
Novel targeted agents for follicular lymphoma
Introduction
Patients with follicular lymphoma (FL) have a diverse spectrum of treatment options in both the frontline and relapsed settings. In addition to various chemoimmunotherapy regimens, molecularly targeted therapies demonstrate an increasing role in routine management. In the frontline setting, immunomodulatory regimens such as lenalidomide and CD20 antibody (MoAb) combinations are now considered as valuable alternative to chemoimmunotherapy. In the relapsed/refractory disease setting, multiple molecularly targeted agents are approved for clinical use and more are under active investigation. The following chapter will focus on agents shown to effectively target relevant intracellular oncogenic pathways in FL but will exclude immune based therapies targeting antigens on the lymphoma surface. For each drug class, we will first review current efficacy and safety data obtained as a single-agent and combined with anti-CD20 MoAbs. A second part will address preliminary data of each class in combination with chemotherapy or other classes of agents.
B cell receptor (BCR) pathway
BCR signaling is chronically upregulated in FL and activates many proliferation and survival pathways including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), Janus kinase/signal transducer and activator of transcription proteins (JAK/STAT), phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) to promote survival. Approximately 30% of FL patients have mutations in the BCR-NF-κB signaling pathway though the functional consequences of these mutations are unknown (1-4). Somatic hypermutation of the variable region of the immunoglobulin heavy and light chains facilitate N-glycosylation of surface immunoglobulin which also activates antigen independent BCR signaling (5-7). Essential downstream components of the BCR pathway include the Bruton’s tyrosine kinase (BTK), PI3K/Akt/mTOR, Notch, and JAK/STAT pathway. Most of these signaling events convert on survival and proliferation signals propagated through the NF-κB pathway.
BTK is the immediate downstream partner of BCR activation. Despite chronic expression of BCR, subpopulations of FL are insensitive to BCR signaling and portend a poor prognosis (8). FL also harbor mutations downstream of BTK thereby bypassing dependency on BTK signal transduction. Furthermore, in contrast to activated B cell DLBCL which rely on BTK, JAK/STAT, and NF-κB signaling, germinal derived lymphomas such as FL and germinal center B cell DLBCL more commonly rely on PI3K/mTOR signaling to transmit survival signals (9).
The PI3K/Akt/mTOR pathway is a crucial downstream partner of BCR signaling regulating intrinsic cellular processes such as proliferation, growth, apoptosis, and cellular migration (10). PI3K inhibition effectively reduces AKT phosphorylation and decreases expression of pro-survival proteins (11). A constitutively active PI3K signal rescues BCR knockout B cells by re-expression of critical transcription factors such as FOXO1 (12). Alternative mechanisms of PI3K lymphomagenesis include regulation of leukocyte trafficking and development of T cell mediated immune responses (13,14). Strategies targeting multiple PI3K isoforms may be beneficial to enhance PI3K signal blockade. In a PI3Kα shRNA knockdown model, dual inhibition of PI3Kα and -δ blockade enhanced lymphoma apoptosis (15). Studies of PI3K isoform specific inhibitors similarly support PI3Kα and PI3Kδ inhibition for adequate suppression of phospho-AKT, and downstream NF-κB and PI3K pathways (15,16). Development of resistance to PI3Kδ inhibition may be reduced with concurrent PI3Kα inhibition (15).
BTK inhibitors
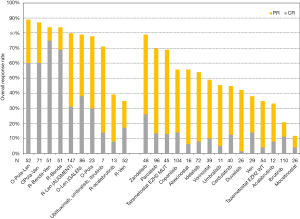
BTK inhibition has been studied in FL initially with ibrutinib and subsequently newer more specific BTK inhibitors such as acalabrutinib. When BCR and its downstream BTK signaling is constitutively activated in FL, FL seemingly is not dependent on BCR signaling for survival. These may be contributory factors to the modest clinical response achieved thus far with BTK inhibitors in three independent FL studies. Ibrutinib at 560 mg daily in 40 patients with relapsed/refractory FL showed a modest response of 37.5% with a complete response rate of 12.5% (Figure 1). The median lines of therapy was 3 and the median progression-free survival (PFS) was 14 months (37). Another phase 2 study enrolling 110 FL patients with a median 3 prior lines of therapy showed overall response of 21%, complete response of 11% and a median PFS of 4.6 months (17). Similarly, single agent acalabrutinib in relapsed/refractory FL demonstrated similar efficacy to rituximab and acalabritunib with overall response of 33% and 39%, respectively, and complete response of 8% in both groups (18). Targeting BTK may be better pursued in combination with other therapies. The results of the ROSEWOOD clinical trial comparing obinutuzumab and zanubrutinib to obinutuzumab alone will be enlightening (NCT03332017).
