
Primary central nervous system lymphoma: a narrative review of ongoing clinical trials and goals for future studies
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare disease affecting the brain, spine, cerebrospinal fluid (CSF), and or/eyes with an incidence of 0.4 per 100,000 person-years (1). While PCNSL is chemo and radiosensitive, relapse is as common as 50% in the first two years (2), highlighting the need for improved durable therapies.
There is little consensus on the management of PCNSL, aside from a general agreement on the importance of high-dose methotrexate (HD-MTX) in the first-line setting. Recommended doses, drug combinations, and duration of therapy are inconsistent, with practitioners often relying on regional and institutional preferences. Variations in treatment can be attributed to the paucity of prospective, randomized clinical trial data. Due to the rarity of PCNSL, phase III studies powered for comparison are difficult to conduct and take many years to produce meaningful results. As a result, many therapeutic trials are single-arm or randomized phase II studies, to be interpreted with caution. Moreover, data generated by successfully completed trials may be of limited generalizability in the clinic. While PCNSL disproportionately affects the elderly with a median age of diagnosis of 67 (3), many trials restrict enrollment to younger patients. As a result, there is a lack of safe and effective treatment options for the elderly population. Additionally, patients may have involvement of multiple central nervous system (CNS) compartments including the CSF or intraocular space. Whether these compartments require dedicated treatment remains unclear as such patients are often underrepresented or under-described in most trials.
This review discusses recently completed and ongoing studies in PCNSL while highlighting the complexity of clinical trial design in this disease. It provides an overview of outstanding questions in the field and the ongoing trials that are attempting to answer them. It also attempts to provide a framework for the design of future studies. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/aol-20-47).
Induction therapy
The difficulties of designing a study for PCSNL are highlighted by the paucity of prospective data. The first attempted randomized study in this disease accrued only 53 patients over 7 years and was ultimately terminated due to poor enrollment (4) (Table 1). It was not until 2009 that the first randomized study in PCNSL was published. The phase II trial compared HD-MTX monotherapy (3.5 g/m2) to HD-MTX (3.5 g/m2) + high-dose cytarabine. While the study was not powered for comparison between arms, data favored combination therapy with a complete remission rate (CRR) of 18% vs. 46% (primary endpoint), a 3-year failure-free survival of 21% vs. 38% and a 3-year overall survival (OS) of 32% vs. 46% (12). Rather than defining a new standard for induction, this study was interpreted to indicate polychemotherapy with HD-MTX-based treatment is superior to HD-MTX alone. Several single arm phase II studies seemingly found success with alternate induction polychemotherapy regimens that combine MTX with an alkylating agent. Popular regimens include HD-MTX, carmustine, etoposide, and prednisone (MBVP); HD-MTX, vincristine, and procarbazine (MVP); and HD-MTX, temozolomide, and rituximab (R-MT) to highlight a few (19,25,30-32). With increasing concern over the inadequacy of HD-MTX monotherapy, the German Primary CNS Lymphoma Study Group (G-PCNSL-SG) amended their ongoing trial in 2006 to include the addition of ifosfamide to MTX 4 g/m2 in the induction regimen (9). Notably, many of the studied combination regimens use MTX dosed 3–4 g/m2. An unanswered question is whether higher doses of MTX may be more effective and obviate the need for combination treatment. In 2003 the New Approaches to Brain Tumor Therapy (NABTT) CNS Consortium published a study of 8 g/m2 MTX monotherapy that demonstrated response rates comparable to historical controls [CRR 52%, overall response rate (ORR) 74%) with only modest toxicity (48% grade 3 or 4 events] (16).
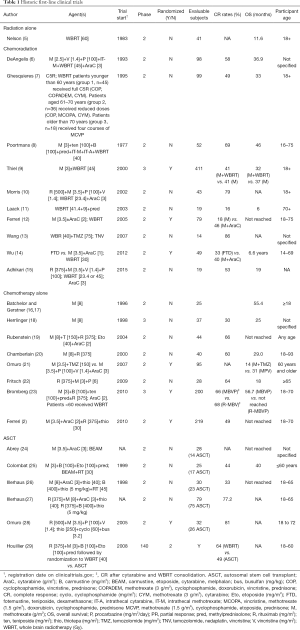
Full table
To date, only a handful of randomized studies comparing induction strategies have been published. In 2016, the International Extranodal Lymphoma Study Group-32 (IELSG32) compared HD-MTX + high-dose cytarabine; HD-MTX, high-dose cytarabine + rituximab; and HD-MTX, high-dose cytarabine, rituximab, + thiotepa (MATRix). The study was not powered for comparison between arms limiting interpretation though CRR of 49% (primary endpoint) appeared superior in the MATRix arm of treatment. The addition of rituximab to HD-MTX and cytarabine appeared to result in improved CRR to 30% from 23% with HD-MTX and cytarabine alone (2). An important criticism of this study is the seemingly poor outcomes of the control arm. While the IELSG32 saw a CRR of 23% and ORR of 53% with HD-MTX + high-dose cytarabine, the same regimen resulted in a CRR 46% with ORR 69% seven years prior (2,12). The authors argue the data reflects different patient populations with the IELSG32 study enrolling more patients with high-risk features such as increased elevated serum LDH and CSF protein, presence of meningeal spread and involvement of deep structures (2). Currently the MATRix protocol is being used as the induction regimen for an ongoing phase III clinical trial comparing consolidation therapies (NCT02531841) (Table 2). It remains to be seen whether its use will be widely adopted as standard practice across the globe.
