
Novel immunotherapy in follicular lymphoma: a narrative review
Introduction
Significant progress has been made in the understanding of the genetic and molecular features of B-cell non-Hodgkin lymphomas (NHL) in the last two decades. The expanding knowledge of the interactions, composition, and role of various immune and non-immune cells within the lymphoma microenvironment in lymphomagenesis, progression, and survival has generated great interest in targeting and modulating the immune microenvironment. Several promising novel agents have thus been developed and approved for B-cell NHL. These non-chemotherapeutic drugs harness the immune system by various mechanisms such as direct targeting of the lymphoma cells and engaging the immune system to eliminate the lymphoma cell, or targeting non-lymphoma immune cells in the tumor and promote their anti-tumor function (1-3). Follicular lymphoma (FL), like other B-cell indolent lymphomas, has an average survival of well over ten years (4). Various frontline treatment strategies include watchful waiting, single-agent rituximab, chemoimmunotherapy such as bendamustine and rituximab (BR), R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), and non-chemotherapy regimen such as lenalidomide and rituximab (R2) (5-10). The treatment choice is individualized and depends on both the patient and disease-related factors and the efficacy and toxicity profile of the drug. Although FL is a very treatable malignancy, the long survival is characterized by multiple relapses necessitating several rounds of treatment. Additionally, a small subset of patients with disease progression within 24 months of chemoimmunotherapy comprises a particularly poor prognostic group that warrants novel treatment strategies (11-13). Therefore, the goal of newer treatment modalities should focus on minimizing both short and long-term toxicities while maintaining efficacy and attempting to achieve a cure. It is in this high-risk population of FL patients where novel immunological agents may be of particular importance.
This review will broadly cover the mechanism and clinical studies of novel agents that utilize the immune system directly or indirectly for an effective anti-tumor response. These include monoclonal antibodies (mAbs), antibody-drug conjugates (ADCs) that deliver cytotoxic payload targeted to the lymphoma cells, and bispecific antibodies that have dual specificity to the tumor and immune cells, bringing them close to each other. Also, antibodies directly targeting the immune effector cells, such as T-cells and macrophages, along with agents that modulate the tumor microenvironment or target the B-cell receptor (BCR) signaling pathways, will be described. Lastly, we will review clinical studies with cellular therapies such as chimeric antigen receptor (CAR) T-cell therapy and adoptive cell therapies in FL. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/aol-20-48).
Monoclonal antibodies and antibody-drug conjugates targeting the malignant B-cell
Monoclonal antibodies targeting CD20 have changed the treatment paradigm for B-cell NHL. In FL, rituximab is used widely as a single-agent or in combination with chemotherapy. The role of rituximab in FL is well established in the frontline, maintenance, and relapsed settings and will not be discussed here (5,6,8,9,14). Mechanisms by which rituximab exerts its anti-tumor response include complement-mediated cytotoxicity, antibody-dependent cell killing (ADCC), and direct cytotoxicity via apoptosis (15-17). To manage patients who progress after receiving rituximab, subsequent therapies have focused on two strategies. First, target a different epitope on CD20 or modify the Fc portion of the antibody to enhance its cell-mediated cytotoxicity. Second, target other B-cell surface antigens such as CD19, CD22, and CD79, which are typically expressed on the FL cell (Figure 1).
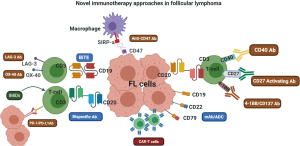
CD20 targeting antibodies
Obinutuzumab, a type II glycoengineered IgG1 mAb, shows enhanced ADCC compared to rituximab in preclinical studies (18,19). It is currently FDA approved in both the frontline and relapsed setting in FL based on the results of two phase III trials (GALLIUM and GADOLIN) (20,21). In the GALLIUM study, obinutuzumab and rituximab-based chemoimmunotherapy regimens were compared in the frontline setting. Responding patients were then continued on maintenance therapy for up to 2 years. While there was no improvement in overall survival (OS) between the two arms, the trial was stopped early as it met the primary endpoint of progression-free survival (PFS). There was an absolute difference of 6.7% in the 3-year PFS rate at the interim analysis in favor of obinutuzumab. Grade 3–5 adverse events (AEs) and serious AE’s were more frequent in the obinutuzumab arm. The GADOLIN study compared obinutuzumab in combination with bendamustine in rituximab-refractory indolent B-NHL patients (n=396, 81% FL). This trial also showed an improvement in PFS (primary endpoint) at the interim analysis, and obinutuzumab received FDA approval for relapsed/refractory (R/R) FL after a rituximab-containing regimen in 2016. A 2018 update of GADOLIN showed an improvement in OS [hazard ratio (HR), 0.67; 95% CI, 0.47–0.96) for the combination treatment (22). Grade 3–5 AEs were also seen more frequently in the combination arm with extended follow up (median 32 months), 72.5% in bendamustine and obinutuzumab combination compared to 65.5% with bendamustine alone. While these approvals have added to the treatment options in both frontline and R/R FL, some concerns remain regarding lack of OS benefit in the frontline setting and increased risk of AEs and long-term safety, especially with maintenance therapy.
Ofatumumab, a fully humanized second generation, type 1 mAb, targets a different CD20 epitope than rituximab. It has been studied both in the frontline and R/R settings in FL in phase I/II trials (23,24). The efficacy and toxicity profile appears to be comparable to the other CD20 targeting mAbs. However, the experience remains limited in FL as obinutuzumab (rather than ofatumumab) was more extensively studied in phase III studies.
Ublituximab, another type 1, chimeric, recombinant IgG1 mAb, targets a unique epitope on CD20 and is also glycoengineered to enhance its affinity for FcγRIIIa variants, thereby enhancing its ADCC effect (25). It has shown single-agent activity of around 40% in the R/R setting when studied in phase I/II trials (26). Currently, combination studies with a phosphatidylinositol 3-kinase (PI3K) inhibitor umbralisib and a Bruton tyrosine kinase (BTK) inhibitor ibrutinib are ongoing, especially in chronic lymphocytic leukemia (CLL) and other indolent B-NHLs (27,28).
CD19 targeting antibodies
MOR208 (tafasitamab) is a novel CD19 targeting humanized Fc engineered mAb with increased affinity for FcγRIIIa on effector cells resulting in enhanced ADCC, antibody-dependent cellular phagocytosis, and apoptosis (29). Single-agent activity in FL was around 30%, with a favorable toxicity profile in the preliminary studies, which makes this drug suitable for testing in combination studies (30). The combination of tafasitamab with lenalidomide was recently approved for R/R diffuse large B-cell lymphoma (DLBCL) based on the phase II L-MIND trial data showing an overall response rate (ORR) of 60%, including 43% patients achieving a complete remission (CR) (31).
Inebilizumab (MEDI-551) is also a CD19 targeting humanized mAb with enhanced ADCC in the preclinical studies (32). Initial trials have been conducted in Japan for dose-finding studies in R/R NHL, where it showed efficacy and favorable toxicity in patients with relapsed or refractory FL and DLBCL (NCT01957579) (33). It has recently been approved in the USA for an autoimmune condition, neuromyelitis optica (34). A phase 1/2 trial (NCT00983619) of inebilizumab alone or in combination with rituximab in FL, CLL, and DLBCL has recently completed recruitment in the US, and the results are currently awaited.
CD22 targeting antibodies
Epratuzumab, a humanized IgG1 mAb, targets CD22 antigen, which is expressed in FL and other B-NHLs. Multiple studies were conducted in the early 2000s evaluating this drug both as monotherapy and in combination with rituximab. Single-agent ORR for epratuzumab was noted to be around 18%, and in combination with rituximab was increased to 55–60% in R/R setting (35-37). The combination was well tolerated with few AEs and drug discontinuations. The ORR was increased to 88% in the frontline setting in FL with a 42% CR rate and a 3-year PFS rate of 60% seen in the CALGB 50701 trial (38). This drug did not move forward in phase III trials due to competing strategies and currently has no ongoing trials in FL.
Antibody drug conjugates
ADCs were developed to improve upon the efficacy of mAbs with the idea of attaching a toxic payload to the mAb and promoting its targeted delivery to the cell of interest. Like mAb, these molecules target cell surface antigens such as CD19, CD22, and CD79 expressed on the B-NHL. The toxic payload can be an anti-mitotic agent such as monomethyl auristatin E (MMAE) or a cytotoxic agent such as a pyrrolobenzodiazepine (PBD) dimer.