PI3K inhibitors
Three PI3K inhibitors are FDA approved in treatment of FL and several are in development (Figure 1). Idelalisib is an oral PI3Kδ specific inhibitor which was the first PI3K inhibitor approved in FL (19). The study treated 125 patients with indolent non-Hodgkin lymphoma (NHL) on a phase 2 study who were “double refractory” to prior therapy which was defined as patients treated with rituximab and an alkylating agent either in successive regimens or together with less than a partial response within 6 months of prior therapy. Seventy-two FL patients included 79% of patients with an intermediate or high FLIPI score. The overall response and complete response rate was 54% and 8% respectively in patients with FL. In the whole study, the median duration of response was 12.5 months and the median PFS was 11 months. The safety analysis identified diarrhea (43%), fatigue (30%), nausea (30%), cough (29%), and pyrexia (28%) occurring in >20% of patients. Notable grade 3 or higher toxicities included neutropenia (27%), transaminase elevations (13%), diarrhea (13%), and pneumonia (7%). These adverse events led to treatment discontinuation in 25 patients (20%) and dose reductions in 42 patients (34%). Guidelines for management of diarrhea/colitis, transaminitis, and pneumonitis have since positively contributed to management of treatment related adverse events (38).
Duvelisib is a second-generation orally administered dual δ/γ PI3K inhibitor that gained accelerated approval in 2018 for the treatment of relapsed/refractory FL after ≥2 prior lines of therapy. The phase 2 DYNAMO study enrolled 129 patients with indolent NHL who were double refractory to rituximab (monotherapy or in combination) and either chemotherapy or radioimmunotherapy (20). The FL population included 75% patients with an intermediate or high FLIPI score. The overall response rate 42.2% and complete response rate 1.2% in patients with FL. Across all NHL histologies, the median duration of response was 10 months and median PFS was 9.5 months. Similar to idelalisib, the adverse events include diarrhea (48.8%), nausea (29.5%), neutropenia (28.7%), fatigue (27.9%), and cough (27.1%) with the most coming grade 3 or higher adverse events being neutropenia (24.8%), diarrhea (14.7%), anemia (14.7%), and thrombocytopenia (11.6%). Grade 3 or higher laboratory elevations of lipase was 7% and ALT elevates was 5.4%. The adverse events led to discontinuation of duvelisib in 31% (39) of patients. Dose interruptions or reductions were observed in 66% of patients.
Early results of frontline activity of duvelisib in combination with an anti-CD20 antibody in FL demonstrated promising activity, with an ORR of 87–90% (40). Of 28 patients in the rituximab and duvelisib arm, 64% experienced an adverse events and 14% discontinued duvelisib from an adverse event. The most common grade 3 or higher adverse events in these patients included transaminitis (21%) and rash (14%). The obinutuzumab and duvelisib experienced higher adverse events with 70% of patients experienced grade 3 or higher events. In this group, 63% of patients experienced an adverse event leading to modification or interruption of duvelisib and 7% discontinued duveslisib from an adverse event. The most common grade 3 or higher adverse events included neutropenia (19%), ALT increase (15%), and aspartate aminotransferase increase (11%) (40).
Copanlisib is an intravenously administered pan-PI3K inhibitor with preference targeting the PI3Kα and PI3Kδ isoform. The expansion component of the phase 2 CHRONOS study enrolled patients with indolent NHL including 104 patients with FL (21). The cohort included 56% patients who were refractory rituximab and 43% patients who were refractory to rituximab and an alkylating agent. In the FL patients, the overall response was 59% with 14% patients achieving a complete response. The median duration of response was 12.2 months and the median PFS was 11.2 months. The most common treatment-emergent adverse events occurring in ≥25% of patients were transient hyperglycemia, transient hypertension, diarrhea, fatigue, decreased neutrophil count, and fever. The highest incidences of grade 3 or higher adverse events were neutropenia (24%), lung infection (15%). While pneumonitis, diarrhea and colitis were observed, they represented <5% of the events. Adverse events leading to cessation of therapy which were attributed to therapy was observed in 16% of patients. Transaminitis was observed in 23–28% of patients and mostly grade 1 (19–25%). Rituximab and copanlisib were evaluated for safety in a small phase I study of multiple NHL including 7 patients with FL. The most common AEs were neutropenia, nausea, thrombocytopenia, and hyperglycemia with no new adverse event profile observed (41). Combination of copanlisib with rituximab are ongoing in a randomized phase 3 study (NCT02367040).
