
Narrative review of primary central nervous system lymphoma: treatment-related neurotoxicity
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare infiltrative tumor that develops most frequently in the subcortical periventricular white matter, with multifocal lesions occurring in 30–40% of patients (1,2). It is a disease with a mean age at diagnosis of 60 years, and it is more common in men (3). Focal neurological signs are the most typical presentation followed by behavioral and cognitive symptoms, headaches and seizures (4,5). The standard treatment for PCNSL often includes high-dose methotrexate (HD-MTX)-based regimens, high-dose cytarabine (HD-ARA-C) and whole-brain radiotherapy (WBRT) (6,7). Although this combined modality treatment approach is effective, with a median survival of 30 to 60 months (1,8), it is often associated with delayed neurotoxicity in most patients (9-12). HD-MTX-based chemotherapy without WBRT has shown to be effective in the treatment of PCNSL and to reduce the risk of delayed neurotoxicity (5). However, the long-term efficacy of HD-MTX-based regimens with or without blood brain barrier disruption (BBBD) or followed by high-dose chemotherapy and autologous stem cell transplantation (ASCT) remains to be confirmed, as many patients relapse and require treatment with WBRT or chemotherapy (4,7,13). Novel agents like the Bruton tyrosine kinase inhibitor ibrutinib or immunomodulatory drugs have shown promising results in the treatment of recurrent/refractory PCNSL (14), and future studies assessing cognitive outcome associated with these regimens would be relevant.
Long-term survivors of PCNSL often experience delayed treatment-related cognitive dysfunction that can interfere with their quality of life (QoL) (15,16). Cognitive dysfunction is likely related to multiple factors including the effects of the tumor itself given its infiltrative pattern, age, and the delayed effects of treatment with HD-MTX-based chemotherapy and WBRT, either combined or alone (17). It is considered the most frequent complication among long-term survivors (18), and may disrupt their ability to function at pre-diagnosis levels professionally and socially (19,20). The pattern of cognitive deficits is often diffuse and the domains disrupted include attention, executive functions, graphomotor speed, and learning and retrieval of new information (15).
The importance of assessing cognitive dysfunction associated with HD-MTX-based regimens with and without WBRT has been recognized by the International Primary CNS Lymphoma Collaborative Group (IPCG) (7,21). Guidelines for standardized cognitive assessments and follow up intervals have been developed (15), and these have been incorporated in recent studies (22-24).
An initial literature review (15) indicated that cognitive function was evaluated systematically with standardized neuropsychological tests in a relatively small number of studies, and methodological problems limited the understanding of the contribution of disease and treatment in early studies. A more recent systematic literature review (16) indicated an increase in the number of studies including standardized neuropsychological tests to assess cognitive outcome; however, several continued to use only mental status screening tools to assess cognitive functions. Brief cognitive screening tests (e.g., mini-mental status exam) have been shown decreased sensitivity to detect cognitive dysfunction in patients with brain tumors (25,26), and provide an underestimation of the incidence and extent of cognitive dysfunction in patients with PCNSL. The current review focuses primarily on cognitive outcome in patients with PCNSL, and it includes studies that reported the results of standardized cognitive assessments.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/aol-20-37).
Methods
This article provides a comprehensive review and update of studies assessing neurocognitive functions in patients with PCNSL according to different treatment modalities. An extensive search for articles published up until October 1, 2020 and available in MEDLINE/PubMed, Cochrane, and Google Scholar was performed. Search terms included “PCNSL”, “cognitive” and “cognition.” Original peer-reviewed articles published in English and reporting the results of standardized cognitive assessments in adult patients with PCNSL were eligible for inclusion. Case studies and articles including only the results of mental status screening examinations or Karnofsky Performance Scale scores were excluded.
Discussion
Treatment-related delayed neurotoxicity
The adverse effects of chemotherapy and radiotherapy have been described to involve demyelination, inflammation, and microvascular injury (27). The pathophysiological mechanisms of radiation injury involve interactions among multiple cell types within the brain including astrocytes, endothelial cells, microglia, neurons and oligodendrocytes (28). Blood-vessel dilatation and wall thickening with hyalinization, increased BBB permeability due to endothelial cell loss and apoptosis, and a decrease in vessel density have been hypothesized to lead to white matter necrosis (28,29). Depletion of oligodendrocytes, microglial inflammation and disruption of hippocampal neurogenesis have been described after radiation (30,31). Radiation may diminish the reproductive capacity of the O-2A progenitors of oligodendrocytes, astrocytes and microglia, disrupting the normal turnover of myelin (32,33). Progressive demyelination may take months to cause symptoms because of the slow turn-over of oligodendrocytes, contributing to the latency in onset of neurotoxicity and its progressive nature. The prevalence of radiation-induced brain injury appears to increase with volume of radiated tissue, dose of radiation, dose per fraction, concomitant administration of chemotherapy, and age >60 years (34,35).
