
Narrative review: secondary central nervous system lymphoma
Introduction
With the term of secondary central nervous system (CNS) lymphoma (SCNSL) we indicate the systemic lymphoproliferative diseases with CNS involvement at presentation or at relapse or at both stages of disease. SCNSL may present as dissemination leptomeningeal, parenchymal, in cranial nerves or more rarely ocular. Several CNS compartments frequently are involved concomitantly or sequentially. CNS involvement in diffuse large B cell lymphoma (DLBCL) represents often a fatal event. The incidence of SCNSL at relapse in DLBCL is rare, around 5%, and possibly further reduced after the introduction of rituximab (1,2). However, the real benefit obtained with the addition of rituximab to chemotherapy is small and controversial (3-5). In the end, consensus opinion supports that the reduction of CNS relapse in the rituximab era is a consequence of improved control of systemic disease (6,7). The pattern of CNS relapse in DLBCL after R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone) appears different compared to the pattern observed in the pre-rituximab era, with relapses increasingly involving the brain parenchyma (70–80%) rather than the leptomeninges (8,9) and occurring earlier. Although a higher proportion of isolated CNS recurrences was reported in rituximab-treated patients (1,10), concurrent CNS and systemic relapses still occur in a significant proportion of cases with SCNSL (46–48%) (11). Some risk factors of CNS relapse were recognized such as high International Prognostic Index (IPI) score, involvement of more than 2 extranodal sites or involvement of specific organs defined at high risk (12-16). In these instances, CNS relapse usually occurs within the first year from diagnosis (3,11). The different scenarios, with whom the SCNSL can occur, influence the choice of therapy. However, being by definition a systemic disease, also in cases without a macroscopic systemic dissemination, the SCNSL treatment needs to be able to tackle both systemic and CNS areas of disease. The treatment usually includes two phases: induction and consolidation. The induction consists of sequential combined regimens containing agents able to cross blood brain barrier (BBB) and to penetrate within brain parenchyma and regimens containing agents that are well-known to be active in extra-CNS DLBCL. The consolidation phase can include myeloablative chemotherapy followed by autologous stem cell transplantation (HDT/ASCT), radiotherapy or standard dose chemotherapy, according to the host characteristics and to previous treatments and their responses.
This review will focus on the managements of the patients with DLBCL at high-risk of CNS relapse and on the diagnostic and therapy approaches that are increasingly widespread in clinical practice for patients with SCNSL.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/aol-20-39).
Discussion
Identification of patients with high-risk of CNS relapse lymphoma and CNS prophylaxis
Clinical risk factors of CNS relapse
SCNSL is a rare but devastating event. The identification of variables and scores with high diagnostic sensitivity to select patients at high risk for CNS relapse could permit the application of CNS prophylaxis to the only subjects with a favorable risk/benefit ratio.
In the last years, several studies tried to identify factors predicting CNS dissemination. A number of clinical characteristics (such as age more than 60 years, elevated LDH level, involvement of more than one extranodal site) were recognized to increase the risk of CNS disease. These studies’ results were often discordant and their level of evidence remain low (1,3,17,18). More studies have suggested a predictive role of the IPI score (3,11,19). In the end, a large retrospective study (>2,000 patients) has analyzed the risk factors for CNS relapse. A six-factor model called ‘CNS-IPI’, based on the five IPI variables and kidney and/or adrenal gland involvement, was developed and validated as toll to predict the risk of CNS relapse in patients with DLBCL. CNS-IPI score permits to identify 3 risk classes: low, intermediate, and high risk, that have showed 2-year rates of CNS relapse of 0.6%, 3.4% and 10.2%, respectively. Patients belonging to low- and intermediate-risk groups, that represent around 90% of DLBCL subjects, have a risk lower than 5% and, in the absence of specific neurological symptoms, any diagnostic and therapeutic intervention may be spared. In contrast, those classified as high-risk for SCNSL have a more than 10% risk of CNS relapse and should undergo CNS-directed investigations and prophylaxis therapy (12).
Both the involvement of some extranodal sites, that are likely to be underrepresented in clinical trials but in retrospective studies have demonstrated to be associated with a high CNS relapse rates (12–16%) (11,20), and the concurrent involvement of three or more extranodal sites have demonstrated to play a crucial role in defining the risk of CNS relapse independently to CNS-IPI score (21). The inclusion of kidney and adrenal gland as the only high-risk extranodal sites in the CNS-IPI score evaluation represents one of the limitations of CNS-IPI score. Even if in large studies numbers become rather small if specific sites are analyzed separately.
More than 80% of CNS relapses seem to occur in patients with extranodal disease (22). Testis (13), breast and female reproductive organs (14,15), kidney and adrenal glands (16,23), paranasal sinus, intra-orbital (24) are among the extranodal sites that showed retrospectively to confer an increased risk of CNS dissemination (with a range incidence of CNS relapse of 10–30%). It is unclear why some of the extranodal localizations have a high risk of SCNSL, but genetic and homing factors, that are yet unknown, must play a role (13-16,23).
Molecular markers predicting CNS tropism
One possible way to improve the identification of patients with high risk of CNS relapse may be the use of biomarkers, which could layer the subjects with aggressive lymphomas on top of the clinical model.
Some biological factors have been described to be associated with an increased risk of CNS relapse in retrospective series. In particular, in high grade B cell lymphoma that harbor a MYC combined with a BCL2 and/or a BCL6 rearrangement (double-hit or triple-hit high-grade B cell lymphoma, DH/TH HGBL) and double-protein expressor lymphomas (DE), have an increased risk of CNS relapse, independent of CNS-IPI. Also, CD5 positivity has been identified as a risk factor of CNS relapse (25,26).
The increased risk in DE lymphoma was not confirmed by a subgroup analysis in patients who were enrolled in the GOYA trial and relapsed in CNS after treatment with R-CHOP or G-CHOP (obinutuzumab-CHOP). Activated B-cell-like (ABC) (HR, 5.2) or unclassified cell of origin (COO) subtypes (HR, 4.2) and high-risk CNS-IPI score were recognized as independent risk factors of CNS relapse. DE status did not demonstrate to impact on CNS relapse risk. Based on these data a consequent molecular CNS-IPI (CNS-IPI-C) was proposed (27). Three risk subgroups were identified based on the presence of high CNS-IPI score and/or ABC/unclassified COO: low risk (no risk factors), intermediate risk (1 factor), and high risk (both factors), that were associated with 2-year CNS relapse rates of 0.5%, 4.4%, and 15.2%, respectively. One of the disadvantages of the use of CNS-IPI-C model, is that the COO classification requires gene expression profiling, that is not used in general (28).
