluorodeoxyglucose (FDG)-positron emission tomography (PET) scans are usually positive in 87% of patients (21) (Figure 4). MRI images demonstrate abnormal enhancement (76%), enlarged nerves (53%) with a minority showing nodular (30%) or diffuse (17%) involvement of the nerve. Imaging is not definitive in approximately 50% of patients, who then required a nerve biopsy, reported to be positive in 88% of patients. Histologically, most cases are B-cell lymphoma with a minority showing T-cell lymphoma.
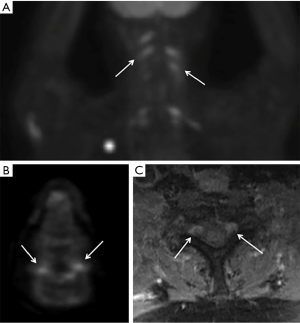
Conclusions
PCNSL is a rare lymphoma that can mimic multiple different neurologic diseases. The incidence of PCNSL continues to increase in the elderly population. Given the wide variety of possible clinical presentations of PCNSL, it is important to have a high degree of clinical suspicion. Primary CNS lymphoma can often mimic a variety of other CNS pathologies, especially demyelinating diseases given their steroid responsive nature. While intracranial PCNSL remains the most common manifestation, PCNSL can also present initially in the leptomeninges, eyes, spinal cord, or in the peripheral nervous system. Additionally, psychiatric manifestations are a common symptom in PCNSL leading to delay in diagnosis. Consequently, it is important to understand the different manifestations of PCNSL for prompt evaluation and treatment of a potentially curable, CNS malignancy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Andrés J. M. Ferreri, Maurilio Ponzoni) for the series “Central Nervous System Lymphomas” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-50). The series “Central Nervous System Lymphomas” was commissioned by the editorial office without any funding or sponsorship. Dr. TB reports SAB Honoraria from Genomicare, personal fees from UpToDate, Inc., Honoraria for CME lectures from Oakstone, Honoraria for CME lecture from Audio Oncology Digest, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985-1999. Neuro Oncol 2006;8:27-37. [Crossref] [PubMed]
- Ostrom QT, Gittleman H, Truitt G, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro Oncol 2018;20:iv1-86. [Crossref] [PubMed]
- Giannini C, Dogan A, Salomão DR. CNS Lymphoma: A Practical Diagnostic Approach. J Neuropathol Exp Neurol 2014;73:478-94. [Crossref] [PubMed]
- Fine HA, Mayer RJ. Primary Central Nervous System Lymphoma. Ann Intern Med 1993;119:1093-104. [Crossref] [PubMed]
- Schabet M. Epidemiology of primary CNS lymphoma. J Neurooncol 1999;43:199-201. [Crossref] [PubMed]
- Penn I, Porat G. Central nervous system lymphomas in organ allograft recipients. Transplantation 1995;59:240-4. [Crossref] [PubMed]
- Villano JL, Koshy M, Shaikh H, et al. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer 2011;105:1414-8. [Crossref] [PubMed]
- Abla O, Weitzman S, Blay JY, et al. Primary CNS lymphoma in children and adolescents: a descriptive analysis from the International Primary CNS Lymphoma Collaborative Group (IPCG). Clin Cancer Res 2011;17:346-52. [Crossref] [PubMed]
- Miller DC, Hochberg FH, Harris NL, et al. Pathology with clinical correlations of primary central nervous system non-Hodgkin’s lymphoma. The Massachusetts General Hospital experience 1958-1989. Cancer 1994;74:1383-97. [Crossref] [PubMed]
- Olson JE, Janney CA, Rao RD, et al. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: a surveillance, epidemiology, and end results analysis. Cancer 2002;95:1504-10. [Crossref] [PubMed]
- Coté TR, Manns A, Hardy CR, et al. Epidemiology of brain lymphoma among people with or without acquired immunodeficiency syndrome. AIDS/Cancer Study Group. J Natl Cancer Inst 1996;88:675-9. [Crossref] [PubMed]
- Kadan-Lottick NS, Skluzacek MC, Gurney JG. Decreasing incidence rates of primary central nervous system lymphoma. Cancer 2002;95:193-202. [Crossref] [PubMed]
- Shiels MS, Pfeiffer RM, Besson C, et al. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol 2016;174:417-24. [Crossref] [PubMed]