There is increasing evidence that chemotherapy has direct toxic effects on progenitor cells and oligodendrocytes, and disrupts gliogenesis and neurogenesis (36). The pathophysiological mechanisms are suggested to include demyelination, secondary inflammatory response, oxidative stress and DNA damage, immune dysregulation, and microvascular injury (37). Neurotoxicity has been reported after treatments including HD-MTX-based chemotherapy and HD-ARA-C (1,38), and intrathecal chemotherapy is more likely to cause CNS toxicity. A recent study demonstrated that MTX can induce long-term tri-glial dysfunction and disrupt oligodendrocyte lineage cells, astrocytes, and microglia homeostasis (33). Decrease in white matter density in the corpus callosum, hippocampal cell death, and memory impairments were reported in rats after administration of HD-MTX (39). Combined treatment with radiotherapy and chemotherapy may have synergistic adverse effects (40), as chemotherapy agents may act as a radiosensitizer.
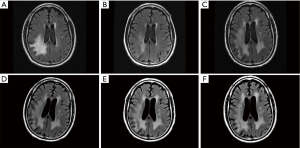
In patients with PCNSL treated with combined modality therapy including HD-MTX-based chemotherapy and WBRT, magnetic resonance imaging (MRI) studies often show diffuse white matter abnormalities (Figure 1) and atrophy, and in some cases, communicating hydrocephalus (27). Several chemotherapeutic agents, particularly HD-MTX-based chemotherapy and HD-ARA-C, have been associated with the development of periventricular white matter abnormalities (36), but often less extensive following combined modality treatment. In a recent prospective study, Estephan et al. (41) reported that 62% of patients with PCNSL developed white matter abnormalities 3–6 months after treatment with HD-MTX-based chemotherapy, and these were more extensive in patients treated with rituximab.
Recent studies suggest that HD-MTX-based regimens without WBRT (11,42,43), or with reduced-dose WBRT (44), and HD-MTX-based chemotherapy followed by high-dose chemotherapy and ASCT (22,45,46), can be efficacious and may diminish the risk for delayed neurotoxicity. However, since disease relapse is relatively common, requiring additional therapy with WBRT or chemotherapy (13), the optimal induction and consolidative treatment for PCNSL remains controversial. The contribution of the disease and the adverse effects of different treatments regimens to cognitive dysfunction requires further study, as the neurotoxicity of combined treatments and individual chemotherapy agents is difficult to determine when each can contribute to CNS damage (40).
HD-MTX-based chemotherapy ± WBRT regimens
Pels et al. (47) studied cognitive functions in twenty-seven patients with PCNSL (age range, 27–74 years) subsequent to treatment with HD-MTX-based chemotherapy alone, WBRT alone, or combined modality therapy. Thirteen patients were evaluated between 1 and 95 months after WBRT either alone or in combination with HD-MTX-based chemotherapy, and eight had cognitive deficits [five had progression of disease (PD), or partial treatment response (PR), and two had complete treatment response (CR)]. On subsequent follow-ups, nine of the thirteen patients had cognitive decline (three had PD or PR, and five had stable disease). Fourteen patients had cognitive evaluations 10 to 38 months post-HD-MTX-based chemotherapy, with ten showing cognitive deficits (two had PR and seven had CR). On subsequent follow-ups, nine of the fourteen patients with CR improved, and two patients with PD declined. The neuropsychological tests and scores were not reported. The findings were interpreted to suggest that WBRT either alone or in combination with chemotherapy was associated with cognitive deterioration. However, the inclusion of patients with PD or PR limited the ability to differentiate tumor and treatment adverse effects.
Harder et al. (20) studied cognitive functions in nineteen PCNSL patients (median age =44 years, range, 24–63 years) treated with HD-MTX-based chemotherapy followed by WBRT. Patients had a CR and were evaluated at a mean of 23 months (SD =14) after treatment completion. Eleven patients had mild to moderate impairment (4/18 test indices impaired), and four patients had severe impairment (>6 test indices impaired). In comparison to a non-CNS cancer control group, patients with PCNSL had lower scores on verbal and visual memory, attention, executive functions, and motor speed. Self-report QoL assessment showed that compared to controls, patients with PCNSL reported lower cognitive, emotional, and social functioning. The authors concluded that combined modality therapy was associated with cognitive impairment even in patients younger than 60 years.