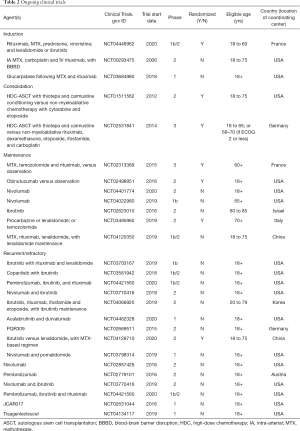
Full table
Challenging the results of the IELSG32 and earlier retrospective data (33), the Cooperative Trial of the Hemato-Oncologie voor Volwassenen Nederland (HOVON) and Australasian Leukaemia and Lymphoma Group (ALLG) recently published a phase III randomized trial (HOVON 105/ALLG NHL 24) addressing the role of rituximab in first-line treatment for PCNSL. The study enrolled 200 patients over 6 years to receive HD-MTX, carmustine, teniposide, and prednisone, with or without rituximab. Patients with response received consolidation with high-dose cytarabine and, if 60 years of age or younger, whole-brain radiotherapy (WBRT). Ultimately, the study found no statistical difference in their primary endpoint of event free survival (EFS) at one year (49% without rituximab vs. 52% with rituximab) (23). While the authors did comment on a trend toward improved EFS with rituximab in younger patients, the significance of this is unclear as the study was not sufficiently powered to address this question. The results of this phase III study highlight some of the difficulties in trial design for a heterogeneous population and the dangers of over-interpreting non-comparative phase II data. With conflicting results and the relative tolerability of rituximab, many practitioners have continued to incorporate rituximab into treatment regimens and it is still being used in many ongoing clinical trials. For example, LOC-R01 will include rituximab with MVP plus lenalidomide or ibrutinib for the treatment of newly diagnosed patients aged 18–60 (NCT04446962).
Rare studies have taken the novel approach of omitting HD-MTX from first-line treatment entirely. After a 2014 study of fotemustine, teniposide, and dexamethasone (FTD) appeared to demonstrate comparable efficacy to historical controls (34), a phase II study compared this regimen to HD-MTX + high-dose cytarabine. The regimens appeared to yield similar results with ORR 88% in the FTD arm vs. 84% with HD-MTX + high-dose cytarabine though the sample size of 49 patients was small, limiting interpretation (14). Interestingly, a higher incidence of cognitive decline following WBRT was reported in the HD-MTX arm. As such, the FTD regimen warrants further study for patients with contra-indication to HD-MTX or planned for radiation.
Other studies are attempting to optimize MTX delivery. Doses required to achieve cytotoxic drug concentrations in the CSF can result in acute renal or hepatic injury, pneumonitis, and bone marrow suppression (35). Dose reduction or premature cessation of therapy is not uncommon, particularly in the elderly population (36,37). Intra-arterial (IA)-MTX with blood brain barrier disruption (BBBD) may allow for increased chemotherapy penetration into the CNS, potentially improving efficacy or reducing dose of MTX required to achieve desired results. A multi-center study of IA MTX with BBBD demonstrated ORR 81.9% (CRR 57.8%) with OS 3.1 years, comparing favorably to historical controls. Notably, approximately half the patients in the study were aged 60 or older and did not undergo consolidation therapy (38). Prospective neuropsychological tests demonstrated preservation of cognitive functioning from the end of treatment to a median follow up 12 years after diagnosis (39). Use of IA MTX and BBBD remains under investigation with an ongoing study enrolling patients with newly diagnosed PCNSL for treatment with IA MTX, rituximab and carboplatin with BBBD (NCT00293475).
Empiric use of glucarpidase is another potential strategy for enhancing MTX delivery. Glucarpidase is a bacterial recombinant enzyme that results in rapid clearance of systemic MTX. It is currently approved by the Food and Drug Administration for use in patients with MTX toxicity and acute renal failure. CSF MTX concentrations do not appear affected by glucarpidase as it does not cross the blood brain barrier (40). Its use is currently being investigated in PCNSL to achieve rapid systemic MTX clearance without impact on efficacy in the CNS. Preliminary data from our ongoing study demonstrates glucarpidase can be given throughout MTX treatment. CSF MTX concentrations appear to remain cytotoxic following glucarpidase administration and clinical response to MTX is appreciated (41). Further investigation is needed to determine whether use of glucarpidase might prevent need for MTX dose reduction, which can be required in as many as 75% of patients (37). Currently we are utilizing glucarpidase to investigate feasibility of outpatient HD-MTX administration (NCT03684980).
Consolidation therapy
The optimal consolidation regimen for PCNSL has yet to be elucidated and likely differs based on patient and disease characteristics. While WBRT was the mainstay of treatment for many years, cognitive deficits and lackluster survival data have led to the exploration of other options. In 2010, the first phase III study conducted in PCNSL attempted to address the need for WBRT. Designed as a non-inferiority trial, the study randomized patients with a complete response (CR) following MTX-based treatment to receive WBRT 36 Gy or undergo observation. Patients without a CR were randomized to WBRT (36–40 Gy with 9 Gy boost) or high-dose cytarabine. While the study detected no significant difference in OS between treatment groups (32.4 months with WBRT vs. 37.1 without WBRT), it failed to meet the pre-determined non-inferiority margin (9). The study collected little cognitive data but did report clinical neurotoxicity in 49% of assessable patients treated with WBRT as compared to 26% of patients who did not receive WBRT. These findings, particularly in patients aged 60 and older, are consistent with those previously reported (42,43).
Whether low dose WBRT may reduce neurotoxicity without compromising disease control remains to be seen. A single arm study of WBRT 23.4 Gy following rituximab-MVP-cytarabine (R-MVP-A) yielded an ORR of 78% and median progression-free survival (PFS) of 7.7 months (10). There was no impact on cognition initially reported however, a decline in attention and executive functioning was detected after 3 years, following an initial improvement (44). RTOG 1114 is an ongoing randomized trial comparing R-MVP-A with WBRT 23.4 Gy to R-MVP-A alone. Preliminary results presented at the American Society of Clinical Oncology (ASCO) 2020 meeting demonstrate improved 2-year PFS in the radiation arm (78% vs. 54%) with median OS not reached in either arm (45). In these preliminary results, neurotoxicity did not appear to differ between the two arms though long term follow up is required.