The molecular analysis, performed on the biopsy samples of the patients with CNS relapse, showed that CDKN2A loss and mutation of MYD88 were most commonly associated with CNS relapse event. In contrast, MYD88 mutations were not identified in SCNSLs in a retrospective study (29).
Lemma et al. have explored the role of biological markers that may confer to lymphoma cells a homing into the CNS due to a highly selective CNS tropism, in order to identify patients with a high risk of CNS recurrence. High levels of Integrin alpha 10 and PTEN on biopsy samples were associated with CNS tropism, while CD44 and cadherin-11 expressions seem to be protective of SCNSL. Due to limitations of the retrospective status and to limited samples, these results are highly preliminary and need to be validated in a larger prospective trial (30).
Prophylaxis to prevent CNS recurrence
CNS prophylaxis in DLBCL is a contentious issue. There is a wide variability in the choice of this therapeutic approach among various centers. The major reason is due to the paucity of robust, prospective studies to drive the selection of the patients who are candidate to CNS prophylaxis and the optimum method of preventing CNS relapse.
The most widely used prophylaxis is intrathecal methotrexate (MTX) (24), although its effectiveness is not established in randomized trials. A hint to at least some effect may be found in some recent studies (11,31) and the reduced incidence in testicular lymphoma (32). Other studies failed to demonstrate a benefit of intrathecal (IT) prophylaxis, probably because the pattern of CNS relapse in DLBCL is predominantly intra-parenchymal (28,33), an area that is inadequately penetrated by IT chemotherapy (34). A recent systematic review tried to solve the issue. The authors conclude that strong evidence for the use of IT prophylaxis was absent (35).
Furthermore, the optimal IT chemotherapy is not known due to the lack of randomized studies comparing the efficacy and toxicity profile of different IT regimens (MTX, Cytarabine, PEGylated Cytarabine, methylprednisolone alone or combined).
Since most of the CNS relapses nowadays present with an intra-parenchymal involvement, there has been increased focus on the use of systemic prophylaxis with intravenous high-dose methotrexate (i.v. HD-MTX). It has demonstrated its protective role in preventing CNS relapse in several studies, with a reduced CNS relapse rate to 2–5% (36-38). Since CNS disease tends to occur early, with a median of 6–8 months after DLBCL diagnosis, some authors suggested that systemic HD-MTX should be administered as early as possible after diagnosis. A recent retrospective study compared the outcome in term of CNS relapse rate and overall disease control with prophylaxis with HD-MTX intercalated to R-CHOP versus HD-MTX at the end of therapy (EOT). A higher incidence of toxicity in the intercalated HD-MTX subgroup (mucositis and febrile neutropenia), with a more frequent delay of the subsequent R-CHOP cycles (20%) was observed, although no difference in efficacy and survival were reported between two approaches. It should be mentioned here that 56% of the patients in the EOT group received also MTX it, compared to 34% of the patients that received intercalated MTX iv. It is uncertain if this has an additional role. The authors concluded that to reduce the risk of very early CNS relapse, intercalating HD-MTX with R-CHOP has a theoretical benefit, and if this approach is used, to give the HD-MTX before day 10 to minimize toxicity and dose delays of R-CHOP. Delivery of HD-MTX at the EOT seems to be a valid alternative strategy, particularly where there is concern about fitness and ability to maintain R-CHOP dose intensity, accepting a risk that early CNS relapse may not be prevented (39).
A more intensive therapeutic approach was investigated in a recent phase 2 trial. Treatment with HD-MTX, given with the first 2 cycles of 14-day R-CHOP therapy, followed by 4 cycles of 14-day R-CHOP plus etoposide with IT cytarabine given as further CNS prophylaxis. Consolidation therapy with HD-cytarabine (HD-ARA-C) was performed in responsive patients (27). After 5 years of follow up, the failure-free survival, overall survival (OS), and CNS progression rates were 74%, 83%, and 2.3%, respectively. Treatment failure due to acute toxicity and treatment-related deaths were 6.5% and 3.6%, respectively.
No worldwide consensus exists about the optimal HD-MTX dosing and frequency. Generally, a dose of 3 g/m2 as short (3.5–6 hours) infusion seems optimal in achieving effective CNS concentrations and avoiding serious toxicities (40).
There has been no randomized study investigating the optimal number of courses of HD-MTX as CNS prophylaxis, and a lack of worldwide consensus exists. However, two to three courses are recommended in patients without cardiac, hepatic and/or renal disfunction (41).
Lenalidomide and ibrutinib, two novel agents that were incorporated into R-CHOP therapy in two large phase 3 trials, have failed to show overall benefit for untreated patients with DLBCL (42,43). The results of a multicenter analysis to detect the potential role of lenalidomide in preventing CNS relapse was promising. Among 136 patients, who received lenalidomide in induction therapy (R2-CHOP), only one patient developed CNS relapse, after a median follow-up of 48.2 months. This promising result needs to be confirmed by larger prospective studies (44).
Whether ibrutinib and lenalidomide as well as other small molecules such as venetoclax and everolimus could specifically confer a potential protection of the CNS relapse in patients at high-risk remains an unanswered question but their ability to cross the BBB presupposes the rational for future studies.