Polatuzumab vedotin is a CD79b targeting ADC with an MMAE payload. CD79 is an attractive target for B-NHL treatment as it forms an essential component of the BCR signaling and is almost exclusively expressed on the B-cells. It showed promising efficacy as a single agent with ORR up to 47% in indolent NHL. These responses were further improved in combination with rituximab in a phase II study (NCT01691898), where the ORR and CR rates were 70% and 45% in R/R FL, respectively (39). Similar responses were seen in another phase II trial (32 FL patients) combining obinutuzumab with polatuzumab vedotin (ORR, 78%, CR, 30%) (40). The most common grade ≥3 AEs were neutropenia, and the most common AEs in > 20% of patients were fatigue, diarrhea, and nausea. Polatuzumab was combined with BR, and when compared to the BR alone arm, no difference was found in the CR rates or PFS for FL (41). However, these were significantly different in the DLBCL arm, which led to the approval of polatuzumab-BR in R/R DLBCL patients (42). This agent has moved in the frontline setting trials in DLBCL (NCT03274492). Multiple combination studies are also ongoing evaluating polatuzumab vedotin with lenalidomide, atezolizumab (NCT02729896), venetoclax (NCT02611323), and bispecific antibody such as mosunetuzumab (NCT03671018) and glofitamab (NCT03533283) in R/R B-NHL, results for which are currently awaited.
Loncastuximab tesirine (ADCT-402) is a CD19 targeting ADC with a PBD dimer payload (43). The phase I study (88 patients) in R/R B-NHL patients (8 FL) showed overall tolerability with most common grade ≥3 AEs of anemia, thrombocytopenia, and febrile neutropenia, along with fatigue, dyspnea, and liver function test abnormalities (44). Despite small numbers, a response was seen in seven of eight FL patients (87.5%), six of whom achieved a CR. A combination phase I trial of loncastuximab tesirine with an anti-PD-L1 agent durvalumab (NCT03685344) in R/R DLBCL, and FL was recently terminated as no additional activity was evident for the combination vs. loncastuximab alone. Ongoing clinical trials evaluating this agent are being performed in DLBCL and mantle cell lymphoma (MCL).
Other ADCs: Other CD19 targeting ADCs previously evaluated are denintuzumab mafodotin (SGN-CD19A) bound to monomethyl auristatin F payload and coltuximab ravtansine (SAR3419) conjugated to potent cytotoxic maytansinoid drug DM4 (45,46). Both the payloads affect microtubule assembly and have ocular side effects (47). Coltuximab ravtansine in the first in human phase I study of 39 patient cohort with R/R B-NHL (44% FL) showed a decrease in tumor size in 74% of patients (48,49). Currently, no ongoing trials are evaluating these two drugs.
Two ADCs targeting CD22 antigen, inotuzumab ozogamicin, and pinatuzumab vedotin have been evaluated in R/R B-NHL, including FL patients. Inotuzumab ozogamicin is an IgG4 anti-CD22mAb with calicheamicin payload, an enediyne antibiotic, and induces DNA damage and cell apoptosis (50). Inotuzumab, when combined with rituximab in a phase I/II trial for R/R B-NHL (119 patients), had an ORR of 87% in the FL cohort (35%) (51). This combination did not show a significant difference when tested in the randomized phase III setting in R/R CD22+ B-NHL (52). Another phase III trial of this agent (NCT00562965) plus rituximab in FL was terminated early due to poor enrollment. Pinatuzumab vedotin, targeting CD22, has the MMAE payload. In the ROMULUS phase II study, R/R FL and DLBCL patients received a combination of rituximab with either pinatuzumab or polatuzumab. The FL patients (n=21) in the pinatuzumab arm, had ORR and CR rates of 62% and 5%, respectively. The most common grade 3–5 AEs were neutropenia and hyperglycemia (39). This drug has not moved forward in trials as polatuzumab vedotin had slightly better outcomes and was preferred.
Other surface antigens currently under investigation are CD37, CD40, CD74, and CD80. The results and toxicity profile of targeting mAbs and completed study results, and ongoing studies are shown in Tables 1 and 2.
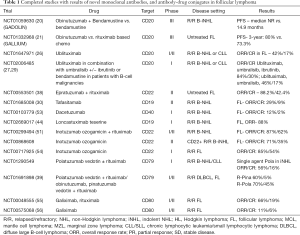
Full table
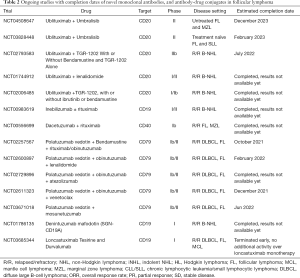
Full table
Bispecific antibodies
A bispecific antibody contains two antigen-binding sites: one directed against a receptor that activates cytotoxic cells and the other against a specific antigen expressed by tumor cells. It is engineered to target both the malignant cell and CD3+ T-cells, bringing them in close proximity for activation of the effector T-cells. It consists of variable domains of two antibodies, one targeting CD3+ cytotoxic T-cells and the other surface antigen such as CD19 or CD20 on the B-NHL cells (57-59). These bispecific antibodies are of various designs, which determines their mechanism of action (60). The majority of bispecific antibodies engage immune cells to destroy tumor cells. One such antibody is blinatumomab, the initial CD19/CD3 bispecific T-cell engager (BiTE) evaluated in R/R B-NHL, showed promising efficacy within the FL cohort (28 patients), with an ORR of 80% and a CR rate of 40% (61). The toxicities, although short-lasting and overall tolerable, were unique such as cytokine release syndrome (CRS) seen in 75% and neurotoxicity in 18% of patients. Phase II (NCT02811679) and phase Ib trials (NCT02961881) are still ongoing in R/R indolent B-NHL.
Mosunetuzumab, a CD20/CD3 bispecific antibody, has shown promising efficacy in the heavily pre-treated R/R B-NHL. Data presented at ASH annual meeting in 2019 from the phase I/Ib study included 69 patients with FL, of whom five had received prior chimeric antigen receptor T-cell (CAR-T) therapy. The ORR and CR rates for the indolent NHL group were 64% and 42%, respectively. The CRs were also durable in the indolent NHL subset, with 93% of patients (25/27) who achieved CR remained in remission at data cut off (62). Responses were also noted in patients progressing post-CAR-T therapy. Most of the CRS and neurotoxicity AEs were grades 1–2 (28.4% and 44%, respectively). This data was further updated at ASH 2020 for patients with at least two prior systemic therapies for FL. The ORR was 68% (42/62), with a 50% CR rate. A consistent CR rate (53%) was observed even in patients who progressed within 24 months of front-line therapy. The median duration of response was 20.4 months (95% CI: 11.7–not reached) for all 42 responders (63). The subcutaneous (SC) administration of this drug as an alternative to minimize CRS risk was evaluated in the phase I/Ib, open-label, multicenter dose-escalation, and expansion study in R/R B-NHL. Five of the 23 patients in this study had R/R FL, with a median of 4 prior lines of therapy in the total cohort. The majority of CRS events were seen in Cycle 1 and were mild, transient, and required minimal intervention. There were no grade ≥3 CRS events, and less frequent grade 2 CRS events were observed with SC dosing even at 7-fold higher dose levels versus the IV fixed-dosing group (64). Mosunetuzumab is currently under evaluation in various combination studies (Table 3) with agents such as polatuzumab vedotin (NCT03671018), CHOP chemotherapy (NCT03677141), lenalidomide (NCT04246086), and atezolizumab (NCT02500407) in B-NHL, including FL.
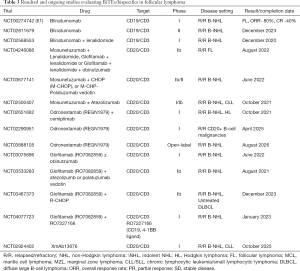
Full table
REGN1979 (odronextamab) is another promising IgG4 CD20/CD3 bispecific antibody in development. Treatment consists of 12 weekly intravenous doses of REGN1979 followed by every 2-week dosing for 12 doses (36 weeks total). Data presented in 2019 from the phase I study included heavily pre-treated patients with B-NHL (25 patients with grade 1–3a FL). An ORR of 95.5% and a CR rate of 77.3% were reported from the 22 evaluable FL patients that received ≥5 mg of the REGN1979. FL patients treated with ≥80 mg of this bispecific antibody had 100% ORR. With a median follow-up of 6.8 months in the FL group, the median PFS in the FL cohort was 11.4 months (95% CI, 6.7–not evaluable). At the time of data cutoff, 14/21 responses were ongoing, and 12/17 CRs were maintained (65). This was further updated at ASH 2020 and included 37 patients with FL grade 1–3a. In patients treated with ≥5 mg of the REGN1979 (n=28), the ORR was 92.9%, and CR rate was 75%, with a median duration of response of 7.7 months (66). The most common AEs were fever (80%), and CRS (59.1%); most common grade 3–4 AEs were anemia (22%), lymphopenia (19%), neutropenia (19%) and hypophosphatemia (19%). Most of the neurological events were grade 1-2, and neurological AEs did not cause any treatment discontinuations. REGN1979 is currently in evaluation in R/R B-NHL in combination with cemiplimab (anti-PD-1 mAb, NCT02651662); a global multi-arm trial is under development.