Based on the ongoing concerns surrounding WBRT, many in the field have sought alternate methods of consolidation. Myeloablative high-dose chemotherapy followed by autosomal stem cell transplant (HDC-ASCT) offers the benefit of increased drug concentrations in the CNS and may allow for disease control without the need for radiation. Recent data on HDC-ASCT suggest this is a promising strategy for patients who are eligible. Two single-arm phase II studies of HDC-ASCT following MTX-based induction yielded ORR >90% with prolonged PFS and median OS not yet reached (27,28). Notably, both these studies utilized thiotepa-based consolidation regimens and yielded superior results to historical studies in which BCNU-based regimens were utilized (24,25,46).
Early results of two randomized studies exploring the efficacy and tolerability of HDC-ASCT were recently published. IELSG32 randomized all patients with stable disease or response after induction therapy (HD-MTX + Ara-C ± rituximab ± thiotepa) to receive 36 Gy WBRT or HDC-ASCT with carmustine and thiotepa conditioning. Initial results suggest no significant difference in the primary end point (2-year PFS 80% WBRT vs. 69% HDC-ASCT) (47). Of note, only 50–60% of patients in each induction arm proceeded to the second phase of randomization, again highlighting the poor outcomes seen in the induction portion of this study (2). The phase II PRECIS study similarly randomized patients to receive WBRT (40 Gy) or HDC-ASCT. Both arms met the pre-determined threshold for efficacy with 2-year PFS 63% in the WBRT arm and 87% in the HDC-ASCT arm (29). Neither study was powered for comparison, limiting interpretation. Cognitive decline was noted after WBRT in both studies while patients treated with HDC-ASCT had improved cognition at two or three years of follow up (29,47). Continued long term assessment will be required as decline in executive functioning more than three years following HDC-ASCT has been reported (44).
Early studies of HDC-ASCT largely limited eligibility to patients younger than 60–70 (26,27,29,47,48). Only recently was this method of consolidation prospectively explored in a population that included patients >70 years of age. Schorb et al. reported that HDC-ASCT with busulfan/thiotepa conditioning was feasible and well tolerated in a carefully selected group of fourteen patients with a median age of 74 (range, 69–79) with no treatment related deaths (49). However only 40% of screened patients met eligibility for enrollment, highlighting the fact that there is a subset of patients, predominantly the elderly, for whom HDC-ASCT is not an option and whom are high risk for neurotoxicity with WBRT. In these patients, consolidation strategies consist of non-myeloablative chemotherapy or maintenance therapy. It is an open question whether either strategy is as efficacious as radiation or HDC-ASCT. Encouragingly, a single arm phase II study of R-MT followed by consolidation with etoposide and cytarabine yielded responses comparable with historical controls (ORR 72%, PFS 48 months) (19). CALGB (Alliance) 51101 randomized patients to consolidation with non-myeloblastic chemotherapy with cytarabine and etoposide vs. HDC-ASCT with thiotepa and carmustine conditioning. Preliminary results reporting response to the induction regimen were disappointing with only two-thirds of patients proceeding to consolidation (37) however, results of the consolidation phase are pending (NCT01511562). IELSG43 is another ongoing study which randomizes patients after response to MATRix induction therapy to receive either HDC-ASCT with carmustine/thiotepa conditioning or a nonmyeloablative regimen of rituximab, dexamethasone, etoposide, ifosfamide, and carboplatin (NCT02531841).
Ongoing maintenance therapy, rather than consolidation, is another potential treatment strategy. Two early phase prospective trials support the use of TMZ maintenance. RTOG 0227 treated patients with R-MT followed by WBRT and temozolomide 200 mg/m2 days 1–5 every 4 weeks. The study reported an ORR of 87.5% and a 2-year OS of 81% (32). Notably the Nordic Lymphoma Group also utilized TMZ maintenance though in an elderly population and following age-adjusted MTX and cytarabine. This study omitted WBRT and saw 2-year OS of 60% (50). Unfortunately a phase III study comparing WBRT to WBRT with concurrent and then maintenance TMZ following HD-MTX was recently terminated due to futility after 134 patients were enrolled (51) raising the question of benefit of maintenance TMZ. Complicating interpretation of this study is the potentially sub-optimal induction chemotherapy regimen of HD-MTX monotherapy. Further light may be shed on this issue with the completion of BLOCAGE-01, a phase III study of patients with CR following HD-MTX, randomized to receive seven cycles of R-MT or no further treatment (NCT02313389).
Multiple studies have also provided evidence for maintenance with rituximab, procarbazine, ibrutinib, and lenalidomide (22,52-55). Based on reports which suggest maintenance rituximab may prolong disease control (52,55) a multi-center randomized phase II study was developed to evaluate the effect of maintenance obinutuzumab on CR duration in patients who attain a CR to first-line HD-MTX-based chemotherapy (NCT02498951). Ongoing studies with obinutuzumab and nivolumab maintenance will further add to our understanding of the role of immunotherapy in this disease and may provide a treatment alternative to prolong response duration for elderly and high risk patients, with minimal toxicity (NCT04401774, NCT04022980). An additional study in the elderly population is examining the role of ibrutinib maintenance following response to HD-MTX based therapy (NCT02623010). FIORELLA, a phase II study of patients ≥70, will eventually provide robust data as patients who are considered eligible for HD-MTX will receive induction HD-MTX, procarbazine, and rituximab then be randomized to receive either procarbazine or lenalidomide maintenance. Alternatively, patients unable to tolerate HD-MTX will receive WBRT with concurrent TMZ and rituximab followed by maintenance TMZ (NCT03495960).
Recurrent/refractory disease
Despite aggressive treatment, 15% of PCNSL cases are refractory to first-line therapy and the probability of recurrence after initial response remains high (56). There is no consensus on disease management in these settings as there are few prospective studies and no randomized trials. A variety of chemotherapeutic options have been studied and have demonstrated modest responses including MTX rechallenge, temozolomide, pemetrexed, topotecan, and rituximab (57-62) (Table 3).