Diagnostic and staging assessment of SCNSL
The clinical symptoms of CNS involvement may vary widely from new onset headache (50%), palsies of cranial nerves III, IV, VI, and VII, changes in neurological status (29%), seizures (23–29%) and even coma (45). CNS relapse typically presents within 8 months from diagnosis of the primary lymphoma, but late CNS relapse occurs up till 79 months (46). The CNS relapse should be confirmed by CSF and/or neuroimaging studies. In some cases, a vitrectomy or brain biopsy is required to confirm the diagnostic suspicious (47). Brain contrast-enhanced magnetic resonance imaging (MRI) and CSF examination including flow cytometry and cytology may detect CNS disease. These assessments permit to guide the choice of CNS-directed therapy and to define therapy response. MRI is the current gold standard for localizing the CNS parenchyma and leptomeningeal recurrence, having shown its superior sensitivity compared to computed tomography (CT) scan in detecting pathological lesion of CNS. Parenchymal lesions usually bright on diffusion weighted imaging, showing homogeneous enhancement. They are often multiple and localize usually in superficial cortex or periventricular sites (48). Although the imaging appearance may mimic infectious, inflammatory, or metastatic disease, a history of systemic lymphoma can orient to SCNSL diagnosis, however a confirmation by biopsy or positive CSF is advisable (49,50). Spinal MRI is recommended only if neurologic symptoms suggest spinal localization and in cases with positive CSF (48,51).
Baseline total body positron emission tomography/CT (PET/CT) should be performed in all CNS lymphoma patients to assess the extent of lymphoma involvement. In addition, PET/ CT is contemplated in the evaluation of therapy response in Non-Hodgkin Lymphomas according to Lugano criteria (52). CNS lymphomatous involvement may present as a pathological 18F-FDG uptake. Preliminary data suggest that PET/CT could represent an additional tool to the MRI in the assessment of therapy response also in CNS lymphoma, conferring metabolic information (53). However, further larger studies are needed to validate this conclusion and before its use in the clinical practice.
Ophthalmological assessment is recommended in all cases with CNS lymphoma and ocular symptoms. It includes direct ophthalmoscopy, fundus examination, fluoro-scintigraphy. In some cases, a histological or cytological confirmation could be assessed with vitrectomy and/or vitreous humor aspiration
Bone marrow biopsy and aspiration should be routinely performed at the CNS lymphoma diagnosis or relapse to detect lymphoma bone marrow involvement and/or impaired bone marrow reserve that may influence the treatment choice (Table 1).

Full table
Treatment strategy
CNS involvement may be diagnosed at presentation with a systemic DLBCL, or later, as relapsed disease, either with or without systemic relapse/progression of the lymphoma. This results in three different situations of SCNSLs that may influence the choice of therapy: the patient may be treatment naïve or not, and in case of relapse it may be relevant if systemic disease is also present. However, some studies have not differentiated between upfront and relapse setting. Anyway, the systemic disease component should be incorporated in the treatment. A keystone in the treatment of CNS lymphoma is MTX. No studies have been performed that randomize between i.v and IT drug delivery. However, it is clear that a parenchymal localization is inadequately treated by intrathecal MTX only, as this penetrates only a few mm in the tumor mass.
Simultaneous CNS and systemic disease at diagnosis.
The standard treatment for systemic DLBCL is R-CHOP. To address the CNS localization, incorporation of MTX in the treatment regimen is an option. Retrospective studies show that several regimens are in use, reflecting the scarcity of well-defined prospective studies. The choice of treatment in the individual patients may have been influenced by host characteristics (comorbidity, PS, age), physician’s experience and other factors.
In case of positive CSF, treatment with IT MTX may be used. Data on the outcome are scarce. In a single center retrospective study investigating the efficacy of intrathecal treatment, that included also 21 DLBCL patients, the response was 86%. The median OS was 15 months (54). To reduce the chance of systemic side-effects, such as mucositis and prolonged neutropenia, 15 mg of folinic acid orally 24 hours after the intrathecal injection is recommended.
One of the systemic treatment options is “intercalating” i.v HD-MTX between R-CHOP. As reported above, this regimen is also in use as CNS prophylaxis and it is feasible and safe (36). The HD-MTX is best given before day 9 of the R-CHOP cycle, to prevent delay of the next cycle (39). Although this regimen is in use in several centers, reports on the outcome for SCNSL are scarce and included only few patients (55). In a larger retrospective study investigating the management and outcome of 44 patients with diagnosis of SCNSL, the majority of them received R-CHOP/HD-MTX. Sixty-six percent of the patients receiving induction therapy achieved a complete remission (CR). The 3-years OS was 60%. Multivariate analysis showed that treatment with R-CHOP/HD-MTX (3.5 g/m2) and achievement of CR were significantly associated with a better OS (56).
Another drug that penetrates the BBB effectively is HD-ARA-C. In a French retrospective study, 52 of 60 patients with SCNSL received anthracycline-based therapy. In addition, 31 of them also received HD-MTX and HD-ARA-C. Forty-one patients (68%) achieved a CR. The 3-year OS was 44% (57).
More intensive regimens than R-CHOP/HD-MTX have been used as induction regimen. In an international, multicenter, retrospective study with 80 SCNSL patients, the authors have divided the regimens used in CNS-intensive and CNS-conservative. R-Hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate and cytarabine) and R-CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, high-dose cytarabine) were the most used CNS-intensive regimens. The CR rate in this subgroup was 69%. The 2-year OS was 54% (58).
It is not clear if consolidation treatment with HDT/ASCT is beneficial for this patient group. The two largest retrospective studies mentioned before, have contradictory results. Damaj et al. describe that almost half of the patients that were in CR after induction therapy were consolidated with ASCT. In 8 patients the conditioning regimen consisted of BEAM (carmustine (BCNU), etoposide, cytarabine and melphalan), in 8 patients a thiotepa based-regimen was used (TBC: thiotepa, busulfan, cyclophosphamide). In univariate analysis, the consolidation with ASCT was strongly associated with a better 3 year-OS (75% vs. 29%). No difference in outcome was found between the conditioning regimens used (57). This positive result of ASCT was not confirmed by another study, in which 19 patients received ASCT, and 14 no additional treatment (56).
CNS lymphoma in the relapse setting
The majority of CNS lymphoma dissemination is found in the relapse setting. It occurs relatively early, at a median of 8 months after initial lymphoma diagnosis. Concurrent systemic relapse is frequent. Isolated CNS relapse has a better prognosis. A retrospective database study reported the final results on 291 patients with SCNSL, of which 161 with isolated CNS relapse. Twenty-eight percent of the patients had received CNS prophylaxis, of whom 61% systemic prophylaxis. The 2-year OS was 20% (59).