Correa et al. (19) studied cognitive functions in twenty-eight patients with PCNSL treated with WBRT ± HD-MTX-based chemotherapy (n=18, median age =53 years, range, 36–73 years) or HD-MTX-based chemotherapy alone (n=10, median age =72, range, 60–85 years). Patients were without evidence of disease progression and the mean time since treatment completion was 61 months (SD =40) for patients who had combined modality therapy, and 18 months (SD =16) for patients treated with chemotherapy alone. Patients treated with WBRT ± HD-MTX-based chemotherapy had impairments (scores 1.5 SD below the normative mean) in attention/executive function, verbal memory, graphomotor speed, and naming. Patients treated with chemotherapy only had impairments in graphomotor speed and scored within 1 SD below the normative mean on other cognitive domains. In patients treated with WBRT ± HD-MTX-based chemotherapy, memory performance did not differ according to time since treatment completion (≤60 months, n=9; >60 months, n=9), but it was significantly more impaired than that of patients treated with chemotherapy only (≤60 months). Patients treated with the combined modality regimen had more extensive white matter abnormalities on MRI, and these were associated with worse performance in attention/executive functions and memory. Self-report QoL showed that 50% of patients were either unemployed or worked at a lower capacity than prior to their illness, with no differences according to treatment modality. The authors concluded that WBRT ± HD-MTX-based chemotherapy was associated with more pronounced cognitive impairment than chemotherapy alone.
In a subsequent study including additional PCNSL survivors, Correa et al. (10) reported cognitive outcome in patients treated with WBRT ± HD-MTX-based chemotherapy (n=24, mean age =53.5 years, SD =8.9) or HD-MTX-based chemotherapy alone (n=26, mean age =70.6 years, SD =8.2). Compared with patients treated with combined regimens, patients treated with HD-MTX-based chemotherapy alone were significantly older and had shorter time since treatment completion (mean =15 months, SD =12.9 vs. 51 months, SD =39.5). Comparisons according to treatment type, controlling for age and time since treatment completion, showed that patients treated with chemotherapy alone had significantly higher scores in attention, executive function, and memory, compared to patients treated with WBRT ± HD-MTX-based chemotherapy, who had impaired scores on most cognitive domains. However, patients treated with chemotherapy alone scored 1 SD below the normative sample. Patients with more extensive white matter abnormalities on MRIs (regardless of treatment modality) had lower scores on tests of set-shifting and memory. Consistent with the cognitive findings, patients treated with chemotherapy alone reported more intact QoL. Thirty-three patients completed an additional follow-up assessment at a mean of 14–16 months after the initial visit. There were no significant changes on any of the cognitive tests among patients treated with WBRT + HD-MTX, and an improvement in auditory attention in patients treated with HD-MTX-based chemotherapy alone. The findings were interpreted to suggest that cognitive dysfunction was more frequent in PCNSL survivors treated with WBRT ± HD-MTX-based chemotherapy.
Blood-brain barrier disruption chemotherapy with WBRT
Neuwelt et al. (48) studied twelve patients with PCNSL (age range, 18–66 years) prior to and one-year post treatment and all patients had a CR. WBRT was completed pre-BBBD chemotherapy for three patients and post-BBBD chemotherapy for two patients, and seven had no WBRT. Among the seven patients treated with BBBD chemotherapy, 6 had stable or improved summary z-scores 1–7 years post-treatment, relative to baseline. Of note, most scores at treatment completion were within the normal range. Specific tests used and scores were not reported. Among patients who received WBRT, results were more equivocal, with two of five patients having significant cognitive decline.
Dahlborg et al. (49) studied twenty-two patients with PCNSL [some were included in Neuwelt et al. (48)] pre- and one-year post-treatment with BBBD chemotherapy with and without WBRT. The results (summary z-scores) showed that of the fifteen patients treated with BBBD chemotherapy, cognitive functioning either remained stable or improved. In contrast, three of eight patients treated with WBRT had declines approaching one standard deviation. There was stability or improvement in cognitive functions following BBBD chemotherapy without WBRT in all age groups, including among patients over the age of 60 (n=7).
Blood-brain barrier disruption chemotherapy without WBRT
Crossen et al. (50) studied eight patients with PCNSL (mean age =55.6, range, 37–69 years) pre- and post-BBBD chemotherapy (median of 1 year, range, 1–7 years) using standardized tests of intellectual functioning, psychomotor speed, executive functioning, verbal and visual memory, and motor speed. Patients had significant impairment on tests of executive functions, learning and memory, and motor speed at baseline. There was no decline in the summary z-score (defined as a difference of at least one SD) post-treatment; two patients improved while six remained stable. All patients had a CR. However, examination of individual test scores, showed that two patients declined on visual memory, and one declined in executive function, delayed memory, and motor speed.
McAllister et al. (51) studied twenty-three patients with PCNSL (age <60 years) before and after BBBD chemotherapy (mean =16.5 months, SD =10.9); all had a CR. The standardized cognitive tests used were described previously (50). The results showed a significant improvement in cognitive functioning post-treatment (summary z-score). An evaluation of individual scores showed improvement in intellectual functioning, learning and memory, attention and visuospatial skills; a non-significant trend was seen for executive functions. Seven patients had declines in individual test scores, primarily in motor speed.