RO7082859 (glofitamab), is a novel T-cell-engaging, bispecific, full-length antibody with a 2:1 molecular configuration that facilitates bivalent binding to CD20 on B-cells and monovalent binding to CD3 on T-cells. Its binding pattern can increase tumor antigen avidity, cause rapid T-cell activation, and enhanced tumor cell killing versus other bispecific formats (67). It is currently under evaluation in a phase I/Ib multicenter, dose-escalation study as a single agent and in combination with obinutuzumab in R/R B-NHL (NCT03075696). Obinutuzumab pre-treatment has been shown to mitigate the risk of CRS with this agent, to allow for rapid step-up dosing (68). Data from the monotherapy dose-escalation arm which had 14 FL patients, showed an ORR in all FL patients of 77%, and 88% in the higher dose cohort (68). The latest data presented at ASH 2020 included 52 patients (24 indolent NHL), with an ORR of 66.7% and 54.2% CR rate in the indolent NHL cohort. The most common AEs in the entire cohort were CRS (63.5%), neutropenia (38.5%), thrombocytopenia, and hypophosphatemia. No treatment discontinuations occurred due to AEs (69). CRS events were only observed in cycles 1 and 2 with a median time to CRS of 14.23 hours after administration of glofitamab and a median duration of 28.7 hours. Tocilizumab was used to manage CRS for 15.4% of patients. Grade ≥2 CRS events were reduced in the step-up dosing cohorts compared to the fixed dosing cohorts (36.3% vs. 30.7%).
GEN3013 (epcoritamab) is a novel, SC administered CD20/CD3 bispecific antibody studied in an open-label, phase I/II trial (NCT03625037). It is given as an SC 1-mL injection of flat-dose epcoritamab in 28-day cycles (q1w: cycles 1–2; q2w: cycles 3–6; q4w thereafter) until disease progression or unacceptable toxicity. Of the 67 patients enrolled, 12 had FL grade 1–3a, with 4.5 (range, 1–18) median prior lines of therapy. There were no dose-limiting toxicities or febrile neutropenia events and no deaths due to treatment-related AEs. The CRS events were all grade 1–2 (58%) in the entire cohort with no grade ≥3 events. The most common AEs were pyrexia (70%), local injection-site reaction (48%), and fatigue (45%). The ORR was 100% for the 8 pts with FL receiving epcoritamab ≥0.76 mg, with 2 pts achieving a CR (PET scans were not mandatory and disease assessment by PET was not available in 4/6 pts who achieved a PR) (70).
Bispecific antibodies provide the advantage of being readily available as off-the-shelf products when compared to autologous CAR-T therapy, which takes a few weeks to manufacture. However, long term data are required for assessing the durability of these agents (Table 3). Additionally, where in the treatment armamentarium these agents will provide the most benefit also remains unclear at this time, given concerns for CD20 antigen escape as a mechanism of resistance in heavily pre-treated patients. Subcutaneous dosing is emerging as the preferred method for its ease of administration and improved safety profile with much fewer grade ≥3 CRS events making these agents a very attractive therapeutic option.
CAR-T therapy
CD19 targeting CAR-T therapy has had a significant impact on the outcomes of R/R aggressive B-NHL, with patients achieving durable remissions in an otherwise poor prognostic group of patients (71-74). It is yet to be known whether such durable remissions can be achieved in indolent lymphomas such as FL and its relative efficacy compared to other novel agents available for treatment. The possibility of long term remission also offers the benefit of potentially avoiding exposure to several treatments required with multiple relapses. Younger FL patients progressing within 24 months of initial chemoimmunotherapy (PFS24) is another situation where there is a need for effective treatments. The toxicities associated with CAR-T therapy are unique such as CRS and neurotoxicity and are more pronounced than those seen with bispecific mAbs. Interim analysis of phase II multicenter trial of axicabtagene ciloleucel (axi-cel), ZUMA-5, enrolled R/R indolent B-NHL patients (FL and MZL). Eighty of the 94 patients were R/R FL patients, the median number of prior therapies was 3, with 66% patients not achieving PFS24 status. Impressive efficacy of CAR-T therapy was seen in this trial with an ORR of 95% and 80% achieving CRs in the FL cohort. The median duration of response was 20.8 months, and the median PFS was 23.5 months. Median OS was not reached in the group. Notable grade ≥3 AEs were neutropenia (33%), anemia (28%), CRS (11%), and neurotoxicity (19%) (75). Based on this data, an FDA approval application has been submitted for axi-cel in R/R FL.
The phase II ELARA trial of tisagenlecleucel in R/R FL patients was presented at ASH 2020. This trial included 97 patients who received tisagenlecleucel, and 52 patients were evaluable for efficacy with a median follow-up of 9.9 months. The median number of prior lines of therapy was 4 (range, 2–13), including prior autologous transplant in 36%, a FLIPI score ≥3 in 60%, and 43% required bridging therapy. The ORR was 82.7% (43/52), with a CR rate of 65.4% (34/52) in the intent-to-treat population. For those achieving CR as the best response, the probability of response over six months was 89.7%. The median duration of response, PFS, OS, and time to next lymphoma treatment were not reached. The safety analysis included all 97 patients, of which 68% experienced grade ≥3 AEs. The CRS rate was 48% for all grades, with no grade ≥3 events. Any grade neurotoxicity events were noted in 10% of patients, of which 2% were grade ≥3. No treatment-related deaths were seen in the trial so far, while 3 patients died from progressive disease (76). Where CAR-T therapy will fit in the treatment landscape for FL patients to ensure maximum benefit is yet to be determined. We anticipate that the coming decade will see a significant upsurge of clinical trials with these cellular therapies to determine their place in the management algorithm of FL treatment.
Monoclonal antibodies targeting immune cells
Immune cells present within the tumor microenvironment of FL play an important role in lymphomagenesis, cell survival, and progression. One of the mechanisms by which the lymphoma cells evade an antitumor immune surveillance is by the upregulation or overexpression of immunosuppressive ligands on the tumor cells or on other cells in the tumor microenvironment (3,77,78). Overexpression of programmed death-ligand 1 (PD-L1; CD274) and PD-L2 (CD273) by malignant lymphoma cells is one such mechanism. As T-cells become activated, they express PD-1 to avoid over activation. The immunosuppressive ligands on the lymphoma cells, namely PD-L1 and PD-L2, signal through PD-1 to inhibit T-cell function and promote immune exhaustion, which ultimately results in T-cell apoptosis. Other immune-inhibitory receptors such as T-cell immunoglobulin mucin-3 and lymphocyte-activation gene 3, which are also found to be expressed on the T cells in the microenvironment and are associated with immune exhaustion (79-81). While the FL cells themselves do not overexpress program death-ligand 1 (PD-L1), they can modulate the microenvironment such that intratumoral effector T-cells are exhausted, and macrophages are polarized towards a suppressive environment (79,82-87). Also, the upregulation of PD-1 has been shown on the intratumoral and peripheral blood CD4 and CD8+ T-cells (88,89). Several mAbs targeting PD-1 or its ligand PD-L1, and other checkpoints are under evaluation as monotherapy or in combination in FL are shown in Table 4.
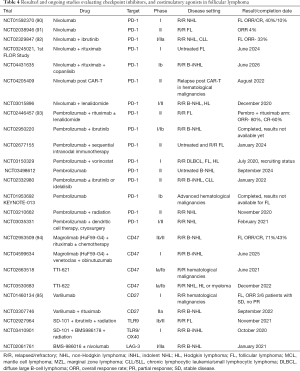
Full table
PD-1/PD-L1
Nivolumab, a PD-1 blocking mAb, showed ORR up to 40% with a relatively favorable toxicity profile in the initial studies in patients with FL (90). However, these results were not recapitulated in the phase II trial in a larger cohort of R/R FL patients. CheckMate 140 evaluated nivolumab monotherapy efficacy and safety in R/R FL patients who had progressed after at least 2 prior lines of therapy, including a CD20 mAb and an alkylating agent. The ORR (which was the primary endpoint) was 4% (4/92), with a median PFS of 2.2 months (95% CI, 1.9–3.6) (91). In an attempt to improve on these results, the combination of nivolumab with rituximab in a phase II trial for previously untreated FL patients is ongoing (NCT03245021). Other ongoing combination studies include drugs such as lenalidomide (NCT03015896) and copanlisib (NCT04431635).
Pembrolizumab, another PD-1 blocking mAb like nivolumab, had disappointing single-agent efficacy with an ORR of 11% based on the partial response in 2 patients and median PFS of 3.4 months (95% CI, 2.1–5.7) in the entire cohort in a phase II trial (96). The combination of pembrolizumab with rituximab appeared more effective in a 30 patient study with R/R FL with ORR of 64% and a CR rate of 48%, suggesting a possible synergistic effect (93).
Other mAbs targeting the PD-1/PD-L1 pathway have shown similar results as monotherapy. A phase I study of atezolizumab (PD-L1) with 3 patients in the FL cohort had only 1 partial response (97). Higher efficacy was seen with atezolizumab in combination with obinutuzumab (26 FL, ORR 57%) and with both obinutuzumab and lenalidomide in a phase Ib/II study (NCT02631577) (29 patients, ORR 85%) (98,99). Other combination studies are ongoing, combining PD-1/PD-L1 blocking mAbs with ibrutinib, venetoclax, chemotherapy, histone deacetylase (HDAC) inhibitors, or other immunotherapies (Table 4).