The largest study into the incidence of CNS relapse analyzed almost 2,000 patients up to 60 years old with aggressive lymphoma, who were included in several prospective studies. It revealed that 56 patients (2.6%) presented a SCNSL. The median time to development of SCNSL in this study was 7 months. Two-third of the patients developed isolated CNS disease, the others had concurrent systemic relapse or progression. The median survival after occurrence of CNS lymphoma was 5 months (3). The poor outcome of these patients is all the more disappointing considering the relatively young age of the patients in this analysis.
Even in isolated CNS relapse, the risk of systemic relapse later on is high, so treatment is usually directed against both systemic disease and the CNS compartment. Several studies have addressed this difficult situation, but they all include relatively few patients, due to the rarity of the disease. A relapse in systemic localization is generally treated with immuno-chemotherapy followed by HDT/ASCT in responsive patients. Consolidation with HDT/ASCT is valid also for CNS relapsed patients, although a conditioning regimen with CNS penetrating drugs should be used. A retrospective study from the International Primary Central Nervous System Lymphoma Study Group reported the outcome of 92 patients with SCNSL, diagnosed between 2000 and 2010. Seventy-nine percent of them received chemotherapy and 29% were consolidated with HDT/ASCT. The main reasons to refrain from ASCT were age, lack of response and poor PS/comorbidities. The median OS was 7 months. The 3-year OS was 22% for all patients, while in the group that had undergone HDT/ASCT the 3-year OS was 42% (60). The Center for International Blood and Marrow Transplant Research published the outcome of 151 patients with SCNSL comparing it with that of transplanted patients without SCNSL. They found no difference in outcome. In both groups the majority of the relapses after HDT/ASCT were outside the CNS. The 3-year OS in patients with active CNS disease at HDT/ASCT was inferior compared to those in CNS remission (31% vs. 58%) (61).
Prospective studies in SCNSL are rare. A German multicenter study in 30 patients used an intensive induction regimen consisting of 2 cycles of HD-MTX/ifosfamide followed by 1 cycle HD-ARA-C/thiotepa, all in combination with intrathecal therapy. This was followed by ASCT after conditioning with BCNU/thiotepa. Eighty percent of the patients had an isolated CNS relapse. A total of 24 patients (80%) received HDT/ASCT, 20 of them (67%) had achieved a response (7 CR) with induction therapy. The 2-year OS was 63% (46).
The Dutch HOVON group included 36 patients in a prospective study, treated with a regimen of R-DHAP and HD-MTX, in combination with intrathecal rituximab. Twenty patients (56%) also had a systemic relapse. Responding patients after two cycles of R-DHAP/MTX received a third cycle, and were then consolidated with ASCT after busulfan/cyclophosphamide conditioning. The overall response rate (ORR) after 2 cycles, combined CNS and systemic responses, was 53% (19/36) with CR in 22% (8/36). Fifteen patients (42%) underwent HDT/ASCT. The main reason HDT/ASCT was not performed was insufficient response. The 2-year OS was 22% (62).
A third prospective study included 38 patients with both CNS localization upfront (42%) and at relapse (58%). Treatment consisted of debulking of systemic disease with R-CHOP, if clinically indicated, induction with 2 courses of HD-MTX/HD-ARA-C/rituximab and intrathecal PEGylated-cytarabine. Patients with responsive disease proceeded with sequential HD of cyclophosphamide/ARA-C/and etoposide. Finally, patients with responsive disease received HDT/ASCT with BCNU/thiotepa–conditioning. Twenty responsive patients (19 in CR, 1 in PR) received HDT/ASCT, all achieved a CR. The 5-year OS rate of the entire population was 41%, while that of patients receiving HDT/ASCT was 68%. The majority of deaths were lymphoma-related (63). This study was the basis for a prospective study of the International Extranodal Lymphoma Study Group (IELSG): IELSG42/MARIETTA trial. The induction was intensified to 3 cycles of MATRix (HD-MTX/HD-ARA-C/thiotepa/rituximab) followed by 3 cycles of intensification R-ICE (rituximab/ifosfamide/carboplatin/etoposide), and intrathecal chemotherapy. Patients in response were consolidated with HDT/ASCT after BCNU/Thiotepa-conditioning. The results of this largest prospective study in SCNSL are recently published. Seventy-five patients with CNS involvement at presentation (43%), as isolated site of relapse (20%) or with concomitant CNS-systemic relapse (37%) received treatment. Thirty-seven patients received HDT/ASCT. The 2-year PFS was estimated at 46% for all population and at 83% for transplanted patients. Most organs involved at relapse or progression were primary sites of disease. The 2-year OS was 46% for all population and 83% for transplanted patients. Major causes of death were lymphoma-related (n=35) and toxicity (n=4). Patients with CNS involvement at presentation had the best outcome, with a 2-year PFS of 71% (64).
The UK Central and Southern lymphoma group developed the R-IDARAM regimen for treatment of SCNSL, consisting of rituximab/HD-MTX/HD-ARA-C/idarubicin/dexamethasone with IT MTX. A retrospective analysis of 23 patients with SCNSL, upfront (n=10) and at relapse (n=13) found that the ORR after 1–4 cycles was 61%. Although the numbers were small, especially the response rate in newly diagnosed patients was promising (ORR 70%). The 2-year estimated PFS and OS were 39% and 52% respectively (65).
Refractory/relapsed SCNSL: treatment options beyond chemotherapy
The result of treatment in SCNSL with curative intent is unfortunately relatively poor, and many patients will progress or relapse. Efficient therapeutic approach for refractory/relapsed (r/r) SCNSL remains a challenge.
Traditionally, symptomatic parenchymal CNS relapse can be treated with whole brain radiotherapy (WBRT). In general, this is not a curative option, but confers an improvement of symptoms. Also, treatment with dexamethasone may reduce symptoms, although for a limited time.
Salvage treatment with chemotherapy is often difficult due to patients’ poor general condition and because the effective drugs that penetrate the BBB have already been used (66,67).
The interest of the clinicians now moves to investigate innovative agents beyond chemotherapy in this setting of patients.
To date, the experience reported in literature in r/r SCNSL patients treated with targeted therapy are limited to few studies on small populations.