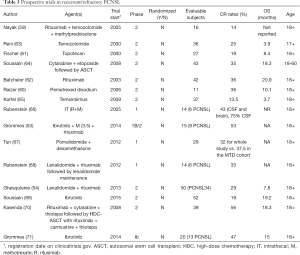
Full table
Increased understanding of the pathophysiology of PCNSL has led to developments in targeted therapy. PCNSL harbors frequent mutations in the B-cell receptor (BCR) and toll-like receptor (TLR) pathways, mediated in part by Bruton tyrosine kinase (BTK). Ibrutinib is a BTK inhibitor that has been shown to induce response in recurrent/refractory PCNSL and is detectable in the CSF at concentrations necessary to induce BTK inhibition (71). Ibrutinib has demonstrated efficacy both as a single agent (69,72), and in combination with alkylating therapy (72) as well as MTX/rituximab (53). It has also been studied as a maintenance agent (53) though data suggests early resistance mechanisms are a concern. Currently, multiple studies are ongoing combining ibrutinib with alternate treatment strategies including rituximab and lenalidomide (NCT03703167), copanlisib (NCT03581942), check point inhibition (NCT04421560, NCT03770416), and traditional chemotherapies such as ifosfamide and etoposide (NCT04066920). Acalabrutinib is a second generation BTK inhibitor approved for treatment of chronic and small lymphocytic leukemias as well as mantle cell lymphoma and will be tested in an upcoming phase I study for newly diagnosed PCNSL patients unable to tolerate HD-MTX (NCT04462328).
Alterations of the PI3K/mTOR pathway are also commonly detected in PCNSL. Early attempts to inhibit mTOR with temsirolimus yielded disappointing results. Initial radiographic findings suggested early response however, PFS was only 2.1 months suggesting development of resistance (65). Notably, CSF concentrations of temsirolimus were detected in only one of nine tested patients, suggesting poor CNS penetration (65). Current studies are ongoing for two additional agents targeting this pathway: PQR309, a dual PI3K/mTOR inhibitor (NCT02669511), and copanlisib, a PI3K inhibitor (NCT03581942). Both drugs are being studied in recurrent/refractory PCNSL. To address concerns regarding resistance, copanlisib is being combined with ibrutinib (73). The study is also open to newly diagnosed patients with contra-indications to HD-MTX.
Immunomodulatory drugs (IMiDs) such as lenalidomide and pomalidomide are also being investigated for use in PCNSL. A phase I study of lenalidomide alone and in combination with intrathecal rituximab demonstrated an objective response in nine of 14 patients. Notably, lenalidomide was detected in the CSF in dose-dependent concentrations (74). In a multi-center phase II study, lenalidomide was administered with rituximab followed by lenalidomide maintenance for responders. The primary objective was ORR at the end of induction therapy. Best observed responses during induction resulted in an ORR 63% (16/43 CR, 11/43 PR) however by the end of induction, ORR was 39%. Results of the maintenance phase are pending (54). Current studies combining lenalidomide with ibrutinib (NCT03703167) and MTX-based regimens, (NCT04129710, NCT04446962) are underway in recurrent/refractory disease. It is also being studied as maintenance therapy for newly diagnosed disease (NCT04120350, NCT03495960). Pomalidomide, a third general IMiD has also demonstrated some efficacy in PCNSL through a phase I dose escalation study (67). It is currently being studied in conjunction with nivolumab for patients with recurrent/refractory PCNSL or primary vitreoretinal disease (NCT03798314).
Immunotherapy is of increasing interest in the treatment of PCNSL. PD-1 ligand deregulation is seen in >50% of PCNSL suggesting a role for PD-1 inhibition (75). A small retrospective study that included 4 patients with PCNSL demonstrated PFS between 14–17 months and in two patients, a durable response >12 months (76). A more recent study of PD-1 blockade in combination with rituximab in 6 patients with recurrent PCNSL or isolated CNS relapse of systemic lymphoma yielded a 50% CRR. One patient in the series experienced progression after cessation of therapy and re-attained CR with re-initiation of PD-1 blockade (77). Currently PD-1 blockade is being studied in the monotherapy setting (NCT02857426, NCT02779101), in conjunction with other agents such as ibrutinib (NCT03770416, NCT04421560) or pomalidomide (NCT03798314) and as a maintenance/consolidation strategy (NCT04401774, NCT04022980). PDL-1 blockade is also being studied in a single institution phase 1 study (NCT04462328). Chimeric antigen receptor-T (CAR-T) cells are an exciting and novel strategy in systemic lymphoma. Initial concerns regarding neurotoxicity and impaired T-cell expansion in the absence of systemic disease led to the exclusion of PCNSL patients from early CAR-T trials. NCT02631044 allowed enrollment of patients with secondary CNS involvement in the presence of systemic disease and reported on a singular case of a patient achieving a CR in the brain following therapy with JCAR017, a CD19-directed CAR-T cell product (78). Since this time, a retrospective report of patients treated with off-label tisagenlecleucel, another CD19-directed CAR-T, yielded responses in 4/8 patients with minimal toxicity and evidence of T-cell expansion even in patients with isolated CNS disease (79). A study investigating the use of tisagenlecleucel in PCNSL is currently recruiting (NCT04134117).