Other costimulatory/checkpoint molecules
Several other checkpoint molecules and costimulatory molecules are involved in the process of tumor antigen presentation and T-cell activation (Figure 2). Given the low clinical benefit of PD-1/PD-L1 as monotherapy in FL, other molecules and combinations are under investigation as potential targets. CD27 is a costimulatory molecule on T-cells, and by interacting with its ligand CD70, it promotes T-cell activation. A CD27 agonist mAb varlilumab (CDX-1127) is under investigation in B-NHL and has shown clinical efficacy in early phase trials (6 FL of 25 B-NHL patients) (95). Combination phase II trials of varlilumab with nivolumab and rituximab are ongoing in R/R B-NHL (100,101). 4-1BB (CD137) is another costimulatory molecule present on the CD8+ T-cells which is being explored as a potential target (102). Urelumab, a fully human, CD137 agonist IgG4 mAb, showed increased hepatic toxicities in solid tumors (103,104). In R/R B-cell NHL, the results from two phase I studies (NCT01471210, NCT01775631) of urelumab alone and in combination with rituximab (n=60), showed an ORR and disease control rate in the FL cohort (n=17) of 12% and 35%, respectively (105). Utolimumab, another 4-1BB targeting IgG2 mAb, was studied in combination with rituximab in R/R B-NHL (33 FL). Within the FL cohort, 24/33 patients were rituximab-refractory; and the ORR in the entire FL cohort and the rituximab refractory cohort was 27% (9/33) and 33% (8/24), respectively (106). Unlike urelumab, no dose-limiting toxicities or hepatic toxicities were seen with this agent. Another costimulatory molecule CD40 was targeted using dacetuzumab, an IgG1 mAb in R/R B-NHL, in a phase I study (53). Monotherapy efficacy of dacetuzumab was not substantial, and a combination study with rituximab in indolent NHL (NCT00556699) is awaiting results.

The results from checkpoint inhibitor or costimulatory agonist mAb trials suggest that the efficacy of monotherapy remains low, and further steps need to be taken to improve outcomes in FL patients. Future strategies will need to include combination studies with agents that show synergy while maintaining a favorable toxicity profile and identifying specific predictive biomarkers associated with these agents to better select patients within the FL subset.
CD47/SIRPα
CD47 and its ligand signal regulatory protein alpha (SIRPα) are part of the innate immune system and play an important role in the anti-tumor immune response. CD47 is upregulated in B-NHL, including FL, and is responsible for sending a “don’t eat me” signal to the innate immune system cells such as macrophages (107,108). The CD47/SIRPα blocking mAb, Hu5F9-G4 combined with rituximab demonstrated an ORR of 71% and a CR rate of 43% in the FL subset (94). At a median follow-up of 8.1 months in the FL cohort, 91% of patients maintained their response. Another anti-CD47 agent, TTI-621 (SIRPαFc), consists of the CD47 binding domain of SIRPα linked to the Fc region of human IgG. This agent is designed to engage the Fcγ receptors on the macrophages and block the CD47 negative signaling, thereby activating macrophage-mediated phagocytosis (109). It is currently under investigation as monotherapy or in combination with rituximab (NCT02663518) in various lymphoma types, including FL; and results for this subset are awaited (110).
Immunomodulatory drugs (IMiDs)
The IMiDs deserve a special mention as lenalidomide with rituximab is an approved indication for R/R FL patients. The IMiDs, by binding to cereblon, an E3 ubiquitin ligase, promote degradation of Ikaros and Aiolos transcription regulators of B- and T-cell development (111). This results in the stimulation of effector T and NK cells and the improvement of ADCC when combined with rituximab (112). Also, the IMiDs, especially lenalidomide, have been shown to affect the immune cells present in the tumor microenvironment in generating an antitumor response (113,114). It augments the effector function of CD8 T-cells, increases immune synapse formation, and antigen presentation by dendritic cells. It also affects the innate immune system by increasing the NK cell numbers and activity and direct NK cell killing. Lastly, it decreases the pro-inflammatory cytokines such as interleukin (IL)2, IL 6, IL12, and tumor necrosis factor α, while increasing the anti-inflammatory cytokines such as IL-10.
The phase III RELEVANCE trial showed similar 3-year PFS rate, ORR, and CR rates between R2 and R-chemo arms in newly diagnosed FL, which makes a compelling argument for its use in the upfront setting as a non-chemotherapy regimen (10). In the relapsed setting phase III, the AUGMENT trial compared R2 vs. rituximab in marginal zone lymphoma (MZL) and FL (n=147) and met its primary endpoint of improvement in PFS while maintaining an acceptable safety profile (115). Several combination studies are ongoing using lenalidomide in FL to develop effective chemo-free regimens for FL. A newer IMiD currently under investigation is avadomide (CC-122) in B-NHL. In combination with obinutuzumab in a phase I study of R/R B-NHL (53/73 FL), the toxicity profile of avadomide was noted to be manageable, and the combination resulted in an ORR of 76%, with 43% patients achieving CR (116). Efficacy was also noted in rituximab-refractory patients when avadomide was combined with rituximab (117).
Agents targeting BCR signaling and transduction pathways
BCR pathway is an appealing treatment strategy with multiple available targets from its downstream signaling mechanisms (118-120). These downstream signaling pathways are responsible for B-cell growth, proliferation, and survival and are critical in lymphomagenesis. Several components of the BCR pathway, such as BTK, PI3K already have effective inhibitors with proven benefits in R/R FL, with more agents in development. The inhibiton of BCR pathway and off-target activity of BTK and PI3K inhibitors affects multiple components of the tumor microenvironment (77). BTK inhibitors decrease the expression of immunosuppressive markers such as PD-1, increase the number of CD8 and CD4+ T-cells while also decreasing the fraction of regulatory T-cells, thereby promoting an anti-tumor response (121). The off-target activity of ibrutinib by inhibiting the IL 2 inducible kinase (ITK), promotes a T helper-1 based immune responses with more cytotoxicity than Th-2 based (122). PI3K inhibitors effect on immune cells in the microenvironment is dependant on the isoforms inhibited by the drug. PI3K inhibitors can downregulate the secretion of chemokines such as CXCL-12 and CXCL-13, thereby impairing the chemotaxis and adhesion of the lymphoma cells to the stromal cells, both promoting an anti-tumor response (123). The PI3Kδ inhibition has been shown to impair the function of T-cells, including the T-regulatory cells, which likely resulted in immune-mediated AEs and increased infection rates seen with idelalisib (124). While inhibition of PI3Kγ isoform polarize tumor-associated macrophages to an anti-inflammatory M1-like state from a protumoral M2 state that restricts tumor expansion (125,126).
Phosphoinositide 3- Kinase (PI3K) Inhibitors
The PI3K enzymes exist in four different isoforms. Their relative distribution within different tissues helps predict their activity and toxicity profile. While PI3Kα and β are present on the majority of the tissues, PI3Kδ and γ are limited predominantly to hematopoietic cells (127-129). Idelalisib, a PI3Kδ inhibitor, received accelerated FDA approval for R/R FL patients after ≥2 lines of therapy (both rituximab and an alkylating agent) in 2014. This was based on the phase II trial, which included 72 FL patients, with ORR of 57%, CR rate of 6%, and median PFS of 11 months (130). Significant side effects (grade ≥3) with idelalisib included neutropenia, transaminitis, diarrhea, and pneumonia. Despite the side effect profile, the approval of idelalisib was thought to be significant due to a lack of options for treatment of such refractory FL patients. Copanlisib, an intravenous (i.v) pan-PI3K inhibitor, with a predominant effect on PI3Kα and δ isoforms, received accelerated FDA approval for R/R FL in 2017. In the CHRONOS 1 part B study with 104 FL patients, ORR was 58% with a 14% CR rate. The treatment-related AEs were unique with (all grades) hyperglycemia (49%) and hypertension (29%) owing mostly to inhibition of PI3Kα isoform (131). The immune-mediated AEs were much fewer than idelalisib, mostly due to i.v dosing and the effect of hepatic first-pass metabolism. A phase III randomized, double-blind, combination study of rituximab with or without copanlisib in indolent NHL (n=458) in second-line therapy was recently announced to have met its primary endpoint of PFS (CHRONOS 3) (132). Duvelisib, inhibits both the PI3Kδ and γ and was shown to have an ORR of 42% in R/R FL patients in the DYNAMO study (133). Its side effect profile appeared to be similar to that of idelalisib. Combination trials of duvelisib in FL have been terminated early or withdrawn by the sponsor to focus on studies that can enable the registration of duvelisib (CLL). Umbralisib, a unique PI3Kδ inhibitor with fewer side effects than other PI3Kδ inhibitors, is currently in clinical trials in various combinations. Phase I monotherapy trial (24% FL) showed an ORR of 53%, with 12% CR (134). Multiple combination studies are ongoing in FL with umbralisib.