Neuwelt et al. (52) studied fifteen patients with PCNSL (mean age =50.5, range, 28–68 years) pre- and one-year post-treatment; nine patients also had long-term follow-up (mean =3.5 years after diagnosis). The neuropsychological test battery was described previously (50). The summary z-scores ranged from −2.59 to 0.46 (mean =−1.1, SD =1.1) at baseline, and from −1.45 to 0.26 (mean =0.35, SD =0.5) at the end of treatment. Paired samples t-tests indicted a significant improvement in cognitive functioning from baseline. Long-term follow up showed no evidence of cognitive decline among patients without disease recurrence for more than two years.
HD-MTX-based chemotherapy regimens
Schlegel et al. (53) studied cognitive functions in ten patients with PCNSL (median age =64 years, range, 27–71 years) pre- and post-treatment with HD-MTX-based chemotherapy (median =32 months, range, 2–59 months). Patients had either a PR (n=2) or CR (n=8). A standardized neuropsychological test battery was used to assess attention, verbal and visual memory, verbal fluency and visuospatial skills (tests and test scores were not included). Nine patients showed stable or improved cognitive summary test scores with a median of 95 (with 100±10 as reference value) at the last follow-up evaluation performed 15–41 months post-treatment; one patient with a history of PD had severe cognitive impairment. The authors reported no evidence of treatment-related cognitive impairment.
Pels et al. (54) assessed cognitive functions in twenty-two patients with PCNSL (median age =62 years, range, 27–75 years) between 4 and 82 months post-HD-MTX-based chemotherapy. There was no decline in attention, verbal memory, visual memory, word fluency or visual-construction abilities in patients who had either a PR or CR (test scores were not included). Although older patients had lower test scores, cognitive performance post-treatment was similar for patients younger and older than 60 years.
Fliessbach et al. (55) studied ten patients with PCNSL (median age =60 years, range, 27–67 years) who completed serial cognitive assessments pre- and post-HD-MTX-based chemotherapy (median =36 months, range, 21–69 months), and had either a CR or PR. At baseline, five of the eight patients who were tested had cognitive impairment. At a 4-month follow-up, four of the ten patients improved, particularly in attention and verbal memory. Patients without impairment at baseline remained stable at follow-up. At the last follow-up, the summary scores (with 100±10 as reference value) ranged from 86 to 109 (median =94), but test scores declined (for each test in at least one patient) in written phonemic verbal fluency and visual and verbal memory. There were no differences in cognitive performance according to age. Cognitive test scores were impaired in four patients with PD and in two patients treated with WBRT or HD-MTX-based chemotherapy. The authors concluded that there was no treatment-related cognitive decline.
In another study, Fliessbach et al. (56) assessed cognitive functions in twenty-three patients with PCNSL (median age =60 years, range 27–67 years) pre- and up to 44 months post-HD-MTX-based chemotherapy (median =36 months, range 21–69 months). All patients were in PR or CR. At baseline, patients had impairments in attention and executive functions, verbal and visual memory, and word fluency (test scores were not included); these were classified as mild in three patients, moderate in ten patients, and severe in six patients. At the last follow-up, impairment (in at least one domain) was mild in five patients, moderate in five patients, and severe in one; twelve patients had no deficits. Twenty-one patients improved, but scores remained in the low average range on tests of attention, visual memory, and word fluency. The authors concluded that the cognitive deficits were mostly disease-related and there was no treatment-related cognitive decline.
Juergens et al. (42) studied cognitive functions in nineteen patients with PCNSL before treatment with systemic and intrathecal HD-MTX-based chemotherapy, and 3–4 months and at a median of 100 months (range, 77–149 months) post-treatment. At baseline, there was impairment in attention, executive functions, memory, word fluency and psychomotor speed. There was improvement at the initial post-treatment evaluation and either stable or improved performance at the long-term follow ups in all cognitive domains, except for visual memory which declined at the last follow up. At the individual level, three patients declined in verbal and visual memory and one patient declined in visual memory and psychomotor speed at the long-term follow up; 72% of patients reported good or very good QoL. The authors concluded that the chemotherapy regimen was not associated with delayed neurotoxicity.