Goals for future studies
Despite the recently completed and ongoing studies in PCNSL, a number of outstanding questions remain unanswered. To advance this field, more well-designed appropriately-powered clinical trials are needed. An important limitation however, is the rarity of PCNSL in the general population. This issue can be addressed in two ways: by increasing the number of patients enrolled onto study and by maximizing efficiency of the studies performed. Whenever possible, PCNSL patients should be referred to a specialized cancer center where they may be enrolled on an investigational protocol. Investigators designing trials should be mindful of overly restrictive eligibility criteria that may unnecessarily exclude patients (80). Randomized control trials offer the most reliable data and serve as the gold standard in evidence-based medicine however, a large number of patients is necessary for their completion. The field of neuro-oncology has begun to circumvent this problem in the treatment of glioma by opening multi-center, multi-arm platform studies with comparison of several experimental therapies to a single contemporary control arm (81). Such platforms are optimal for rare diseases as they allow for a reduction in the overall number of contemporary control patients necessary to answer multiple scientific questions simultaneously. Such a strategy may be considered in the future of PCNSL but would require some consensus in the field regarding an appropriate control. In the meantime, multi-stage studies such as IELSG32 with more than one randomization, maximize efficiency by attempting to answer two different questions with the same study group (2).
An important factor to consider in trial design is the achievability of the primary endpoint. While demonstration of improved OS is optimal, such studies can take years to mature before yielding meaningful data. As a result, many studies are appropriately opting for shorter endpoints such as CRR, ORR, or pre-determined points of assessment of PFS or OS. This problem may be aided by the identification of novel bio or imaging markers that could be used to assess response. For example, detection of ct-DNA in the CSF of PCNSL patients appears associated with treatment response and/or relapse (53). This biomarker is now being used to determine eligibility for maintenance therapy in an ongoing phase II study (NCT04401774). Further data is needed to determine whether its presence might serve as a surrogate endpoint. Concentration of interleukin-10 in the CSF has also been associated with treatment response and duration (68,82). Imaging biomarkers are also the source of much interest with recent work suggesting correlations between perfusion imaging, diffusion-weighted sequences, or texture parameters to outcome measures (83-86).
To further optimize clinical studies, it is essential we are studying the correct treatments. Only therapeutics with sound biologic rationale and promising early phase clinical data should be brought to a larger setting. Efficacy stopping rules should be in place in the event of an unsuccessful therapy. In the study of novel agents, it is crucial to incorporate tissue sampling (CSF or biopsy if able) to gather pharmacokinetic data. CSF is often used as a surrogate for brain parenchyma but pharmacokinetics are likely different between these spaces. Notably, temsirolimus was detected at adequate concentrations in glioma tissue (87) however was not detectable in the CSF in eight of nine patients with PCNSL (65).
Trials involving new therapies or enrolling the elderly should include robust assessment of neurocognitive functioning and quality of life metrics as the minimization of neurotoxicity is a primary goal in PCNSL treatment. As PCNSL treatments intensify, durable remission rates and survival are expected to increase. Increased survival in combination with intact cognitive abilities is an important benchmark for determining optimum PCNSL therapies. While early studies relied on the Mini Mental Status Exam (MMSE), this is an insensitive test for cognitive decline in PCNSL (88). A standard battery of cognitive tests would allow for comparison across trials. The specific battery proposed by Correa et al. in conjunction with the International PCNSL Collaborative Group (IPCG) has the benefit of testing across multiple cognitive domains, is easy to administer, has been translated into several languages, and has demonstrated high retest reliability (88). Imaging biomarkers including quantification of T2 signal change on magnetic resonance imaging (MRI) and analysis of diffusion tensor imaging (DTI) should be assessed whenever feasible as they have been associated with cognitive decline after cancer-directed treatment (43,89). Analysis of functional MRI may be beneficial in predicting patients at risk for treatment-related cognitive impairment and following change in brain function (90,91). All studies should include prospective pre-treatment and long term follow up whenever possible.
Summary
While there is no consensus on the exact management of PCNSL, general guidelines are agreed upon. These include the use of HD-MTX for induction therapy when possible, followed by consolidation or maintenance. Recommendations for consolidation/maintenance should ideally be tailored to individual patients, taking into account age, performance status, general medical health, and response to induction treatment.
The use of novel targeted agents and immunotherapies are currently being investigated in PCNSL and hold much promise. Optimization of clinical trial design and enrollment is needed to streamline study of these new approaches and hopefully render a new standard of care.
Acknowledgments
Funding: This article was supported, in part, by the MSK Cancer Center Support Grant (P30 CA008748), National Institute of Health. CG was supported by grants from Cycle for Survival Equinox and the Leukemia & Lymphoma Society.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Andrés J. M. Ferreri, Maurilio Ponzoni) for the series “Central Nervous System Lymphomas” published in Annals of Lymphoma. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/aol-20-47
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-47). The series “Central Nervous System Lymphomas” was commissioned by the editorial office without any funding or sponsorship. Dr. LRS reports the institutional NIH MSK Cancer Center Support Grant (P30 CA008748) partially supported this review; personal fees from Debiopharm, outside the submitted work. In addition, Dr. LRS has a patent related to low-dose glucarpidase pending. Dr. CG reports the institutional NIH MSK Cancer Center Support Grant (P30 CA008748) partially supported this review; personal fees from BTG, personal fees from Kite, personal fees from ONO, outside the submitted work. In addition, Dr. CG has a patent related to low-dose glucarpidase pending. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mendez JS, Ostrom QT, Gittleman H, et al. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol 2018;20:687-94. [Crossref] [PubMed]
- Ferreri AJ, Cwynarski K, Pulczynski E, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 2016;3:e217-27. [Crossref] [PubMed]
- Shiels MS, Pfeiffer RM, Besson C, et al. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol 2016;174:417-24. [Crossref] [PubMed]