BTK inhibitor: ibrutinib
Ibrutinib, a first-class inhibitor of Bruton tyrosine kinase (BTK), has been shown to cross the BBB and to distribute into brain tissue (68). It has been explored in r/r CNS lymphoma patients alone or in combination with chemotherapy. In a dose escalation and dose expansion phase 1 trial, ibrutinib was administered continuously (with a maximum dose of 840 mg OD) until disease progression, intolerable toxicity, or death. Twenty patients were analyzed: 13 with PCNSL and 7 with SCNSL. Ibrutinib as single agent was well tolerated both at 560 mg and at 840 mg dose level with the exception of 1 case of pulmonary aspergillosis. After a median follow-up of 1.5-year, median PFS was 4.6 months. Considering the SCNSL subgroup, 5/7 patients (71%) responded, 4 achieved CR. Median PFS for SCNSLs was 7.4 months (69).
Ibrutinib was also combined with chemotherapy after having demonstrated their synergism in killing cells of DLBCL in vitro (70). A phase 1b trial explored the sequential combination of ibrutinib (560 or 840 mg daily) with HD-MTX (3.5 g/m2 every 2 weeks) for 8 courses in 9 patients with r/r PCNSL and 6 with r/r SCNSL, without active extra-CNS disease. Single-agent ibrutinib daily was administered continuously after completion of induction therapy until disease progression, intolerable toxicity, or death. Considering the subgroup of SCNSL patients, ORR was 67% (2 PR, 2 CR) (71).
Immunomodulatory drugs (IMIDs)
In systemic DLBCLs, ABC or unclassified cell of origin (COO) subtypes were recognized to have a higher risk of CNS relapse (28). The genetic alterations of ABC subtype involve the activation of B-cell receptor (BCR), Toll-like receptor (TLR), and nuclear factor-kb (NF-kB) pathways. Lenalidomide (a second-generation IMID), that carries out part of its activity inhibiting nuclear factor-kb (NF-kB) (72,73) was investigated in r/r systemic DLBCL, demonstrating to be more active in ABC than GCB subtype lymphoma.
A phase 1 study investigating safety, dose limiting toxicities (DLT) and CSF dose concentration of Lenalidomide in patients with SCNSL achieved a high CSF penetration with a minimal dose of 15 mg daily, while DLT was 20 mg (administered 21/28 days). The recommended dose was 15 mg daily. Fourteen patients were enrolled (6 r/r PCNSL and 8 r/r SCNSL, including 3 patients with active systemic lymphoma). Six SCNSLs were evaluated, 4 responded (2CR and 2 PR) and 2 progressed in CNS. The maintenance therapy demonstrated of conferring a response duration 6 times longer than which obtained after CR1 with induction therapy (74).
More recently, 2 clinical trials reported a promising efficacy and good tolerability of maintenance lenalidomide both in PCNSL and in systemic DLBCL (75,76). These results can represent the basis for future studies exploring lenalidomide treatment or maintenance in SCNSL.
Checkpoint inhibitors
The use of checkpoint inhibitors in r/r SCNSL is rarely reported. A retrospective study described the experience in 6 r/r CNS lymphoma patients (3 with r/r PCNSL and 3 with r/r SCNSL) treated with rituximab/pembrolizumab (5 cases) or rituximab/nivolumab (1 case). An ORR of 50% with 3 CR was achieved (77). Prospective clinical trials evaluating efficacy, optimal duration, and dose of combined Checkpoint inhibitors-based regimens in r/r SCNSL are ongoing.
CAR-T cells
CAR-T cells targeting CD19 showed to be highly effective in r/r B cell lymphoproliferative diseases with ORR >80% and CR >50% (78-80). Neurological toxicity in the form of CAR-T related encephalopathy (CRE) occurred in 19% to 64% of patients treated with CAR-T cells. Due to the potential mortality by CRES, patients with CNS lymphoma were excluded from nearly all clinical trials of CAR-T cell therapy. Recent experiences in SCNSL patients treated with CAR-T-cells reported no increased incidence of CRE (81-83). These data are encouraging and hopefully may improve the dismal outcome of SCNSL.
Conclusions
SCNSL represents a strong challenge due to its rarity, the poor outcome, and the difficulty to define a standard treatment. Comparison between available studies is inherently difficult due to their heterogeneity in selection criteria of patients, that preclude a strong recommendation regarding the best therapy. Furthermore, variation in the primary treatment regimen used for systemic disease and/or for CNS prophylaxis complicates data interpretation of the results observed after the first line therapy applied for SCNSL.
The early identification of patients at high risk of CNS relapse and an efficient prophylaxis reducing the recurrence in CNS represent, to date, the more promising therapy approaches. Randomized clinical trials will be required to determine the optimal therapeutic approach for CNS prophylaxis in high-risk patients, assessing also the integration of new drugs, able to cross the BBB and to prevent CNS relapse in DLBCL.
Despite recent knowledge of the biology of CNS lymphoma and improvements in its management, SCNSL outcome is still poor. The cornerstone of treatment includes regimens with BBB penetrating drugs and regimens active for extra-CNS disease (Figure 1).