Studies comparing multiple treatment modalities
Doolittle et al. (24) studied cognitive and neuroimaging outcomes in eighty patients with PCNSL evaluated at a median of 5.5 years (range, 2 to 26 years) after diagnosis, and in stable remission. Treatment modalities included: HD-MTX-based chemotherapy (n=32), HD-MTX-based intra-arterial chemotherapy in conjunction with BBBD chemotherapy (n=25), HD-MTX-based chemotherapy followed by high-dose chemotherapy and ASCT (n=8), and HD-MTX-based chemotherapy followed by WBRT (median dose =4,500 cGy) (n=15); five of these patients also received high-dose chemotherapy and ASCT prior to WBRT. Patients treated with HD-MTX-based chemotherapy and WBRT had significantly lower mean test scores in attention, executive function and motor speed compared to patients treated with HD-MTX-based chemotherapy alone or in conjunction with BBBD chemotherapy, and to all patients treated without WBRT combined. Among patients treated with BBBD chemotherapy evaluated at a median of 12 years post-treatment, there was a significant improvement in executive functions compared to baseline (pre-treatment), and no changes or decline in other cognitive domains (57). White mater abnormalities were more extensive in the patients treated with WBRT, compared to patients treated with chemotherapy alone regimens. The findings were consistent with other studies suggesting a greater risk for delayed neurotoxicity with regimens including WBRT.
Correa et al. (23) assessed cognitive functions prospectively up to five years in patients with PCNSL treated with induction HD-MTX-based chemotherapy followed by consolidation with either reduced-dose WBRT (2,340 cGy) and ARA-C (n=14, mean age =59 years, SD =7.2), or with high-dose chemotherapy and ASCT (n=15, mean age =52 years, SD =13.2), who were without evidence of disease progression. At baseline (pre-treatment), cognitive test scores were impaired in all patients, likely related to disease burden. There were no significant longitudinal group differences in cognitive functions. There was a significant improvement in attention/executive functions, memory and motor speed from baseline up to three years, regardless of treatment modality. However, there was a significant decline in attention/executive functions and memory after year 3 in both treatment groups. Patients treated with reduced-dose WBRT had lower scores in memory (learning and delayed recall) than patients treated with high-dose chemotherapy and ASCT, particularly after year 3, although the group comparisons were not significant. Like the cognitive findings, there was an improvement in self-reported QoL in both groups, and no significant group differences. White matter abnormalities increased over time in both groups, but more patients treated with reduced-dose WBRT had cortical atrophy and white matter abnormalities. The results suggested that both treatment regimens may be associated with long delayed neurotoxicity, although the adverse effects of reduced-dose WBRT may be less severe than those described after full-dose WBRT (10,24,44). The importance of long-term follow up to characterize cognitive functions and treatment adverse effects was highlighted.
Ferreri et al. (22) studied cognitive functions prospectively in the context of the International Extranodal Lymphoma Study Group-32 (IELSG-32) in fifty-seven patients with PCNSL treated with induction HD-MTX-based chemotherapy and subsequently randomized to consolidation with either WBRT (3,600 cGy) (n=30, mean age =58 years, range, 18–70 years) or myeloablative high-dose chemotherapy and ASCT (n=27, mean age =56 years, range, 26–69 years); patients had stable disease. Patients were evaluated at baseline (pre-treatment), after completion of consolidation therapy, and every six months up to two years. Compared to baseline, there was improvement in most cognitive domains after consolidation treatment in all patients, with a significant improvement in attention, executive functions and visuospatial abilities in patients treated with high-dose chemotherapy and ASCT. A comparison between assessments performed after consolidation and at two years post-treatment (median =28 months, range, 21–31 months for WBRT, range, 23–32 months for ASCT), showed a significant decline in attention and executive functions in patients treated with consolidation WBRT and a significant improvement in attention, executive functions and memory, and QoL in patients treated with consolidation high-dose chemotherapy and ASCT. The findings suggested that WBRT was associated with more neurotoxicity than chemotherapy-only regimens, although less severe than reported in patients treated with higher radiation doses.
Houillier et al. (46) studied cognitive functions prospectively in the context of the PRECIS study in patients with PCNSL (age range, 22–60 years) treated with HD-MTX-based induction chemotherapy and randomized to receive consolidation with either WBRT (4,000 cGy) or high-dose chemotherapy and ASCT, and with stable disease. Patients were evaluated at baseline (pre-treatment) (n=104), at the end of induction chemotherapy (n=68), and at 6 months (n=57), 12 months (n=52), 18 months (n=48), 24 months (n=43), and 36 months (n=14) after consolidation treatment. Compared to baseline, there was an improvement on timed tests of graphomotor speed and cognitive flexibility, and in memory immediately following induction chemotherapy in both treatment arms. After consolidation therapy, a substantial proportion of patients treated with WBRT had lower scores in timed cognitive flexibility and memory, compared to patients treated with high-dose chemotherapy and ASCT who either remained stable or improved over time in graphomotor speed and cognitive flexibility and memory. The findings were thought to be consistent with prior studies suggesting cognitive decline after WBRT.