Finally, there are a number of emerging PI3K inhibitors with promising activity and safety profile. Umbralisib is a dual PI3Kδ/casein kinase 1ε inhibitor which has demonstrated efficacy in FL (42). The subset of 17 patients with FL demonstrated an overall response of 53% including 12% with complete response. The most common treatment associated adverse events were diarrhea (43%), nausea (42%), and fatigue (31%). Transaminitis and colitis represented <5% of grade 3 or higher adverse events. A larger study of various NHL showed consistent response of 45%, 5% in complete response, and 10.6 months PFS in 117 FL patients (22). Of note, umbralisib has limited CYP450 inhibition and few pharmacologic interactions. Zandelisib, also known as ME-401, is an oral selective PI3kδ inhibitor undergoing early phase trials with an intermittent schedule designed to minimize long term toxicity. In a study of healthy volunteers, zandelisib demonstrated favorable pharmacokinetic and pharmacodynamic characteristics with near maximal inhibition of PI3Kδ after a single 60 mg dose. No serious adverse events were observed and only three events, one each of pain, headache, and upper abdominal pain, were attributed to zandelisib (43). Early data in 48 FL patient demonstrate a high overall response of 79% with a mild toxicity profile (23,44). Parsaclisib is a selective potent PI3Kδ inhibitor being studied in indolent lymphomas. Of 106 treated FL patients, the early activity showing overall response of 70% and complete response of 13% are promising in context of manageable adverse events (45). These new generations of PI3K inhibitors demonstrate similar efficacy to other agents in their class with improvements in toxicity profile rendering these next generation PI3K inhibitors promising for combinations with other agents in the FL armamentarium.
Areas of investigation in BCR signaling
Other components of the BCR signaling includes Notch, mTOR, and JAK/STAT. Activating mutations in Notch, JAK/STAT pathways are common events which may contribute to follicular lymphomagenesis. Notch mutations typically in the PEST domain are seen in approximately 20% of FL (2,46,47). Recurrent activating genomic alternations of the mTOR pathway leading to mTOR mediated resistance to nutrient deprivation occur in 17% of FL (48,49). Similarly, mutations in JAK/STAT pathway are observed in approximately 20% of FL and contribute to STAT6 activation and promotion of cell survival (3).
Pharmacologic targeting of BCR signaling components have varied success. Despite strong interest in Notch pathway inhibition, gamma-secretase inhibitors targeting the PEST domain of Notch have largely been unsuccessful. JAK/STAT inhibitors have varied success. Cerdulatinib is a dual SYK and JAK inhibitor studied in FL. A phase 2 study comparing single agent cerdulatinib to rituximab and cerdulatinib demonstrated similar overall response of 45% and 59%, and complete response of 12.5% and 11.7%, respectively (24). Agents targeting mTOR such as temsirolimus and everolimus have shown some degree of efficacy, but further studies are needed in larger groups of FL patients (50,51).
Epigenetic modulators
FL is characterized by epigenetic dysregulation. Approximately 80-90% of FL cases harbors genetic alterations of epigenetic regulators the most common being MLL2 (89%), CREBBP (68%), EP300 (9%), and EZH2 (12–20%) (4,39,52,53). Inactivating mutations in KMT2D, CREBBP and EP300 and gain of function mutations in EZH2 all function to suppress transcription. Both types of transcriptional defects suppress transcription, the former by favoring loss of active transcription marks by methylation and acetylation (54,55) while the latter increases the repressive trimethylation marker (56). HDAC inhibitors have been extensively studied in FL however none have been able to obtain regulatory approval. Enhancer of zeste homolog 2 (EZH2) inhibitors are the first epigenetic regulators to be FDA approved in FL.
EZH2 inhibitor
EZH2 has an essential role in germinal center development which is a key component of FL development. EZH2 regulates transcriptional repression by catalyzing the addition of methyl groups to histone H3 at lysine 27 (56,57). EZH2 and its partner proteins are required for coordinated differentiation and proliferation of embryonic stem cells. Conditional knockouts in early B cell differentiation identify EZH2 as crucial for normal immunoglobulin VDJ recombination (58). Heterozygous point mutations affecting tyrosine 641 (Y641) within the catalytic domain of EZH2 have been identified in FL and GCB‐DLBCL, with an incidence of approximately 15% to 20% in both tumor types (4,39,52,53). These series of gain of function activating mutations Y641H/N/S/F and A687 leads to increased methylation markers and suppression of gene expression (53,59). EZH2 methylation also repressed production of T helper 1 chemokines by lymphoma cells effectively regulating T-cell trafficking to the tumor microenvironment (60).