Bruton Tyrosine Kinase Inhibitors (BTKi)
BTKi, while having a direct anti-tumoral effect on the survival of the malignant B-cells, also affect the T-cells in the lymphoma microenvironment. They decrease the expression of inhibitory molecules such as PD-1/CTLA-4 while simultaneously enhancing the effector and memory function of T-cells (121). They have also been shown to decrease inflammatory cytokines, reverse T-cell exhaustion, and disrupt the stromal-tumor interactions mediated by chemokines and cell adhesion (135). In addition, BTKi, in combination with PD-L1 blockade has been shown in mouse models to be synergistic (136,137). Single-agent ibrutinib in a phase I study (16/56 FL patients) showed an ORR of 37% with a favorable toxicity profile (138). A similar ORR was seen in a phase 2 study in R/R FL patients (n=40), with a median PFS of 14 months and a 2-year PFS rate of 20.4%. Patients that were rituximab sensitive were noted to have a better response to ibrutinib (139). Monotherapy efficacy of ibrutinib was not considered significant to be further pursued as a single agent in the R/R setting. However, given its effect on the lymphoma microenvironment, multiple combination strategies have been developed and are under investigation. Ibrutinib plus rituximab in treatment-naïve FL patients was investigated in a multicenter phase II study, evaluating two different treatment schedules for the combination. Arm 1 with concurrent administration had an ORR of 85%, with a 35% CR rate, and arm 2 with an 8-week lead-in phase of ibrutinib had an ORR of 75%, with 35% CRs as well (140). The most common grade ≥3 AEs were rash (10%), fatigue (7%), and fever (10%). A randomized phase III trial (NCT02947347) of rituximab with or without ibrutinib is ongoing in untreated FL. Another combination of ibrutinib, rituximab, and lenalidomide was explored in the phase I (Alliance A051103) setting in newly diagnosed FL. Although the ORR in the 22 patients was 95% and the 1-year PFS was 80%, this regimen showed a significant rate of dose-limiting rash, with 82% of patients experienced a rash of any grade (36% grade 3) and half of the patients requiring a dose reduction (141). The efficacy was similar to R2 in this setting with significantly more toxicity and dose modifications, and therefore this combination is no longer moving forward in this setting. When combined with BR in a phase 1b study for both previously untreated and R/R B-NHL, the combination was found to be safe and tolerable with a 50% CR rate in the FL cohort (142). There is an ongoing combination study in previously untreated FL, including ibrutinib in combination with either BR or R-CHOP (NCT01974440). Other more specific BTKi such as acalabrutinib, and zanubrutinib while approved for other lymphoma types, are currently under investigation in FL.
Conclusions
The natural history of indolent B-NHL, such as FL, has significantly benefitted from the advances in therapeutics. The treatment options for FL continue to expand with the introduction of novel immunotherapies described in this review. Ongoing studies will further advance the understanding of disease biology, mechanisms of resistance, and the role of microenvironment. However, the challenge is identifying the most strategic ways of combining, sequencing, and predicting the response to these newer therapies while minimizing the toxicities and potentially aiming for a cure. Research is also needed to evaluate the financial and quality of life implications of such newer therapies while making them accessible to those most in need.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mark Roschewski, Carla Casulo) for the series “Follicular Lymphoma” published in Annals of Lymphoma. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/aol-20-48
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-48). The series “Follicular Lymphoma” was commissioned by the editorial office without any funding or sponsorship. SMA served as an unpaid editorial board member of Annals of Lymphoma from Nov 2019 to Oct 2021, and reports clinical trial funding from Bristol-Myers Squibb, Seattle Genetics, Takeda, AI Therapeutics, Regeneron, Affimed Therapeutics, Trillium Therapeutics, ADC Therapeutics, outside the submitted work. AK has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ansell SM, Vonderheide RH. Cellular Composition of the Tumor Microenvironment. Am Soc Clin Oncol Educ Book 2013;33:e91-7. [Crossref] [PubMed]
- Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer 2014;14:517-34. [Crossref] [PubMed]
- Khurana A, Ansell SM. Role of microenvironment in non-hodgkin lymphoma: Understanding the composition and biology. Cancer J 2020;26:206-16. [Crossref] [PubMed]
- Al-Hamadani M, Habermann TM, Cerhan JR, et al. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol 2015;90:790-5. [Crossref] [PubMed]
- Ardeshna KM, Qian W, Smith P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: An open-label randomised phase 3 trial. Lancet Oncol 2014;15:424-35. [Crossref] [PubMed]
- Kahl BS, Hong F, Williams ME, et al. Rituximab extended schedule or re-treatment trial for low-tumor burden follicular lymphoma: Eastern cooperative oncology group protocol E4402. J Clin Oncol 2014;32:3096-102. [Crossref] [PubMed]
- Nastoupil LJ, Sinha R, Byrtek M, et al. Outcomes following watchful waiting for stage II-IV follicular lymphoma patients in the modern era. Br J Haematol 2016;172:724-34. [Crossref] [PubMed]
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203-10. [Crossref] [PubMed]
- Flinn IW, Van Der Jagt R, Kahl B, et al. First-line treatment of patients with indolent non-hodgkin lymphoma or mantle-cell lymphoma with bendamustine plus rituximab versus R-CHOP or R-CVP: Results of the BRIGHT 5-year follow-up study. J Clin Oncol 2019;37:984-91. [Crossref] [PubMed]
- Morschhauser F, Fowler NH, Feugier P, et al. Rituximab plus Lenalidomide in Advanced Untreated Follicular Lymphoma. N Engl J Med 2018;379:934-47. [Crossref] [PubMed]
- Maurer MJ, Bachy E, Ghesquières H, et al. Early event status informs subse-quent outcome in newly diagnosed follicular lymphoma. Am J Hematol 2016;91:1096-101. [Crossref] [PubMed]
- Sarkozy C, Maurer MJ, Link BK, et al. Cause of death in follicular lymphoma in the first decade of the rituximab era: A pooled analysis of French and US cohorts. J Clin Oncol 2019;37:144-52. [Crossref] [PubMed]
- Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: An analysis from the National LymphoCare Study. J Clin Oncol 2015;33:2516-22. [Crossref] [PubMed]
- Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet 2011;377:42-51. [Crossref] [PubMed]
- Bellosillo B, Villamor N, López-Guillermo A, et al. Complement-mediated cell death induced by rituximab in B-cell lymphoproliferative disorders is mediated in vitro by a caspase-independent mechanism involving the generation of reactive oxygen species. Blood 2001;98:2771-7. [Crossref] [PubMed]
- Manches O, Lui G, Chaperot L, et al. In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. Blood 2003;101:949-54. [Crossref] [PubMed]
- Shan D, Ledbetter JA, Press OW. Signaling events involved in an-ti-CD20-induced apoptosis of malignant human B cells. Cancer Immunol Immunother 2000;48:673-83. [Crossref] [PubMed]
- Mössner E, Brünker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell - mediated B-cell cytotoxicity. Blood 2010;115:4393-402. [Crossref] [PubMed]
- Alduaij W, Ivanov A, Honeychurch J, et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lyso-some-mediated cell death in B-cell malignancies. Blood 2011;117:4519-29. [Crossref] [PubMed]
- Sehn LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multi-centre, phase 3 trial. Lancet Oncol 2016;17:1081-93. [Crossref] [PubMed]
- Marcus R, Davies A, Ando K, et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med 2017;377:1331-44. [Crossref] [PubMed]
- Cheson BD, Chua N, Mayer J, et al. Overall survival benefit in patients with rituximab-refractory indolent non-hodgkin lymphoma who received obinutuzumab plus bendamustine induction and obinutuzumab maintenance in the GADOLIN study. J Clin Oncol 2018;36:2259-66. [Crossref] [PubMed]
- Czuczman MS, Fayad L, Delwail V, et al. Ofatumumab monotherapy in rituximab-refractory follicular lymphoma: Results from a multicenter study. Blood 2012;119:3698-704. [Crossref] [PubMed]
- Czuczman MS, Hess G, Gadeberg OV, et al. Chemoimmunotherapy with ofa-tumumab in combination with CHOP in previously untreated follicular lymphoma. Br J Haematol 2012;157:438-45. [Crossref] [PubMed]