- Mead GM, Bleehen NM, Gregor A, et al. A medical research council randomized trial in patients with primary cerebral non-Hodgkin lymphoma: cerebral radiotherapy with and without cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy. Cancer 2000;89:1359-70. [Crossref] [PubMed]
- Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin's lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys 1992;23:9-17. [Crossref] [PubMed]
- DeAngelis LM, Seiferheld W, Schold SC, et al. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol 2002;20:4643-8. [Crossref] [PubMed]
- Ghesquieres H, Ferlay C, Sebban C, et al. Long-term follow-up of an age-adapted C5R protocol followed by radiotherapy in 99 newly diagnosed primary CNS lymphomas: a prospective multicentric phase II study of the Groupe d'Etude des Lymphomes de l'Adulte (GELA). Ann Oncol 2010;21:842-50. [Crossref] [PubMed]
- Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H, et al. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol 2003;21:4483-8. [Crossref] [PubMed]
- Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol 2010;11:1036-47. [Crossref] [PubMed]
- Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol 2013;31:3971-9. [Crossref] [PubMed]
- Laack NN, Ballman KV, Brown PB, et al. Whole-brain radiotherapy and high-dose methylprednisolone for elderly patients with primary central nervous system lymphoma: Results of North Central Cancer Treatment Group (NCCTG) 96-73-51. Int J Radiat Oncol Biol Phys 2006;65:1429-39. [Crossref] [PubMed]
- Ferreri AJ, Reni M, Foppoli M, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet 2009;374:1512-20. [Crossref] [PubMed]
- Wang Y, Liu B, Xu D, et al. Phase II trial of temozolomide plus concurrent whole-brain radiation followed by TNV regimen as adjuvant therapy for patients with newly diagnosed primary CNS lymphoma. Neurol India 2013;61:260-4. [Crossref] [PubMed]
- Wu J, Duan L, Zhang L, et al. Fotemustine, teniposide and dexamethasone versus high-dose methotrexate plus cytarabine in newly diagnosed primary CNS lymphoma: a randomised phase 2 trial. J Neurooncol 2018;140:427-34. [Crossref] [PubMed]
- Adhikari N, Biswas A, Gogia A, et al. A prospective phase II trial of response adapted whole brain radiotherapy after high dose methotrexate based chemotherapy in patients with newly diagnosed primary central nervous system lymphoma-analysis of acute toxicity profile and early clinical outcome. J Neurooncol 2018;139:153-66. [Crossref] [PubMed]
- Batchelor T, Carson K, O'Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol 2003;21:1044-9. [Crossref] [PubMed]
- Gerstner ER, Carson KA, Grossman SA, et al. Long-term outcome in PCNSL patients treated with high-dose methotrexate and deferred radiation. Neurology 2008;70:401-2. [Crossref] [PubMed]
- Herrlinger U, Kuker W, Uhl M, et al. NOA-03 trial of high-dose methotrexate in primary central nervous system lymphoma: final report. Ann Neurol 2005;57:843-7. [Crossref] [PubMed]
- Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol 2013;31:3061-8. [Crossref] [PubMed]
- Chamberlain MC, Johnston SK. High-dose methotrexate and rituximab with deferred radiotherapy for newly diagnosed primary B-cell CNS lymphoma. Neuro Oncol 2010;12:736-44. [Crossref] [PubMed]
- Omuro A, Chinot O, Taillandier L, et al. Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol 2015;2:e251-9. [Crossref] [PubMed]
- Fritsch K, Kasenda B, Schorb E, et al. High-dose methotrexate-based immuno-chemotherapy for elderly primary CNS lymphoma patients (PRIMAIN study). Leukemia 2017;31:846-52. [Crossref] [PubMed]
- Bromberg JEC, Issa S, Bakunina K, et al. Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): a randomised, open-label, phase 3 intergroup study. Lancet Oncol 2019;20:216-28. [Crossref] [PubMed]
- Abrey LE, Moskowitz CH, Mason WP, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol 2003;21:4151-6. [Crossref] [PubMed]
- Colombat P, Lemevel A, Bertrand P, et al. High-dose chemotherapy with autologous stem cell transplantation as first-line therapy for primary CNS lymphoma in patients younger than 60 years: a multicenter phase II study of the GOELAMS group. Bone Marrow Transplant 2006;38:417-20. [Crossref] [PubMed]
- Illerhaus G, Marks R, Ihorst G, et al. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol 2006;24:3865-70. [Crossref] [PubMed]
- Illerhaus G, Kasenda B, Ihorst G, et al. High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: a prospective, single-arm, phase 2 trial. Lancet Haematol 2016;3:e388-97. [Crossref] [PubMed]
- Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood 2015;125:1403-10. [Crossref] [PubMed]
- Houillier C, Taillandier L, Dureau S, et al. Radiotherapy or Autologous Stem-Cell Transplantation for Primary CNS Lymphoma in Patients 60 Years of Age and Younger: Results of the Intergroup ANOCEF-GOELAMS Randomized Phase II PRECIS Study. J Clin Oncol 2019;37:823-33. [Crossref] [PubMed]
- Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol 2000;18:3144-50. [Crossref] [PubMed]
- Ferreri AJ, Reni M, Dell'Oro S, et al. Combined treatment with high-dose methotrexate, vincristine and procarbazine, without intrathecal chemotherapy, followed by consolidation radiotherapy for primary central nervous system lymphoma in immunocompetent patients. Oncology 2001;60:134-40. [Crossref] [PubMed]
- Glass J, Won M, Schultz CJ, et al. Phase I and II Study of Induction Chemotherapy With Methotrexate, Rituximab, and Temozolomide, Followed By Whole-Brain Radiotherapy and Postirradiation Temozolomide for Primary CNS Lymphoma: NRG Oncology RTOG 0227. J Clin Oncol 2016;34:1620-5. [Crossref] [PubMed]
- Holdhoff M, Ambady P, Abdelaziz A, et al. High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology 2014;83:235-9. [Crossref] [PubMed]
- Wu JJ, Wang XH, Li L, et al. Fotemustine, teniposide and dexamethasone in treating patients with CNS lymphoma. Asian Pac J Cancer Prev 2014;15:4733-8. [Crossref] [PubMed]
- Green MR, Chamberlain MC. Renal dysfunction during and after high-dose methotrexate. Cancer Chemother Pharmacol 2009;63:599-604. [Crossref] [PubMed]
- Jahnke K, Korfel A, Martus P, et al. High-dose methotrexate toxicity in elderly patients with primary central nervous system lymphoma. Ann Oncol 2005;16:445-9. [Crossref] [PubMed]
- Batchelor T, Giri S, Ruppert AS, et al. Myeloablative versus non-myeloablative consolidative chemotherapy for newly diagnosed primary central nervous system lymphoma: Results of induction therapy in Alliance 51101. J Clin Oncol 2020;38:8042. [Crossref]
- Angelov L, Doolittle ND, Kraemer DF, et al. Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: a multi-institutional experience. J Clin Oncol 2009;27:3503-9. [Crossref] [PubMed]
- Doolittle ND, Dosa E, Fu R, et al. Preservation of cognitive function in primary CNS lymphoma survivors a median of 12 years after enhanced chemotherapy delivery. J Clin Oncol 2013;31:4026-7. [Crossref] [PubMed]
- DeAngelis LM, Tong WP, Lin S, et al. Carboxypeptidase G2 rescue after high-dose methotrexate. J Clin Oncol 1996;14:2145-9. [Crossref] [PubMed]
- Schaff LR, Sener U, Gavrilovic I, et al. Pilot Study of Glucarpidase in Combination with Rituximab and Methotrexate (MTX) in CNS Lymphoma (CNSL). 72nd Annual Meeting of the American Academy of Neurology (AAN). Toronto, Canada. 2020.