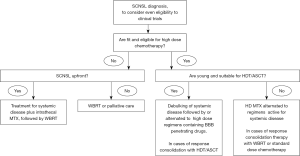
The role of HDT/ASCT consolidation for patients with upfront CNS localization remains at now controversial. In the relapse setting it is generally used. A conditioning regimen with BBB penetrating drugs is important.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Andrés J. M. Ferreri, Maurilio Ponzoni) for the series “Central Nervous System Lymphomas” published in Annals of Lymphoma. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/aol-20-39
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-39). The series “Central Nervous System Lymphomas” was commissioned by the editorial office without any funding or sponsorship. JKD reports grants and non-financial support from Roche, non-financial support from Celgene, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Boehme V, Schmitz N, Zeynalova S, et al. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: An analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood 2009;113:3896-902. [Crossref] [PubMed]
- Ghose A, Elias HK, Guha G, et al. Influence of rituximab on central nervous system relapse in diffuse large B-cell lymphoma and role of prophylaxis: a systematic review of prospective studies. Clin Lymphoma Myeloma Leuk 2015;15:451-7. [Crossref] [PubMed]
- Schmitz N, Zeynalova S, Glass B, et al. CNS disease in younger patients with aggressive B-cell lymphoma: an analysis of patients treated on the Mabthera International Trial and trials of the German High-Grade Non-Hodgkin Lymphoma Study Group. Ann Oncol 2012;23:1267-73. [Crossref] [PubMed]
- Tai WM, Chung J, Tang PL, et al. Central nervous system (CNS) relapse in diffuse large B cell lymphoma (DLBCL): pre- and post-rituximab. Ann. Hematol 2011;90:809-18. [Crossref] [PubMed]
- Feugier P, Virion JM, Tilly H, et al. Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large-B-cell lymphoma: influence of rituximab. Ann Oncol 2004;15:129-33. [Crossref] [PubMed]
- McMillan A, Ardeshna KM, Cwynarski K, et al. Guideline on the prevention of secondary central nervous system lymphoma: British Committee for Standards in Haematology. Br J Haematol 2013;163:168-81. [Crossref] [PubMed]
- Zhang J, Chen B, Xu X. Impact of rituximab on incidence of and risk factors for central nervous system relapse in patients with diffuse large Bcell lymphoma: a systematic review and meta-analysis. Leuk Lymphoma 2014;55:509-14. [Crossref] [PubMed]
- Guirguis HR, Cheung MC, Mahrous M, et al. Impact of central nervous system (CNS) prophylaxis on the incidence and risk factors for CNS relapse in patients with diffuse large B-cell lymphoma treated in the rituximab era: a single centre experience and review of the literature. Br J Haematol 2012;159:39-49. [Crossref] [PubMed]
- Mitrovic Z, Bast M, Bierman PJ, et al. The addition of rituximab reduces the incidence of secondary central nervous system involvement in patients with diffuse large B-cell lymphoma. Br J Haematol 2012;157:401-3. [Crossref] [PubMed]
- Villa D, Connors JM, Shenkier TN, et al. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol 2010;21:1046-52. [Crossref] [PubMed]
- Gleeson M, Counsell N, Cunningham D, et al. Central nervous system relapse of diffuse large B-cell lymphoma in the rituximab era: results of the UK NCRI R-CHOP-14 versus 21 trial. Ann Oncol 2017;28:2511-6. [Crossref] [PubMed]
- Schmitz N, Zeynalova S, Nickelsen M, et al. CNS International Prognostic Index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol 2016;34:3150-6. [Crossref] [PubMed]
- Zucca E, Conconi A, Mughal TI, et al. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol 2003;21:20-7. [Crossref] [PubMed]
- El-Galaly TC, Cheah CY, Hutchings M, et al. Uterine, but not ovarian, female reproductive organ involvement at presentation by diffuse large B-cell lymphoma is associated with poor outcomes and a high frequency of secondary CNS involvement. Br J Haematol 2016;175:876-83. [Crossref] [PubMed]
- Ryan G, Martinelli G, Kuper-Hommel M, et al. Primary diffuse large Bcell lymphoma of the breast: prognostic factors and outcomes of a study by the International Extranodal Lymphoma Study Group. Ann Oncol 2008;19:233-41. [Crossref] [PubMed]
- Villa D, Connors JM, Sehn LH, et al. Diffuse large B-cell lymphoma with involvement of the kidney: Outcome and risk of central nervous system relapse. Haematologica 2011;96:1002-7. [Crossref] [PubMed]
- Kridel R, Dietrich PY. Prevention of CNS relapse in diffuse large B-cell lymphoma. Lancet Oncol 2011;12:1258-66. [Crossref] [PubMed]
- Fletcher CD, Kahl BS. Central nervous system involvement in diffuse large B-cell lymphoma: An analysis of risks and prevention strategies in the post rituximab era. Leuk Lymphoma 2014;55:2228-40. [Crossref] [PubMed]
- Tomita N, Yokoyama M, Yamamoto W, et al. The standard international prognostic index for predicting the risk of CNS involvement in DLBCL without specific prophylaxis. Leuk Lymphoma 2018;59:97-104. [Crossref] [PubMed]
- Hosein PJ, Maragulia JC, Salzberg MP, et al. A multicentre study of primary breast diffuse large B-cell lymphoma in the rituximab era. Br J Haematol 2014;165:358-63. [Crossref] [PubMed]
- El-Galaly TC, Villa D, Michaelsen TY, et al. The number of extranodal sites assessed by PET/CT scan is a powerful predictor of CNS relapse for patients with diffuse large B-cell lymphoma: an international multicenter study of 1532 patients treated with chemoimmunotherapy. Eur J Cancer 2017;75:195-203. [Crossref] [PubMed]
- Ferreri AJ. Risk of CNS dissemination in extranodal lymphomas. Lancet Oncol 2014;15:e159-69. [Crossref] [PubMed]