- Le Garff-Tavernier M, Herbi L, De Romeuf C, et al. Antibody-dependent cel-lular cytotoxicity of the optimized anti-CD20 monoclonal antibody ublituximab on chronic lymphocytic leukemia cells with the 17p deletion. Leukemia 2014;28:230-3. [Crossref] [PubMed]
- Sawas A, Farber CM, Schreeder MT, et al. A phase 1/2 trial of ublituximab, a novel anti-CD20 monoclonal antibody, in patients with B-cell non-Hodgkin lym-phoma or chronic lymphocytic leukaemia previously exposed to rituximab. Br J Haematol 2017;177:243-53. [Crossref] [PubMed]
- Nastoupil LJ, Lunning MA, Vose JM, et al. Tolerability and activity of ublituximab, umbralisib, and ibrutinib in patients with chronic lymphocytic leu-kaemia and non-Hodgkin lymphoma: a phase 1 dose escalation and expansion trial. Lancet Haematol 2019;6:e100-9. [Crossref] [PubMed]
- Lunning M, Vose J, Nastoupil L, et al. Ublituximab and umbralisib in re-lapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2019;134:1811-20. [Crossref] [PubMed]
- Horton HM, Bernett MJ, Pong E, et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res 2008;68:8049-57. [Crossref] [PubMed]
- Jurczak W, Zinzani PL, Gaidano G, et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non- Hodgkin’s lymphoma. Ann Oncol 2018;29:1266-72. [Crossref] [PubMed]
- Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol 2020;21:978-88. [Crossref] [PubMed]
- Ward E, Mittereder N, Kuta E, et al. A glycoengineered anti-CD19 antibody with potent antibody-dependent cellular cytotoxicity activity in vitro and lymphoma growth inhibition in vivo. Br J Haematol 2011;155:426-37. [Crossref] [PubMed]
- Ohmachi K, Ogura M, Suehiro Y, et al. A multicenter phase I study of inebili-zumab, a humanized anti-CD19 monoclonal antibody, in Japanese patients with re-lapsed or refractory B-cell lymphoma and multiple myeloma. Int J Hematol 2019;109:657-64. [Crossref] [PubMed]
- Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neu-romyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet 2019;394:1352-63. [Crossref] [PubMed]
- Leonard JP, Coleman M, Ketas JC, et al. Phase I/II trial of epratuzumab (hu-manized anti-CD22 antibody) in indolent non-Hodgkin’s lymphoma. J Clin Oncol 2003;21:3051-9. [Crossref] [PubMed]
- Leonard JP, Coleman M, Ketas J, et al. Combination antibody therapy with epratuzumab and rituximab in relapsed or refractory non-Hodgkin’s lymphoma. J Clin Oncol 2005;23:5044-51. [Crossref] [PubMed]
- Strauss SJ, Morschhauser F, Rech J, et al. Multicenter phase II trial of immu-notherapy with the humanized anti-CD22 antibody, epratuzumab, in combination with rituximab, in refractory or recurrent non-Hodgkin’s lymphoma. J Clin Oncol 2006;24:3880-6. [Crossref] [PubMed]
- Grant BW, Jung SH, Johnson JL, et al. A phase 2 trial of extended induction epratuzumab and rituximab for previously untreated follicular lymphoma: CALGB 50701. Cancer 2013;119:3797-804. [Crossref] [PubMed]
- Morschhauser F, Flinn IW, Advani R, et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMU-LUS). Lancet Haematol 2019;6:e254-65. [Crossref] [PubMed]
- Phillips T, Brunvand M, Chen A, et al. Polatuzumab Vedotin Combined with Obinutuzumab for Patients with Relapsed or Refractory Non-Hodgkin Lymphoma: Preliminary Safety and Clinical Activity of a Phase Ib/II Study. Blood 2016;128:622. [Crossref]
- Sehn LH, Kamdar M, Herrera AF, et al. Randomized phase 2 trial of po-latuzumab vedotin (pola) with bendamustine and rituximab (BR) in re-lapsed/refractory (r/r) FL and DLBCL. J Clin Oncol 2018;36:7507. [Crossref]
- Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2020;38:155-65. [Crossref] [PubMed]
- Zammarchi F, Corbett S, Adams L, et al. ADCT-402, a PBD dimer-containing antibody drug conjugate targeting CD19-expressing malignancies. Blood 2018;131:1094-105. [Crossref] [PubMed]
- Kahl BS, Hamadani M, Radford J, et al. A phase I study of ADCT-402 (lon-castuximab tesirine), a novel pyrrolobenzodiazepine-based antibody-drug conjugate, in relapsed/ refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res 2019;25:6986-94. [Crossref] [PubMed]
- Moskowitz CH, Fanale MA, Shah BD, et al. A Phase 1 Study of Denintuzumab Mafodotin (SGN-CD19A) in Relapsed/Refactory B-Lineage Non-Hodgkin Lym-phoma. Blood 2015;126:182. [Crossref]
- Blanc V, Bousseau A, Caron A, et al. SAR3419: An anti-CD19-maytansinoid immunoconjugate for the treatment of B-cell malignancies. Clin Cancer Res 2011;17:6448-58. [Crossref] [PubMed]
- Trnĕný M, Verhoef G, Dyer MJ, et al. A phase II multicenter study of the an-ti-CD19 antibody drug conjugate coltuximab ravtansine (SAR3419) in patients with relapsed or refractory diffuse large B-cell lymphoma previously treated with rituximab-based immunotherapy. Haematologica 2018;103:1351-8. [Crossref] [PubMed]
- Ribrag V, Dupuis J, Tilly H, et al. A dose-escalation study of SAR3419, an an-ti-CD19 antibody maytansinoid conjugate, administered by intravenous infusion once weekly in patients with relapsed/refractory B-cell non-hodgkin lymphoma. Clin Cancer Res 2014;20:213-20. [Crossref] [PubMed]
- Younes A, Kim S, Romaguera J, et al. Phase I multidose-escalation study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered by intravenous infusion every 3 weeks to patients with relapsed/refractory B-cell lymphoma. J Clin Oncol 2012;30:2776-82. [Crossref] [PubMed]
- DiJoseph JF, Armellino DC, Boghaert ER, et al. Antibody-targeted chemo-therapy with CMC-544: A CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood 2004;103:1807-14. [Crossref] [PubMed]
- Fayad L, Offner F, Smith MR, et al. Safety and clinical activity of a combination therapy comprising two antibody-based targeting agents for the treatment of non-hodgkin lymphoma: Results of a phase I/II study evaluating the immunoconju-gate inotuzumab ozogamicin with rituximab. J Clin Oncol 2013;31:573-83. [Crossref] [PubMed]
- Dang NH, Ogura M, Castaigne S, et al. Randomized, phase 3 trial of inotuzumab ozogamicin plus rituximab versus chemotherapy plus rituximab for re-lapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Br J Haematol 2018;182:583-6. [Crossref] [PubMed]
- Advani R, Forero-Torres A, Furman RR, et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin’s lymphoma. J Clin Oncol 2009;27:4371-7. [Crossref] [PubMed]
- Ogura M, Tobinai K, Hatake K, et al. Phase I study of inotuzumab ozogamicin (CMC-544) in Japanese patients with follicular lymphoma pretreated with rituxi-mab-based therapy. Cancer Sci 2010;101:1840-5. [Crossref] [PubMed]
- Leonard JP, Friedberg JW, Younes A, et al. A phase I/II study of galiximab (an anti-CD80 monoclonal antibody) in combination with rituximab for relapsed or re-fractory, follicular lymphoma. Ann Oncol 2007;18:1216-23. [Crossref] [PubMed]
- Czuczman MS, Thall A, Witzig TE, et al. Phase I/II study of galiximab, an an-ti-CD80 antibody, for relapsed or refractory follicular lymphoma. J Clin Oncol 2005;23:4390-8. [Crossref] [PubMed]
- Dreier T, Baeuerle PA, Fichtner I, et al. T Cell Costimulus-Independent and Very Efficacious Inhibition of Tumor Growth in Mice Bearing Subcutaneous or Leukemic Human B Cell Lymphoma Xenografts by a CD19-/CD3- Bispecific Sin-gle-Chain Antibody Construct. J Immunol 2003;170:4397-402. [Crossref] [PubMed]
- Gruen M, Bommert K, Bargou RC. T-cell-mediated lysis of B cells induced by a CD19xCD3 bispecific single-chain antibody is perforin dependent and death receptor independent. Cancer Immunol Immunother 2004;53:625-32. [Crossref] [PubMed]
- Smits NC, Sentman CL. Bispecific T-cell engagers (BiTES) as treatment of B-cell lymphoma. J Clin Oncol 2016;34:1131-3. [Crossref] [PubMed]
- Suurs FV, Lub-de Hooge MN, de Vries EGE, et al. A review of bispecific an-tibodies and antibody constructs in oncology and clinical challenges. Pharmacol Ther 2019;201:103-19. [Crossref] [PubMed]
- Goebeler ME, Knop S, Viardot A, et al. Bispecific T-cell engager (BiTE) anti-body construct Blinatumomab for the treatment of Patients with relapsed/refractory non-Hodgkin lymphoma: Final results from a phase I study. J Clin Oncol 2016;34:1104-11. [Crossref] [PubMed]
- Schuster SJ, Bartlett NL, Assouline S, et al. Mosunetuzumab Induces Complete Remissions in Poor Prognosis Non-Hodgkin Lymphoma Patients, Including Those Who Are Resistant to or Relapsing After Chimeric Antigen Receptor T-Cell (CAR-T) Therapies, and Is Active in Treatment through Multiple Lines. Blood 2019;134:6. [Crossref]
- Assouline SE, Kim WS, Sehn LH, et al. Mosunetuzumab Shows Promising Ef-ficacy in Patients with Multiply Relapsed Follicular Lymphoma: Updated Clinical Experience from a Phase I Dose-Escalation Trial. Blood 2020;136:42-4. [Crossref]
- Matasar M. Subcutaneous Mosunetuzumab in Relapsed or Refractory B-Cell Lymphoma: Promising Safety and Encouraging Efficacy in Dose Escalation Cohorts 2020.