- Gavrilovic IT, Hormigo A, Yahalom J, et al. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol 2006;24:4570-4. [Crossref] [PubMed]
- Doolittle ND, Korfel A, Lubow MA, et al. Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology 2013;81:84-92. [Crossref] [PubMed]
- Correa DD, Braun E, Kryza-Lacombe M, et al. Longitudinal cognitive assessment in patients with primary CNS lymphoma treated with induction chemotherapy followed by reduced-dose whole-brain radiotherapy or autologous stem cell transplantation. J Neurooncol 2019;144:553-62. [Crossref] [PubMed]
- Omuro AMP, DeAngelis LM, Karrison T, et al. Randomized phase II study of rituximab, methotrexate (MTX), procarbazine, vincristine, and cytarabine (R-MPV-A) with and without low-dose whole-brain radiotherapy (LD-WBRT) for newly diagnosed primary CNS lymphoma (PCNSL). J Clin Oncol 2020;38:2501. [Crossref]
- Montemurro M, Kiefer T, Schuler F, et al. Primary central nervous system lymphoma treated with high-dose methotrexate, high-dose busulfan/thiotepa, autologous stem-cell transplantation and response-adapted whole-brain radiotherapy: results of the multicenter Ostdeutsche Studiengruppe Hamato-Onkologie OSHO-53 phase II study. Ann Oncol 2007;18:665-71. [Crossref] [PubMed]
- Ferreri AJM, Cwynarski K, Pulczynski E, et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol 2017;4:e510-e523. [Crossref] [PubMed]
- Illerhaus G, Muller F, Feuerhake F, et al. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica 2008;93:147-8. [Crossref] [PubMed]
- Schorb E, Kasenda B, Ihorst G, et al. High-dose chemotherapy and autologous stem cell transplant in elderly patients with primary CNS lymphoma: a pilot study. Blood Adv 2020;4:3378-81. [Crossref] [PubMed]
- Pulczynski EJ, Kuittinen O, Erlanson M, et al. Successful change of treatment strategy in elderly patients with primary central nervous system lymphoma by de-escalating induction and introducing temozolomide maintenance: results from a phase II study by the Nordic Lymphoma Group. Haematologica 2015;100:534-40. [Crossref] [PubMed]
- Mishima K, Nishikawa R, Narita Y, et al. Randomized phase III study of high-dose methotrexate and whole brain radiotherapy with or without concomitant and adjuvant temozolomide in patients with newly diagnosed primary central nervous system lymphoma: JCOG1114C. J Clin Oncol 2020;38:2500. [Crossref]
- Ney DE, Abrey LE. Maintenance therapy for central nervous system lymphoma with rituximab. Leuk Lymphoma 2009;50:1548-51. [Crossref] [PubMed]
- Grommes C, Tang SS, Wolfe J, et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood 2019;133:436-45. [Crossref] [PubMed]
- Ghesquieres H, Chevrier M, Laadhari M, et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective 'proof of concept' phase II study of the French Oculo-Cerebral lymphoma (LOC) Network and the Lymphoma Study Association (LYSA)dagger. Ann Oncol 2019;30:621-8. [Crossref] [PubMed]
- Ambady P, Fu R, Szidonya L, et al. Impact of maintenance rituximab on duration of response in primary central nervous system lymphoma. J Neurooncol 2020;147:171-6. [Crossref] [PubMed]
- Jahnke K, Thiel E, Martus P, et al. Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol 2006;80:159-65. [Crossref] [PubMed]
- Plotkin SR, Betensky RA, Hochberg FH, et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res 2004;10:5643-6. [Crossref] [PubMed]
- Pentsova E, Deangelis LM, Omuro A. Methotrexate re-challenge for recurrent primary central nervous system lymphoma. J Neurooncol 2014;117:161-5. [Crossref] [PubMed]
- Nayak L, Abrey LE, Drappatz J, et al. Multicenter phase II study of rituximab and temozolomide in recurrent primary central nervous system lymphoma. Leuk Lymphoma 2013;54:58-61. [Crossref] [PubMed]
- Raizer JJ, Rademaker A, Evens AM, et al. Pemetrexed in the treatment of relapsed/refractory primary central nervous system lymphoma. Cancer 2012;118:3743-8. [Crossref] [PubMed]
- Fischer L, Thiel E, Klasen HA, et al. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol 2006;17:1141-5. [Crossref] [PubMed]
- Batchelor TT, Grossman SA, Mikkelsen T, et al. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology 2011;76:929-30. [Crossref] [PubMed]
- Reni M, Zaja F, Mason W, et al. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer 2007;96:864-7. [Crossref] [PubMed]
- Soussain C, Hoang-Xuan K, Taillandier L, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. J Clin Oncol 2008;26:2512-8. [Crossref] [PubMed]
- Korfel A, Schlegel U, Herrlinger U, et al. Phase II Trial of Temsirolimus for Relapsed/Refractory Primary CNS Lymphoma. J Clin Oncol 2016;34:1757-63. [Crossref] [PubMed]
- Rubenstein JL, Li J, Chen L, et al. Multicenter phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood 2013;121:745-51. [Crossref] [PubMed]