- Vélayoudom FL, Cardot-Bauters C, Decouvelaere AV, et al. Non Hodgkin’s lymphoma involving the adrenal glands and the central nervous system (CNS): a particular evolution after chemotherapy. Ann Endocrinol (Paris) 2005;66:527-31. [Crossref] [PubMed]
- Cheung CW, Burton C, Smith P, et al. Central nervous system chemoprophylaxis in non-Hodgkin lymphoma: current practice in the UK. Br J Haematol 2005;131:193-200. [Crossref] [PubMed]
- Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol 2014;166:891-901. [Crossref] [PubMed]
- Savage KJ, Slack GW, Mottok A, et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood 2016;127:2182-8. [Crossref] [PubMed]
- Leppä S, Jørgensen J, Tierens A, et al. Patients with high-risk DLBCL benefit from dose-dense immunochemotherapy combined with early systemic CNS prophylaxis. Blood Adv 2020;4:1906-15. [Crossref] [PubMed]
- Klanova M, Sehn LH, Bence-Bruckler I, et al. Integration of cell of origin into the clinical CNS International Prognostic Index improves CNS relapse prediction in DLBCL. Blood 2019;133:919-26. [Crossref] [PubMed]
- Kersten MJ, Kraan W, Doorduijn J, et al. Diffuse large B cell lymphomas relapsing in the CNS lack oncogenic MYD88 and CD79B mutations. Blood Cancer J 2014;4:e266 [Crossref] [PubMed]
- Lemma SA, Kuusisto M, Haapasaari KM, et al. Integrin alpha 10, CD44, PTEN, cadherin-11 and lactoferrin expressions are potential biomarkers for selecting patients in need of central nervous system prophylaxis in diffuse large B-cell lymphoma. Carcinogenesis 2017;38:812-20. [Crossref] [PubMed]
- Wilson WH, Bromberg JE, Stetler-Stevenson M, et al. Detection and outcome of occult leptomeningeal disease in diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica 2014;99:1228-35. [Crossref] [PubMed]
- Vitolo U, Chiappella A, Ferreri AJ, et al. First-line treatment for primary testicular diff use large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international phase II trial. J Clin Oncol 2011;29:2766-72. [Crossref] [PubMed]
- Kansara R, Villa D, Gerrie AS, et al. Site of central nervous system (CNS) relapse in patients with diffuse large B-cell lymphoma (DLBCL) by the CNS-IPI risk model. Br J Haematol 2017;179:508-10. [Crossref] [PubMed]
- Blasberg RG, Patlak C, Fenstermacher JD. Intrathecal chemotherapy: brain tissue profiles after ventriculocisternal perfusion. J Pharmacol Exp Ther 1975;195:73-83. [PubMed]
- Eyre TA, Djebbari F, Kirkwood AA, et al. Efficacy of central nervous system prophylaxis with stand-alone intrathecal chemotherapy in diffuse large B-cell lymphoma patients treated with anthracycline-based chemotherapy in the rituximab era: a systematic review. Haematologica 2020;105:1914-24. [Crossref] [PubMed]
- Abramson JS, Hellmann M, Barnes JA, et al. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer 2010;116:4283-90. [Crossref] [PubMed]
- Ferreri AJ, Bruno-Ventre M, Donadoni G, et al. Risk-tailored CNS prophylaxis in a mono-institutional series of 200 patients with diffuse large B-cell lymphoma treated in the rituximab era. Br J Haematol 2015;168:654-62. [Crossref] [PubMed]
- Kuitunen H, Kaprio E, Karihtala P, et al. Impact of central nervous system (CNS) prophylaxis on the incidence of CNS relapse in patients with high-risk diffuse large B cell/follicular grade 3B lymphoma. Ann Hematol 2020;99:1823-31. [Crossref] [PubMed]
- Wilson MR, Eyre TA, Martinez-Calle N, et al. Timing of high-dose methotrexate CNS prophylaxis in DLBCL: an analysis of toxicity and impact on R-CHOP delivery. Blood Adv 2020;4:3586-93. [Crossref] [PubMed]
- Ferreri AJ, Guerra E, Regazzi M, et al. Area under the curve of methotrexate and creatinine clearance are outcome-determining factors in primary CNS lymphomas. Br J Cancer 2004;90:353-8. [Crossref] [PubMed]
- McKay P, Wilson MR, Chaganti S, et al. British Society of Haematology. The prevention of central nervous system relapse in diffuse large B-cell lymphoma: a British Society for Haematology good practice paper. Br J Haematol 2020;190:708-14. [Crossref] [PubMed]
- Younes A, Sehn LH, Johnson PPHOENIX investigators, et al. Randomized Phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol 2019;37:1285-95. [Crossref] [PubMed]
- Vitolo U, Witzig TE, Gascoyne RD, et al. ROBUST: First report of phase III randomized study of lenalidomide/R-CHOP (R2-CHOP) vs placebo/R-CHOP in previously untreated ABC-type diffuse large B-cell lymphoma. Haematol Oncol 2019;37:36-7. [Crossref]
- Ayed AO, Chiappella A, Pederson L, et al. CNS relapse in patients with DLBCL treated with lenalidomide plus R-CHOP (R2CHOP): analysis from two phase 2 studies. Blood Cancer J 2018;8:63. [Crossref] [PubMed]
- MacKintosh FR, Colby TV, Podolsky WJ, et al. Central nervous system involvement in non-Hodgkin’s lymphoma: an analysis of 105 cases. Cancer 1982;49:586-95. [Crossref] [PubMed]
- Korfel A, Elter T, Thiel E, et al. Phase II study of central nervous system (CNS)-directed chemotherapy including high-dose chemotherapy with autologous stem cell transplantation for CNS relapse of aggressive lymphomas. Haematologica 2013;98:364-70. [Crossref] [PubMed]
- Bashir RM, Bierman PJ, Vose JM, et al. Central nervous system involvement in patients with diffuse aggressive non-Hodgkin’s lymphoma. Am J Clin Oncol 1991;14:478-82. [Crossref] [PubMed]
- Jayaraman MV, Boxerman JL. Adult brain tumours. In: Atlas SW, editor. Magnetic Resonance Imaging of the Brain and Spine. 4th ed. Philadelphia: Lippincott Williams and Wilkins, 2009:525e8.