Conclusions
Treatment-related neurotoxicity is a significant problem in patients with PCNSL as improvements in treatment have prolonged survival. Cognitive dysfunction often limits the patients’ ability to resume their pre-diagnosis social and professional activities. A literature review indicated that cognitive outcome was assessed systematically in a relatively small number of studies, and that recent studies and clinical trials (22-24,46) have begun to incorporate standardized cognitive assessments as outcome measures, as proposed by the IPCG guidelines (15).
Most studies reported diffuse cognitive impairments at diagnosis and prior to treatment, likely related to the disease, and improvement following induction HD-MTX-based chemotherapy. Studies that assessed cognitive outcome in patients months to years after treatment with HD-MTX-based chemotherapy and WBRT, or with BBBD chemotherapy and WBRT reported cognitive impairment and extensive white matter abnormalities in most patients (10,20,24), consistent with the neurotoxicity reported after WBRT and combined modality regimens (27,28,58). Cognitive domains most likely to be disrupted include attention, executive functions, memory, and graphomotor speed. Deficits in these domains have also been documented in diseases that affect the white matter or cortical-subcortical circuitry (59). The retrospective designs in some studies limited the ability to examine the specific contributions of tumor and the delayed adverse effects of treatment.
Studies describing cognitive outcome in patients treated with HD-MTX-based regimens, or BBBD chemotherapy alone were mostly prospective. The majority reported either stable or improved cognitive performance and QoL over time in most patients (42,54,56); although declines in attention, memory, and graphomotor speed were seen in subsets of patients across studies. Of note, some of the earlier studies included patients with PD, limiting the ability to identify the specific contributions of disease and treatment. Several studies reported white matter abnormalities in patients treated with chemotherapy only regimens (23,24,41), consistent with evidence that chemotherapy, particularly HD-MTX and HD-ARA-C, is associated with neurotoxicity (33,36,38).
Recent prospective randomized studies have reported stable or improved cognitive functions over time in patients treated with induction HD-MTX-based chemotherapy followed by consolidation with HD-chemotherapy and ASCT, and cognitive decline in patients treated with consolidation WBRT (22,46); albeit the decline was less pronounced after reduced-dose WBRT compared to full-dose regimens (22,23), suggesting that radiation dose may be proportionally associated with neurotoxicity risk. Drop-out rates over time in some longitudinal studies limited the evaluation of the long-term cognitive adverse effects of different treatment modalities.
Additional collaborative, prospective, randomized studies including standardized evaluations of cognitive functions, and long-term follow ups are needed to determine the incidence of cognitive dysfunction associated with various treatment modalities in patients with PCNSL. The use of similar standardized instruments and follow up assessment intervals, as suggested by the IPCG guidelines, would facilitate the comparison of results across different studies. The findings from such studies would improve our understanding of the neurotoxicity of various treatment modalities and would provide important information to facilitate treatment decision-making and the development of interventions to prevent and improve cognitive dysfunction.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Andrés J. M. Ferreri, Maurilio Ponzoni) for the series “Central Nervous System Lymphomas” published in Annals of Lymphoma. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/aol-20-37
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-37). The series “Central Nervous System Lymphomas” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- DeAngelis LM, Seiferheld W, Schold SC, et al. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol 2002;20:4643-8. [Crossref] [PubMed]
- Grommes C, Rubenstein JL, DeAngelis LM, et al. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro Oncol 2019;21:296-305. [Crossref] [PubMed]
- Villano JL, Koshy M, Shaikh H, et al. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer 2011;105:1414-8. [Crossref] [PubMed]
- Rubenstein J, Ferreri AJ, Pittaluga S. Primary lymphoma of the central nervous system: epidemiology, pathology and current approaches to diagnosis, prognosis and treatment. Leuk Lymphoma 2008;49:43-51. [Crossref] [PubMed]
- Batchelor TT, Neuwelt E, Wang DL, et al. Clinical and diagnostic considerations in primary central nervous system lymphoma. In: Batchelor T, DeAngelis L. editors. Lymphoma and Leukemia of the Nervous System. New York: Springer; 2012:113-28.
- Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol 1998;16:859-63. [Crossref] [PubMed]
- Ferreri AJ, Zucca E, Armitage J, et al. Ten years of international primary CNS lymphoma collaborative group studies. J Clin Oncol 2013;31:3444-5. [Crossref] [PubMed]
- Shiels MS, Pfeiffer RM, Besson C, et al. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol 2016;174:417-24. [Crossref] [PubMed]
- Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H, et al. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol 2003;21:4483-8. [Crossref] [PubMed]
- Correa DD, Shi W, Abrey LE, et al. Cognitive functions in primary CNS lymphoma after single or combined modality regimens. Neuro Oncol 2012;14:101-8. [Crossref] [PubMed]
- Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol 2010;11:1036-47. [Crossref] [PubMed]
- Omuro AM, Ben-Porat LS, Panageas KS, et al. Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol 2005;62:1595-600. [Crossref] [PubMed]
- Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol 2000;18:3144-50. [Crossref] [PubMed]
- Grommes C, Nayak L, Tun HW, et al. Introduction of novel agents in the treatment of primary CNS lymphoma. Neuro Oncol 2019;21:306-13. [Crossref] [PubMed]
- Correa DD, Maron L, Harder H, et al. Cognitive functions in primary central nervous system lymphoma: literature review and assessment guidelines. Ann Oncol 2007;18:1145-51. [Crossref] [PubMed]
- van der Meulen M, Dirven L, Habets EJJ, et al. Cognitive functioning and health-related quality of life in patients with newly diagnosed primary CNS lymphoma: a systematic review. Lancet Oncol 2018;19:e407-e418. [Crossref] [PubMed]
- O'Neill BP. Neurocognitive outcomes in primary CNS lymphoma (PCNSL). Neurology 2004;62:532-3. [Crossref] [PubMed]
- Behin A, DeLattre JY. Neurologic sequelae of radiotherapy on the nervous system. In: Schiff D, Wen PY. editors. Cancer Neurology in Clinical Practice. Totowa, New Jersey: Humana Press; 2003:173-91.
- Correa DD, DeAngelis LM, Shi W, et al. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology 2004;62:548-55. [Crossref] [PubMed]
- Harder H, Holtel H, Bromberg JE, et al. Cognitive status and quality of life after treatment for primary CNS lymphoma. Neurology 2004;62:544-7. [Crossref] [PubMed]
- Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 2005;23:5034-43. [Crossref] [PubMed]
- Ferreri AJM, Cwynarski K, Pulczynski E, et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol 2017;4:e510-e523. [Crossref] [PubMed]
- Correa DD, Braun E, Kryza-Lacombe M, et al. Longitudinal cognitive assessment in patients with primary CNS lymphoma treated with induction chemotherapy followed by reduced-dose whole-brain radiotherapy or autologous stem cell transplantation. J Neurooncol 2019;144:553-62. [Crossref] [PubMed]
- Doolittle ND, Korfel A, Lubow MA, et al. Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology 2013;81:84-92. [Crossref] [PubMed]
- Meyers CA, Wefel JS. The use of the mini-mental state examination to assess cognitive functioning in cancer trials: no ifs, ands, buts, or sensitivity. J Clin Oncol 2003;21:3557-8. [Crossref] [PubMed]
- Weitzner MA, Meyers CA. Cognitive functioning and quality of life in malignant glioma patients: a review of the literature. Psychooncology 1997;6:169-77. [Crossref] [PubMed]
- DeAngelis LM, Posner JB. Side effects of radiation therapy. In: DeAngelis LM, Posner JB. editors. Neurologic Complications of Cancer. 2nd ed. New York: Oxford University Press; 2009:551-5.
- Greene-Schloesser D, Robbins ME, Peiffer AM, et al. Radiation-induced brain injury: A review. Front Oncol 2012;2:73. [Crossref] [PubMed]
- Nordal RA, Wong CS. Molecular targets in radiation-induced blood-brain barrier disruption. Int J Radiat Oncol Biol Phys 2005;62:279-87. [Crossref] [PubMed]
- Dietrich J, Han R, Yang Y, et al. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol 2006;5:22. [Crossref] [PubMed]
- Monje ML, Vogel H, Masek M, et al. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol 2007;62:515-20. [Crossref] [PubMed]
- van der Maazen RW, Kleiboer BJ, Verhagen I, et al. Repair capacity of adult rat glial progenitor cells determined by an in vitro clonogenic assay after in vitro or in vivo fractionated irradiation. Int J Radiat Biol 1993;63:661-6. [Crossref] [PubMed]
- Gibson EM, Nagaraja S, Ocampo A, et al. Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell 2019;176:43-55.e13. [Crossref] [PubMed]
- Lai R, Abrey LE, Rosenblum MK, et al. Treatment-induced leukoencephalopathy in primary CNS lymphoma: a clinical and autopsy study. Neurology 2004;62:451-6. [Crossref] [PubMed]
- Constine LS, Konski A, Ekholm S, et al. Adverse effects of brain irradiation correlated with MR and CT imaging. Int J Radiat Oncol Biol Phys 1988;15:319-30. [Crossref] [PubMed]
- Dietrich J. Chemotherapy associated central nervous system damage. In: Raffa RB, Tallarida RJ. editors. Chemo Fog: Cancer Chemotherapy-Related Cognitive Impairment. Austin, Texas: Landes Bioscience and Springer Science and Business Media; 2010:77-85.