- Bannerji R, Allan JN, Arnason JE, et al. Clinical Activity of REGN1979, a Bispecific Human, Anti-CD20 x Anti-CD3 Antibody, in Patients with Re-lapsed/Refractory (R/R) B-Cell Non-Hodgkin Lymphoma (B-NHL). Blood 2019;134:762. [Crossref]
- Bannerji R, Allan JN, Arnason JE, et al. Odronextamab (REGN1979), a Human CD20 x CD3 Bispecific Antibody, Induces Durable, Complete Responses in Patients with Highly Refractory B-Cell Non-Hodgkin Lymphoma, Including Patients Re-fractory to CAR T Therapy. Blood 2020;136:42-3. [Crossref]
- Bacac M, Colombetti S, Herter S, et al. Cancer Therapy: Preclinical CD20-TCB with Obinutuzumab Pretreatment as Next-Generation Treatment of Hematologic Malignancies 2018;doi:
10.1158/1078-0432.CCR-18-0455 . - Dickinson JM, Morschhauser Franck, Iacoboni Gloria, et al. CD20-TCB in relapsed or refractory non-Hodgkin lymphoma: durable complete responses and manageable safety observed at clinically relevant doses in phase I dose escalation. (accessed November 14, 2020). Available online: https://library.ehaweb.org/eha/2020/eha25th/293690/michael.j.dickinson.cd20-tcb.in.relapsed.or.refractory.non-hodgkin.lymphoma.html?f=listing%3D3%2Abrowseby%3D8%2Asortby%3D1%2Amedia%3D1
- Hutchings M, Carlo-Stella C, Bachy E, et al. Glofitamab Step-up Dosing Induces High Response Rates in Patients with Hard-to-Treat Refractory or Relapsed Non-Hodgkin Lymphoma. Blood 2020;136:46-8. [Crossref]
- Hutchings M, Mous R, Clausen MR, et al. Subcutaneous Epcoritamab Induces Complete Responses with an Encouraging Safety Profile across Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma Subtypes, Including Patients with Prior CAR-T Therapy: Updated Dose Escalation Data. Blood 2020;136:45-6. [Crossref]
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood 2017;130:1800-8. [Crossref] [PubMed]
- Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a sin-gle-arm, multicentre, phase 1-2 trial. Lancet Oncol 2019;20:31-42. [Crossref] [PubMed]
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380:45-56. [Crossref] [PubMed]
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 2020;396:839-52. [Crossref] [PubMed]
- Jacobson CA, Chavez JC, Sehgal AR, et al. Interim analysis of ZUMA-5: A phase II study of axicabtagene ciloleucel (axi-cel) in patients (pts) with re-lapsed/refractory indolent non-Hodgkin lymphoma (R/R iNHL). J Clin Oncol 2020;38:8008. [Crossref]
- Fowler NH, Dickinson M, Dreyling M, et al. Efficacy and Safety of Tisagen-lecleucel in Adult Patients with Relapsed/Refractory Follicular Lymphoma: Interim Analysis of the Phase 2 Elara Trial. Blood 2020;136:1-3. [Crossref]
- Fowler NH, Cheah CY, Gascoyne RD, et al. Role of the tumor microenviron-ment in mature B-cell lymphoid malignancies. Haematologica 2016;101:531-40. [Crossref] [PubMed]
- Roemer MGM, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 Genetic Alter-ations Define Classical Hodgkin Lymphoma and Predict Outcome. J Clin Oncol 2016;34:2690-7. [Crossref] [PubMed]
- Yang ZZ, Kim HJ, Villasboas JC, et al. Expression of LAG-3 defines exhaustion of intratumoral PD-1+ T cells and correlates with poor outcome in follicular lymphoma. Oncotarget 2017;8:61425-39. [Crossref] [PubMed]
- Kumar D, Xu ML. Microenvironment Cell Contribution to Lymphoma Immunity. Front Oncol 2018;8:288. [Crossref] [PubMed]
- Yang ZZ, Kim HJ, Wu H, et al. TIGIT Expression Is Associated with T-cell Suppression and Exhaustion and Predicts Clinical Outcome and Anti-PD-1 Response in Follicular Lymphoma. Clin Cancer Res 2020;26:5217-31. [Crossref] [PubMed]
- Yang ZZ, Grote DM, Ziesmer SC, et al. PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J 2015;5:e281 [Crossref] [PubMed]
- Yang ZZ, Novak AJ, Ziesmer SC, et al. Attenuation of CD8+ T-cell function by CD4+CD25 + regulatory T cells in B-cell non-Hodgkin’s lymphoma. Cancer Res 2006;66:10145-52. [Crossref] [PubMed]
- Yang ZZ, Grote DM, Xiu B, et al. TGF-β upregulates CD70 expression and in-duces exhaustion of effector memory T cells in B-cell non-Hodgkin’s lymphoma. Leukemia 2014;28:1872-84. [Crossref] [PubMed]
- Gravelle P, Do C, Franchet C, et al. Impaired functional responses in follicular lymphoma CD8+TIM-3+ T lymphocytes following TCR engagement. Oncoimmunology 2016;5:e1224044 [Crossref] [PubMed]
- Ramsay AG, Clear AJ, Kelly G, et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: Implica-tions for the tumor microenvironment and immunotherapy. Blood 2009;114:4713-20. [Crossref] [PubMed]
- Dave SS, Wright G, Tan B, et al. Prediction of Survival in Follicular Lymphoma Based on Molecular Features of Tumor-Infiltrating Immune Cells. N Engl J Med 2004;351:2159-69. [Crossref] [PubMed]
- Carreras J, Lopez-Guillermo A, Fox BC, et al. High numbers of tu-mor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood 2006;108:2957-64. [Crossref] [PubMed]
- Carreras J, Lopez-Guillermo A, Roncador G, et al. High numbers of tu-mor-infiltrating programmed cell death 1-positive regulatory lymphocytes are as-sociated with improved overall survival in follicular lymphoma. J Clin Oncol 2009;27:1470-6. [Crossref] [PubMed]
- Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: Preliminary results of a phase ib study. J Clin Oncol 2016;34:2698-704. [Crossref] [PubMed]
- Armand P, Janssens AM, Gritti G, et al. Efficacy and safety results from CheckMate 140, a phase 2 study of nivolumab for relapsed/refractory follicular lymphoma. Blood 2021;137:637-45. [Crossref] [PubMed]
- Younes A, Brody J, Carpio C, et al. Safety and activity of ibrutinib in combi-nation with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: a phase 1/2a study. Lancet Haematol 2019;6:e67-78. [Crossref] [PubMed]
- Nastoupil LJ, Westin JR, Fowler NH, et al. Response rates with pembrolizumab in combination with rituximab in patients with relapsed follicular lymphoma: In-terim results of an on open-label, phase II study. J Clin Oncol 2017;35:7519. [Crossref]
- Advani R, Flinn I, Popplewell L, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N Engl J Med 2018;379:1711-21. [Crossref] [PubMed]
- Ansell SM, Flinn I, Taylor MH, et al. Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, for hematologic malignancies. Blood Adv 2020;4:1917-26. [Crossref] [PubMed]
- Ding W, Laplant B, Witzig TE, et al. PD-1 Blockade with Pembrolizumab in Relapsed Low Grade Non-Hodgkin Lymphoma. Blood 2017;130:4055.