- Quijano S, Lopez A, Manuel Sancho J, et al. Identification of leptomeningeal disease in aggressive B-cell non-Hodgkin’s lymphoma: improved sensitivity of flow cytometry. J Clin Oncol 2009;27:1462-9. [Crossref] [PubMed]
- Hegde U, Filie A, Little RF, et al. High incidence of occult leptomeningeal disease detected by flow cytometry in newly diagnosed aggressive B-cell lymphomas at risk for central nervous system involvement: the role of flow cytometry versus cytology. Blood 2005;105:496-502. [Crossref] [PubMed]
- Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol 2011;32:984-92. [Crossref] [PubMed]
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059-68. [Crossref] [PubMed]
- Akkas BE, Vural GU. The incidence of secondary central nervous system involvement in patients with non-Hodgkin's lymphoma as detected by 18F-FDG PET/CT. Nucl Med Commun 2013;34:50-6. [Crossref] [PubMed]
- Dara A, Mook BB, Doorduijn JK, et al. Efficacy of intrathecal chemotherapy in patients with central nervous system involvement of hematological malignancies: a retrospective analysis. J Neurooncol 2018;139:117-23. [Crossref] [PubMed]
- Nijland M, Jansen A, Doorduijn JK, et al. Treatment of initial parenchymal central nervous system involvement in systemic aggressive B-cell lymphoma. Leuk Lymphoma 2017;58:1-6. [Crossref] [PubMed]
- Perry C, Ben Barouch S, Goldschmidt N, et al. Characteristics, management and outcome of DLBCL patients, presenting with simultaneous systemic and CNS disease at diagnosis: A retrospective multicenter study. Am J Hematol 2019;94:992-1001. [Crossref] [PubMed]
- Damaj G, Ivanoff S, Coso D, et al. Concomitant systemic and central nervous system non-Hodgkin lymphoma: the role of consolidation in terms of high dose therapy and autologous stem cell transplantation. A 60-case retrospective study from LYSA and the LOC network. Haematologica 2015;100:1199-206. [Crossref] [PubMed]
- Wight JC, Yue M, Keane C, et al. Outcomes of synchronous systemic and central nervous system (CNS) involvement of diffuse large B-cell lymphoma are dictated by the CNS disease: a collaborative study of the Australasian Lymphoma Alliance. Br J Haematol 2019;187:174-84. [Crossref] [PubMed]
- El-Galaly TC, Cheah CY, Bendtsen MD, et al. Treatment strategies, outcomes and prognostic factors in 291 patients with secondary CNS involvement by diffuse large B-cell lymphoma. Eur J Cancer 2018;93:57-68. [Crossref] [PubMed]
- Bromberg JE, Doorduijn JK, Illerhaus G, et al. Central nervous system recurrence of systemic lymphoma in the era of stem cell transplantation--an International Primary Central Nervous System Lymphoma Study Group project. Haematologica 2013;98:808-13. [Crossref] [PubMed]
- Maziarz RT, Wang Z, Zhang MJ, et al. Autologous haematopoietic cell transplantation for non-Hodgkin lymphoma with secondary CNS involvement. Br J Haematol 2013;162:648-56. [Crossref] [PubMed]
- Doorduijn JK, van Imhoff GW, van der Holt B, et al. Treatment of secondary central nervous system lymphoma with intrathecal rituximab, high-dose methotrexate, and R-DHAP followed by autologous stem cell transplantation: results of the HOVON 80 phase 2 study. Hematol Oncol 2017;35:497-503. [Crossref] [PubMed]
- Ferreri AJ, Donadoni G, Cabras MG, et al. High Doses of Antimetabolites Followed by High-Dose Sequential Chemoimmunotherapy and Autologous Stem-Cell Transplantation in Patients With Systemic B-Cell Lymphoma and Secondary CNS Involvement: Final Results of a Multicenter Phase II Trial. J Clin Oncol 2015;33:3903-10. [Crossref] [PubMed]
- Ferreri AJM, Doorduijn JK, Re A, et al. MATRix–RICE therapy and autologous haematopoietic stem-cell transplantation in diffuse large B-cell lymphoma with secondary CNS involvement (MARIETTA): an international, single-arm, phase 2 trial. Lancet Haematol 2021;8:e110-21. [Crossref] [PubMed]
- Maciocia P, Badat M, Cheesman S, et al. Treatment of diffuse large B-cell lymphoma with secondary central nervous system involvement: encouraging efficacy using CNS-penetrating R-IDARAM chemotherapy. Br J Haematol 2016;172:545-53. [Crossref] [PubMed]
- Plotkin SR, Betensky RA, Hochberg FH, et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res 2004;10:5643-6. [Crossref] [PubMed]
- Pentsova E, Deangelis LM, Omuro A. Methotrexate re-challenge for recurrent primary central nervous system lymphoma. J Neurooncol 2014;117:161-5. [Crossref] [PubMed]
- Goldwirt L, Beccaria K, Ple A, et al. Ibrutinib brain distribution: a preclinical study. Cancer Chemother Pharmacol 2018;81:783-9. [Crossref] [PubMed]
- Grommes C, Pastore A, Palaskas N, et al. Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov 2017;7:1018-29. [Crossref] [PubMed]
- Mathews Griner LA, Guha R, Shinn P, et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci U S A 2014;111:2349-54. [Crossref] [PubMed]
- Grommes C, Tang SS, Wolfe J, et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood 2019;133:436-45. [Crossref] [PubMed]
- Dredge K, Horsfall R, Robinson SP, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res 2005;69:56-63. [Crossref] [PubMed]
- Hernandez-Ilizaliturri FJ, Reddy N, Holkova B, et al. Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clin Cancer Res 2005;11:5984-92. [Crossref] [PubMed]
- Rubenstein JL, Geng H, Fraser EJ, et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv 2018;2:1595-607. [Crossref] [PubMed]
- Vu K, Mannis G, Hwang J, et al. Low-dose lenalidomide maintenance after induction therapy in older patients with primary central nervous system lymphoma. Br J Haematol 2019;186:180-3. [Crossref] [PubMed]
- Ferreri AJM, Sassone M, Angelillo P, et al. Long-lasting efficacy and safety of lenalidomide maintenance in patients with relapsed diffuse large B-cell lymphoma who are not eligible for or failed autologous transplantation. Hematol Oncol 2020;38:257-65. [Crossref] [PubMed]
- Ambady P, Szidonya L, Firkins J, et al. Combination immunotherapy as a non-chemotherapy alternative for refractory or recurrent CNS lymphoma. Leuk Lymphoma 2019;60:515-8. [Crossref] [PubMed]
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380:45-56. [Crossref] [PubMed]
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531-44. [Crossref] [PubMed]
- Abramson SJ, Gordon L, Lia Palomba M, et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. J Clin Oncol 2018;36:7505. [Crossref]
- Abramson JS, McGree B, Noyes S, et al. Anti-CD19 CAR T Cells in CNS Diffuse Large-B-Cell Lymphoma. N Engl J Med 2017;377:783-4. [Crossref] [PubMed]
- Frigault MJ, Dietrich J, Martinez-Lage M, et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood 2019;134:860-6. [Crossref] [PubMed]
- Novo M, Ruff MW, Skrabek PJ, et al. Axicabtagene Ciloleucel Chimeric Antigen Receptor T Cell Therapy in Lymphoma With Secondary Central Nervous System Involvement. Mayo Clin Proc 2019;94:2361-4. [Crossref] [PubMed]
Cite this article as: Steffanoni S, Doorduijin JK. Narrative review: secondary central nervous system lymphoma. Ann Lymphoma 2021;5:5.