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer 2007;7:192-201. [Crossref] [PubMed]
- Salkade PR, Lim TA. Methotrexate-induced acute toxic leukoencephalopathy. J Cancer Res Ther 2012;8:292-6. [Crossref] [PubMed]
- Seigers R, Schagen SB, Coppens CM, et al. Methotrexate decreases hippocampal cell proliferation and induces memory deficits in rats. Behav Brain Res 2009;201:279-84. [Crossref] [PubMed]
- Keime-Guibert F, Napolitano M, Delattre JY. Neurological complications of radiotherapy and chemotherapy. J Neurol 1998;245:695-708. [Crossref] [PubMed]
- Estephan F, Ye X, Dzaye O, et al. White matter changes in primary central nervous system lymphoma patients treated with high-dose methotrexate with or without rituximab. J Neurooncol 2019;145:461-6. [Crossref] [PubMed]
- Juergens A, Pels H, Rogowski S, et al. Long-term survival with favorable cognitive outcome after chemotherapy in primary central nervous system lymphoma. Ann Neurol 2010;67:182-9. [Crossref] [PubMed]
- Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol 2013;31:3061-8. [Crossref] [PubMed]
- Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol 2013;31:3971-9. [Crossref] [PubMed]
- Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood 2015;125:1403-10. [Crossref] [PubMed]
- Houillier C, Taillandier L, Dureau S, et al. Radiotherapy or Autologous Stem-Cell Transplantation for Primary CNS Lymphoma in Patients 60 Years of Age and Younger: Results of the Intergroup ANOCEF-GOELAMS Randomized Phase II PRECIS Study. J Clin Oncol 2019;37:823-33. [Crossref] [PubMed]
- Pels H, Deckert-Schluter M, Glasmacher A, et al. Primary central nervous system lymphoma: a clinicopathological study of 28 cases. Hematol Oncol 2000;18:21-32. [Crossref] [PubMed]
- Neuwelt EA, Goldman DL, Dahlborg SA, et al. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: prolonged survival and preservation of cognitive function. J Clin Oncol 1991;9:1580-90. [Crossref] [PubMed]
- Dahlborg SA, Henner WD, Crossen JR, et al. Non-AIDS primary CNS lymphoma: first example of a durable response in a primary brain tumor using enhanced chemotherapy delivery without cognitive loss and without radiotherapy. Cancer J Sci Am 1996;2:166-74. [PubMed]
- Crossen JR, Goldman DL, Dahlborg SA, et al. Neuropsychological assessment outcomes of nonacquired immunodeficiency syndrome patients with primary central nervous system lymphoma before and after blood-brain barrier disruption chemotherapy. Neurosurgery 1992;30:23-9. [Crossref] [PubMed]
- McAllister LD, Doolittle ND, Guastadisegni PE, et al. Cognitive outcomes and long-term follow-up results after enhanced chemotherapy delivery for primary central nervous system lymphoma. Neurosurgery 2000;46:51-60; discussion 60-1. [Crossref] [PubMed]
- Neuwelt EA, Guastadisegni PE, Varallyay P, et al. Imaging changes and cognitive outcome in primary CNS lymphoma after enhanced chemotherapy delivery. AJNR Am J Neuroradiol 2005;26:258-65. [PubMed]
- Schlegel U, Pels H, Glasmacher A, et al. Combined systemic and intraventricular chemotherapy in primary CNS lymphoma: a pilot study. J Neurol Neurosurg Psychiatry 2001;71:118-22. [Crossref] [PubMed]
- Pels H, Schmidt-Wolf IG, Glasmacher A, et al. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol 2003;21:4489-95. [Crossref] [PubMed]
- Fliessbach K, Urbach H, Helmstaedter C, et al. Cognitive performance and magnetic resonance imaging findings after high-dose systemic and intraventricular chemotherapy for primary central nervous system lymphoma. Arch Neurol 2003;60:563-8. [Crossref] [PubMed]
- Fliessbach K, Helmstaedter C, Urbach H, et al. Neuropsychological outcome after chemotherapy for primary CNS lymphoma: a prospective study. Neurology 2005;64:1184-8. [Crossref] [PubMed]
- Doolittle ND, Dosa E, Fu R, et al. Preservation of cognitive function in primary CNS lymphoma survivors a median of 12 years after enhanced chemotherapy delivery. J Clin Oncol 2013;31:4026-7. [Crossref] [PubMed]
- Dietrich J, Monje M, Wefel J, et al. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist 2008;13:1285-95. [Crossref] [PubMed]
- Bobholz JA, Rao SM. Cognitive dysfunction in multiple sclerosis: a review of recent developments. Curr Opin Neurol 2003;16:283-8. [Crossref] [PubMed]
Cite this article as: Correa DD. Narrative review of primary central nervous system lymphoma: treatment-related neurotoxicity. Ann Lymphoma 2021;5:13.