- Till BG, Park SI, Popplewell LL, et al. Safety and Clinical Activity of Ate-zolizumab (Anti-PDL1) in Combination with Obinutuzumab in Patients with Re-lapsed or Refractory Non-Hodgkin Lymphoma. Blood 2015;126:5104. [Crossref]
- Morschhauser F, Ghosh N, Lossos I, et al. Efficacy and safety of obinutuzumab + lenalidomide + atezolizumab in patients with relapsed or refractory follicular lymphoma: primary analysis of a phase 1b/2 trial. Hematol Oncol 2019;37:113-4. [Crossref]
- Salles G, Ghosh N, Lossos IS, et al. Atezolizumab in Combination with Obinutuzumab and Lenalidomide Demonstrates Favorable Activity and Manageable Toxicity in Patients with Relapsed/Refractory Follicular Lymphoma (FL): An In-terim Analysis of a Phase Ib/II Trial. Blood 2018;132:1603. [Crossref]
- Villasboas JC, Reeder CB, Tun HW, et al. The DIAL Study (Dual Immuno-modulation in Aggressive Lymphoma): A randomized phase 2 study of CDX-1127 (varlilumab) in combination with nivolumab in patients with relapsed or refractory aggressive B-cell lymphomas (NCI 10089/NCT03038672). J Clin Oncol 2019;37:TPS7570 [Crossref]
- Lim SH, Linton KM, Collins GP, et al. RIVA - a phase IIa study of rituximab and varlilumab in relapsed or refractory B-cell malignancies: study protocol for a randomized controlled trial. Trials 2018;19:619. [Crossref] [PubMed]
- Houot R, Goldstein MJ, Kohrt HE, et al. Therapeutic effect of CD137 im-munomodulation in lymphoma and its enhancement by Treg depletion. Blood 2009;114:3431-8. [Crossref] [PubMed]
- Wilcox RA, Tamada K, Flies DB, et al. Ligation of CD137 receptor prevents and reverses established anergy of CD8+ cytolytic T lymphocytes in vivo. Blood 2004;103:177-84. [Crossref] [PubMed]
- Sznol M, Hodi FS, Margolin K, et al. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA). J Clin Oncol 2008;26:3007. [Crossref]
- Timmerman J, Herbaux C, Ribrag V, et al. Urelumab alone or in combination with rituximab in patients with relapsed or refractory B-cell lymphoma. Am J Hematol 2020;95:510-20. [Crossref] [PubMed]
- Gopal A, Levy R, Houot R, et al. A phase I study of utomilumab (pf-05082566), a 4-1bb/cd137 agonist, in combination with rituximab in patients with cd20 + non-Hodgkin’s lymphoma. Hematol Oncol 2017;35:260. [Crossref]
- Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012;109:6662-7. [Crossref] [PubMed]
- Barclay AN, van den Berg TK. The Interaction Between Signal Regulatory Protein Alpha (SIRP α) and CD47: Structure, Function, and Therapeutic Target. Annu Rev Immunol 2014;32:25-50. [Crossref] [PubMed]
- Lin GHY, Chai V, Lee V, et al. TTI-621 (SIRPαFc), a CD47-blocking cancer immunotherapeutic, triggers phagocytosis of lymphoma cells by multiple polarized macrophage subsets. PLoS One 2017;12:e0187262 [Crossref] [PubMed]
- Ansell SM, Flinn IW, Maris MB, et al. TTI-621 (SIRPαFc), an Immune Checkpoint Inhibitor Blocking the CD47 “Do Not Eat” Signal, Induces Objective Responses in Patients with Advanced, Relapsed/Refractory Diffuse Large B-Cell Lymphoma (DLBCL). Blood 2017;130:4116.
- Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4CRBN. Br J Haematol 2014;164:811-21. [Crossref] [PubMed]
- Wu L, Adams M, Carter T, et al. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res 2008;14:4650-7. [Crossref] [PubMed]
- Gribben JG, Fowler N, Morschhauser F. Mechanisms of action of lenalidomide in B-cell non-hodgkin lymphoma. J Clin Oncol 2015;33:2803-11. [Crossref] [PubMed]
- Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: Establishing a reversible immune evasion mechanism in human cancer. Blood 2012;120:1412-21. [Crossref] [PubMed]
- Leonard JP, Trneny M, Izutsu K, et al. AUGMENT: A Phase III Study of Le-nalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refrac-tory Indolent Lymphoma. J Clin Oncol 2019;37:1188-99. [Crossref] [PubMed]
- Michot JM, Bouabdallah R, Vitolo U, et al. Avadomide plus obinutuzumab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma (CC-122-NHL-001): a multicentre, dose escalation and expansion phase 1 study. Lancet Haematol 2020;7:e649-59. [Crossref] [PubMed]
- Nastoupil LJ, Bijou F, Ribrag V, et al. Avadomide (CC-122), a Novel Cereblon Modulating Agent, Plus Rituximab in Patients with Relapsed or Refractory Follic-ular Lymphoma. Blood 2018;132:1602. [Crossref]
- Niemann CU, Wiestner A. B-cell receptor signaling as a driver of lymphoma development and evolution. Semin Cancer Biol 2013;23:410-21. [Crossref] [PubMed]
- Myklebust JH, Brody J, Kohrt HE, et al. Distinct patterns of B-cell receptor signaling in non-Hodgkin lymphomas identified by single-cell profiling. Blood 2017;129:759-70. [Crossref] [PubMed]
- Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010;463:88-92. [Crossref] [PubMed]
- Long M, Beckwith K, Do P, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest 2017;127:3052-64. [Crossref] [PubMed]
- Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013;122:2539-49. [Crossref] [PubMed]
- Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood 2011;118:3603-12. [Crossref] [PubMed]
- Patton DT, Garden OA, Pearce WP, et al. Cutting Edge: The Phosphoinositide 3-Kinase p110δ Is Critical for the Function of CD4 + CD25 + Foxp3 + Regulatory T Cells . J Immunol 2006;177:6598-602. [Crossref] [PubMed]
- Fruman DA. Targeting PI3K-gamma in non-hodgkin lymphoma. J Clin Oncol 2019;37:932-4. [Crossref] [PubMed]
- Kaneda MM, Messer KS, Ralainirina N, et al. PI3Kγ 3 is a molecular switch that controls immune suppression. Nature 2016;539:437-42. [Crossref] [PubMed]
- Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, et al. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 2010;11:329-41. [Crossref] [PubMed]
- Vanhaesebroeck B, Vogt PK, Rommel C. PI3K: from the bench to the clinic and back. Curr Top Microbiol Immunol 2010;347:1-19. [Crossref] [PubMed]
- Bilancio A, Okkenhaug K, Camps M, et al. Key role of the p110δ isoform of PI3K in B-cell antigen and IL-4 receptor signaling: Comparative analysis of genetic and pharmacologic interference with p110δ function in B cells. Blood 2006;107:642-50. [Crossref] [PubMed]
- Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ Inhibition by Idelalisib in Patients with Relapsed Indolent Lymphoma. N Engl J Med 2014;370:1008-18. [Crossref] [PubMed]
- Dreyling M, Santoro A, Mollica L, et al. Long-term safety and efficacy of the PI3K inhibitor copanlisib in patients with relapsed or refractory indolent lymphoma: 2-year follow-up of the CHRONOS-1 study. Am J Hematol 2020;95:362-71. [Crossref] [PubMed]
- Combination of copanlisib and rituximab significantly prolonged progres-sion-free survival of patients with relapsed indolent non-Hodgkin’s Lymphoma. (accessed November 15, 2020). Available online: https://media.bayer.com/baynews/baynews.nsf/id/Combination-copanlisib-rituximab-significantly-prolonged-progression-free-survival-patients-relapsed?OpenDocument&sessionID=1605467659
- Flinn IW, Miller CB, Ardeshna KM, et al. DYNAMO: A Phase II Study of Duvelisib (IPI-145) in Patients With Refractory Indolent Non-Hodgkin Lymphoma. J Clin Oncol 2019;37:912-22. Erratum in: J Clin Oncol 2019;37:1448. [Crossref] [PubMed]
- Burris HA, Flinn IW, Patel MR, et al. Umbralisib, a novel PI3Kδ and casein kinase-1ε inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol 2018;19:486-96. [Crossref] [PubMed]
- Niemann CU, Herman SEM, Maric I, et al. Disruption of in vivo chronic lymphocytic leukemia tumor-microenvironment interactions by ibrutinib - Findings from an investigator-initiated phase II study. Clin Cancer Res 2016;22:1572-82. [Crossref] [PubMed]
- Sagiv-Barfi I, Kohrt HEK, Czerwinski DK, et al. Therapeutic antitumor im-munity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci U S A 2015;112:E966-72. [Crossref] [PubMed]
- Sagiv-Barfi I, Kohrt HE, Burckhardt L, et al. Ibrutinib enhances the antitumor immune response induced by intratumoral injection of a TLR9 ligand in mouse lymphoma. Blood 2015;125:2079-86. [Crossref] [PubMed]
- Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ib-rutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 2013;31:88-94. [Crossref] [PubMed]
- Bartlett NL, Costello BA, LaPlant BR, et al. Single-agent ibrutinib in relapsed or refractory follicular lymphoma: A phase 2 consortium trial. Blood 2018;131:182-90. [Crossref] [PubMed]
- Fowler N, Nastoupil L, de Vos S, et al. Ibrutinib Plus Rituximab in Treat-ment-Naive Patients with Follicular Lymphoma: Results from a Multicenter, Phase 2 Study. Blood 2015;126:470. [Crossref]
- Ujjani CS, Jung SH, Pitcher B, et al. Phase 1 trial of rituximab, lenalidomide, and ibrutinib in previously untreated follicular lymphoma: Alliance A051103. Blood 2016;128:2510-6. [Crossref] [PubMed]
- Maddocks K, Christian B, Jaglowski S, et al. A phase 1/1b study of rituximab, bendamustine, and ibrutinib in patients with untreated and relapsed/refractory non-Hodgkin lymphoma. Blood 2015;125:242-8. [Crossref] [PubMed]
Cite this article as: Khurana A, Ansell SM. Novel immunotherapy in follicular lymphoma: a narrative review. Ann Lymphoma 2021;5:9.