- O’Neill BP, Decker PA, Tieu C, et al. The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkin’s lymphoma. Am J Hematol 2013;88:997-1000. [Crossref] [PubMed]
- Haldorsen IS, Krossnes BK, Aarseth JH, et al. Increasing incidence and continued dismal outcome of primary central nervous system lymphoma in Norway 1989-2003 : time trends in a 15-year national survey. Cancer 2007;110:1803-14. [Crossref] [PubMed]
- Enblad G, Martinsson G, Baecklund E, et al. Population-based experience on primary central nervous system lymphoma 2000–2012: the incidence is increasing. Acta Oncol 2017;56:599-607. [Crossref] [PubMed]
- O’Connell K, Looby S, Gou P, et al. CNS lymphoma, the Irish experience: A retrospective review of neuropathologically confirmed cases over 10 years. Clin Neuropathol 2020;39:212-20. [Crossref] [PubMed]
- Mendez JS, Ostrom QT, Gittleman H, et al. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol 2018;20:687-94. [Crossref] [PubMed]
- Furst T, Hoffman H, Chin LS. All-cause and tumor-specific mortality trends in elderly primary central nervous system lymphoma (PCNSL) patients: a surveillance, epidemiology, and end results (SEER) analysis. J Neurosurg Sci 2019; Epub ahead of print. [Crossref] [PubMed]
- Hochberg FH, Miller DC. Primary central nervous system lymphoma. J Neurosurg 1988;68:835-53. [Crossref] [PubMed]
- Grisariu S, Avni B, Batchelor TT, et al. Neurolymphomatosis: an International Primary CNS Lymphoma Collaborative Group report. Blood 2010;115:5005-11. [Crossref] [PubMed]
- Shenkier TN, Blay JY, O'Neill BP, et al. Primary CNS lymphoma of T-cell origin: a descriptive analysis from the international primary CNS lymphoma collaborative group. J Clin Oncol 2005;23:2233-9. [Crossref] [PubMed]
- Momota H, Narita Y, Miyakita Y, et al. Intravascular Lymphoma of the Central Nervous System Presenting as Multiple Cerebral Infarctions. Nagoya J Med Sci 2012;74:353-8. [PubMed]
- Bots GT. Angioendotheliomatosis of the central nervous system. Acta Neuropathol (Berl) 1974;28:75-8. [Crossref] [PubMed]
- Bataille B, Delwail V, Menet E, et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg 2000;92:261-6. [Crossref] [PubMed]
- Hoffman PM, McKelvie P, Hall AJ, et al. Intraocular lymphoma: a series of 14 patients with clinicopathological features and treatment outcomes. Eye (Lond) 2003;17:513-21. [Crossref] [PubMed]
- Helle TL, Britt RH, Colby TV. Primary lymphoma of the central nervous system. Clinicopathological study of experience at Stanford. J Neurosurg 1984;60:94-103. [Crossref] [PubMed]
- Fisher R, Harper C. Depressive illness as a presentation of primary lymphoma of the central nervous system. Aust N Z J Psychiatry 1983;17:84-90. [Crossref] [PubMed]
- Grommes C, Rubenstein JL, DeAngelis LM, et al. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro Oncol 2019;21:296-305. [Crossref] [PubMed]
- Herrlinger U, Schabet M, Clemens M, et al. Clinical presentation and therapeutic outcome in 26 patients with primary CNS lymphoma. Acta Neurol Scand 1998;97:257-64. [Crossref] [PubMed]
- Coulon A, Lafitte F, Hoang-Xuan K, et al. Radiographic findings in 37 cases of primary CNS lymphoma in immunocompetent patients. Eur Radiol 2002;12:329-40. [Crossref] [PubMed]
- Zhang D, Hu LB, Henning TD, et al. MRI findings of primary CNS lymphoma in 26 immunocompetent patients. Korean J Radiol 2010;11:269-77. [Crossref] [PubMed]
- Sutherland T, Yap K, Liew E, et al. Primary central nervous system lymphoma in immunocompetent patients: a retrospective review of MRI features. J Med Imaging Radiat Oncol 2012;56:295-301. [Crossref] [PubMed]
- Taylor JW, Flanagan EP, O’Neill BP, et al. Primary leptomeningeal lymphoma: International Primary CNS Lymphoma Collaborative Group report. Neurology 2013;81:1690-6. [Crossref] [PubMed]
- Kiewe P, Fischer L, Martus P, et al. Meningeal dissemination in primary CNS lymphoma: diagnosis, treatment, and survival in a large monocenter cohort. Neuro Oncol 2010;12:409-17. [Crossref] [PubMed]