- Tun HW, Johnston PB, DeAngelis LM, et al. Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood 2018;132:2240-8. [Crossref] [PubMed]
- Rubenstein JL, Geng H, Fraser EJ, et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv 2018;2:1595-607. [Crossref] [PubMed]
- Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II 'proof-of-concept' iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer 2019;117:121-30. [Crossref] [PubMed]
- Kasenda B, Ihorst G, Schroers R, et al. High-dose chemotherapy with autologous haematopoietic stem cell support for relapsed or refractory primary CNS lymphoma: a prospective multicentre trial by the German Cooperative PCNSL study group. Leukemia 2017;31:2623-9. [Crossref] [PubMed]
- Grommes C, Pastore A, Palaskas N, et al. Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov 2017;7:1018-29. [Crossref] [PubMed]
- Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell 2017;31:833-43.e5. [Crossref] [PubMed]
- Grommes C, Gavrilovic I, Miller AM, et al. Phase Ib of Copanlisib in Combination with Ibrutinib in Recurrent/Refractory Primary CNS Lymphoma (PCNSL). Blood 2019;134:1598. [Crossref]
- Rubenstein JL, Fridlyand J, Abrey L, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 2007;25:1350-6. [Crossref] [PubMed]
- Chapuy B, Roemer MG, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood 2016;127:869-81. [Crossref] [PubMed]
- Nayak L, Iwamoto FM, LaCasce A, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood 2017;129:3071-3. [Crossref] [PubMed]
- Ambady P, Szidonya L, Firkins J, et al. Combination immunotherapy as a non-chemotherapy alternative for refractory or recurrent CNS lymphoma. Leuk Lymphoma 2019;60:515-8. [Crossref] [PubMed]
- Abramson JS, McGree B, Noyes S, et al. Anti-CD19 CAR T Cells in CNS Diffuse Large-B-Cell Lymphoma. N Engl J Med 2017;377:783-4. [Crossref] [PubMed]
- Frigault MJ, Dietrich J, Martinez-Lage M, et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood 2019;134:860-6. [Crossref] [PubMed]
- Lee EQ, Weller M, Sul J, et al. Optimizing eligibility criteria and clinical trial conduct to enhance clinical trial participation for primary brain tumor patients. Neuro Oncol 2020;22:601-12. [Crossref] [PubMed]
- Alexander BM, Ba S, Berger MS, et al. Adaptive Global Innovative Learning Environment for Glioblastoma: GBM AGILE. Clin Cancer Res 2018;24:737-43. [Crossref] [PubMed]
- Nguyen-Them L, Costopoulos M, Tanguy ML, et al. The CSF IL-10 concentration is an effective diagnostic marker in immunocompetent primary CNS lymphoma and a potential prognostic biomarker in treatment-responsive patients. Eur J Cancer 2016;61:69-76. [Crossref] [PubMed]
- Hatzoglou V, Oh JH, Buck O, et al. Pretreatment dynamic contrast-enhanced MRI biomarkers correlate with progression-free survival in primary central nervous system lymphoma. J Neurooncol 2018;140:351-8. [Crossref] [PubMed]
- Chen C, Zhuo H, Wei X, et al. Contrast-Enhanced MRI Texture Parameters as Potential Prognostic Factors for Primary Central Nervous System Lymphoma Patients Receiving High-Dose Methotrexate-Based Chemotherapy. Contrast Media Mol Imaging 2019;2019:5481491 [Crossref] [PubMed]
- Valles FE, Perez-Valles CL, Regalado S, et al. Combined diffusion and perfusion MR imaging as biomarkers of prognosis in immunocompetent patients with primary central nervous system lymphoma. AJNR Am J Neuroradiol 2013;34:35-40. [Crossref] [PubMed]
- Baek DW, Lee SJ, Kang BW, et al. Apparent diffusion coefficient as a valuable quantitative parameter for predicting clinical outcomes in patients with newly diagnosed primary CNS lymphoma. Blood Res 2020;55:99-106. [Crossref] [PubMed]
- Kuhn JG, Chang SM, Wen PY, et al. Pharmacokinetic and tumor distribution characteristics of temsirolimus in patients with recurrent malignant glioma. Clin Cancer Res 2007;13:7401-6. [Crossref] [PubMed]
- Correa DD, Maron L, Harder H, et al. Cognitive functions in primary central nervous system lymphoma: literature review and assessment guidelines. Ann Oncol 2007;18:1145-51. [Crossref] [PubMed]
- Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol 2012;30:274-81. [Crossref] [PubMed]
- Askren MK, Jung M, Berman MG, et al. Neuromarkers of fatigue and cognitive complaints following chemotherapy for breast cancer: a prospective fMRI investigation. Breast Cancer Res Treat 2014;147:445-55. [Crossref] [PubMed]
- Deprez S, Vandenbulcke M, Peeters R, et al. Longitudinal assessment of chemotherapy-induced alterations in brain activation during multitasking and its relation with cognitive complaints. J Clin Oncol 2014;32:2031-8. [Crossref] [PubMed]
Cite this article as: Schaff LR, Ambady P, Doolittle ND, Grommes C. Primary central nervous system lymphoma: a narrative review of ongoing clinical trials and goals for future studies. Ann Lymphoma 2021;5:8.