- Lachance DH, O’Neill BP, Macdonald DR, et al. Primary leptomeningeal lymphoma: report of 9 cases, diagnosis with immunocytochemical analysis, and review of the literature. Neurology 1991;41:95-100. [Crossref] [PubMed]
- Grove A, Vyberg M. Primary leptomeningeal T-cell lymphoma: a case and a review of primary T-cell lymphoma of the central nervous system. Clin Neuropathol 1993;12:7-12. [PubMed]
- Park JS, Park H, Park S, et al. Primary Central Nervous System ALK Positive Anaplastic Large Cell Lymphoma with Predominantly Leptomeningeal Involvement in an Adult. Yonsei Med J 2013;54:791-6. [Crossref] [PubMed]
- King A, Wilson H, Penney C, et al. An unusual case of primary leptomeningeal marginal zone B-cell lymphoma. Clin Neuropathol 1998;17:326-9. [PubMed]
- Karschnia P, Batchelor TT, Jordan JT, et al. Primary dural lymphomas: Clinical presentation, management, and outcome. Cancer 2020;126:2811-20. [Crossref] [PubMed]
- Tu PH, Giannini C, Judkins AR, et al. Clinicopathologic and genetic profile of intracranial marginal zone lymphoma: a primary low-grade CNS lymphoma that mimics meningioma. J Clin Oncol 2005;23:5718-27. [Crossref] [PubMed]
- Iwamoto FM, DeAngelis LM, Abrey LE. Primary dural lymphomas: a clinicopathologic study of treatment and outcome in eight patients. Neurology 2006;66:1763-5. [Crossref] [PubMed]
- Giordano A, Perrone T, Guarini A, et al. Primary intracranial dural B cell small lymphocytic lymphoma. Leuk Lymphoma 2007;48:1437-43. [Crossref] [PubMed]
- Hormigo A, Abrey L, Heinemann MH, et al. Ocular presentation of primary central nervous system lymphoma: diagnosis and treatment. Br J Haematol 2004;126:202-8. [Crossref] [PubMed]
- Farrall AL, Smith JR. Eye involvement in primary central nervous system lymphoma. Surv Ophthalmol 2020;65:548-61. [Crossref] [PubMed]
- Peterson K, Gordon KB, Heinemann MH, et al. The clinical spectrum of ocular lymphoma. Cancer 1993;72:843-9. [Crossref] [PubMed]
- Hong JT, Chae JB, Lee JY, et al. Ocular involvement in patients with primary CNS lymphoma. J Neurooncol 2011;102:139-45. [Crossref] [PubMed]
- Grimm SA, Pulido JS, Jahnke K, et al. Primary intraocular lymphoma: an International Primary Central Nervous System Lymphoma Collaborative Group Report. Ann Oncol 2007;18:1851-5. [Crossref] [PubMed]
- Salomão DR, Pulido JS, Johnston PB, et al. Vitreoretinal presentation of secondary large B-cell lymphoma in patients with systemic lymphoma. JAMA Ophthalmol 2013;131:1151-8. [Crossref] [PubMed]
- Grimm SA, McCannel CA, Omuro AMP, et al. Primary CNS lymphoma with intraocular involvement: International PCNSL Collaborative Group Report. Neurology 2008;71:1355-60. [Crossref] [PubMed]
- Choi JY, Kafkala C, Foster CS. Primary Intraocular Lymphoma: A Review. Semin Ophthalmol 2006;21:125-33. [Crossref] [PubMed]
- Faia LJ, Chan CC. Primary Intraocular Lymphoma. Arch Pathol Lab Med 2009;133:1228-32. [Crossref] [PubMed]
- Coupland SE, Heimann H, Bechrakis NE. Primary intraocular lymphoma: a review of the clinical, histopathological and molecular biological features. Graefes Arch Clin Exp Ophthalmol 2004;242:901-13. [Crossref] [PubMed]
- Park S, Abad S, Tulliez M, et al. Pseudouveitis: a clue to the diagnosis of primary central nervous system lymphoma in immunocompetent patients. Medicine (Baltimore) 2004;83:223-32. [Crossref] [PubMed]
- Pochat-Cotilloux C, Bienvenu J, Nguyen AM, et al. Use of a Threshold of Interleukin-10 and IL-10/IL-6 Ratio in Ocular Samples for the Screening of Vitreoretinal Lymphoma. Retina 2018;38:773-81. [Crossref] [PubMed]
- Flanagan EP, O’Neill BP, Porter AB, et al. Primary intramedullary spinal cord lymphoma. Neurology 2011;77:784-91. [Crossref] [PubMed]
- Guzzetta M, Drexler S, Buonocore B, et al. Primary CNS T-cell lymphoma of the spinal cord: case report and literature review. Lab Med 2015;46:159-63. [Crossref] [PubMed]
- Baehring JM, Damek D, Martin EC, et al. Neurolymphomatosis. Neuro Oncol 2003;5:104-15. [Crossref] [PubMed]
Cite this article as: Song KW, Issa S, Batchelor T. Primary central nervous system lymphoma: epidemiology and clinical presentation. Ann Lymphoma 2021;5:16.