Tazemetostat selectively targets EZH2 and is the first FDA approved epigenetic modulator for relapsed/refractory FL. The phase I study of tazemetostat in relapsed lymphoma or solid tumors demonstrated reasonable safety profile (61). Based on the early safety and response profiles, tazemetostat was furthered studied in a phase II clinical trial of 156 patients with FL or DLBCL with stratification of patients into EZH2 mutation status. The FL cohorts included 45 patients with EZH2 mutations and 54 patients with EZH2 wildtype (25). Patients considered to be double refractory comprised 20% of EZH2 mutated population and 28% of EZH2 wildtype population. Patients with early progression of disease within 24 months represented 42% and 59% of EZH2 mutated and wildtype population. Patients with EZH2 mutation demonstrated a higher response rate of 69% compared to 35% in the EZH2 wildtype cohort (Figure 1). The median duration of response was 10.9 months in the EZH2 mutated cohort and 13.0 months in the EZH2 wildtype cohort. The time to response was 3.7 months in FL which is comparably slower than PI3K inhibitors. Late responses were noted to occur. The most common treated related adverse events are nausea (19%), alopecia (14%) and diarrhea (12%). Treatment-related adverse events of grade 3 or higher included thrombocytopenia (3%), neutropenia (3%), and anemia (2%). Dose adjustments were minimal as demonstrated by 9% of patients needing dose reductions and 5% of patients having treatment associated adverse events leading to drug discontinuation. Comparing to PI3K inhibitors, tazemetostat is well tolerated even with adjusting for baseline population differences across multiple studies (62).
Other EZH2 inhibitors are in development. DS‐3201b, valemetostat, is a dual EZH 1/2 inhibitor with demonstrated promising clinical activity across a range of B‐cell and T‐cell NHL subtypes (63) being studied in a phase I study. Other agents include PF-06821497 which is a potent, selective EZH2 inhibitor, being studied in both solid tumors and FL (NCT03460977).
HDAC inhibitor
Epigenetic imbalance is a hallmark of FL. Inactivating genomic alterations in histone acetyltransferases such as CREBBP and EP300 are observed in both DLBCL and FL (4,39,64). The inactivation mutations in these proteins lead to impaired BCL6 acetylation and suppression of p53. Histone deacetyltransfersase (HDAC) inhibitors potentially restore HDAC imbalance to inactivate BCL6 and normalize p53. Several HDAC inhibitors have been evaluated in lymphoma with three HDAC inhibitors, mocetinostat (26), vorinostat (27,65-67) and abexinostat (28,68,69), having FL specific clinical data (Figure 1). In small series of patients, overall response have ranged from 10–56%. Interestingly, while HDAC inhibitors may display a slower time to response, patients who respond may have prolonged duration of remissions. Abexinostat as third line therapy remains actively investigated in a phase 2 study open to accrual in China (NCT03934567) (64).
Sirtuin epigenetic modulation
Alternative epigenetic mechanisms may also contribute to lymphoma development. Sirtuins are nicotinamide adenine di-nucleotide dependent lysine deacetylases which localize to mitochondria and deacetylase metabolic proteins (70). Seven mammalian sirtuin proteins (SIRT1-7) effects lysine deacetylation, desuccinylation, and defatty-acylation to modulate the metabolic status of lymphoma cells. SIRT1 is known to be overexpressed in FL (71). SIRT3 is overexpressed in DLBCL and depletion induces acetylation of mitochondrial proteins, inhibited proliferation and induced autophagy (72). To date, modulators of sirtuins have pre-clinical data in animals but have yet to reach human studies.
BCL2 inhibitor
Translocation of t(14,18) is a characteristic of FL as an early clonal event and identified in 90% of patients (73). In this oncogenic event, BCL2 is displaced to the transcriptional control of the immunoglobulin heavy chain regulatory region (IGH) leading to constitutive expression of the anti-apoptotic protein BCL2 (74,75). However, single agent activity of the BCL2 inhibitor, venetoclax, appears limited. While mutations of BCL2 are commonly in FL, they are typically outside of the venetoclax binding domain (76). While BCL2 overexpression may be important for early development of FL, the BCL2 pathway may be less critical during later timeline of FL development when therapy is required. In a phase I study of venetoclax in multiple NHL subtypes, 29 patients of the FL cohort demonstrated overall response rates of 38%, complete responses of 14%, and median PFS 11 months (29) (Figure 1). A phase 2 study evaluated the combinations of rituximab-venetoclax, bendamustine-venetoclax, and rituximab-bendamustine in FL (30). The study enrolled similar patients including stage, refractoriness to last therapy, and disease burden by GELF. The rituximab-venetoclax arm enrolled 52 patients and observed an overall response of 35% and complete response of 17% that was sustained at 1 year. In this limited comparison, rituximab-venetoclax did not appear to have additive synergism (30).
Lenalidomide
Lenalidomide is an immunomodulator with multiple mechanisms of action. The best known anti-cancer effects of imides is through direct binding to cereblon (CRBN) (77). Direct binding of lenalidomide to cereblon leads to rapid ubiquitination and degradation of key B cell transcription factors such as Ikaros and Aiolos (78). In multiple myeloma, lenalidomide effects such as IRF4 suppression were diminished when CRBN was depleted supporting the concept that CRBN is required for anti-cancer effects of lenalidomide (79). In FL, lenalidomide has several downstream effects including reactivation of dysfunctional T and NK cells through T-helper cell type 1 cytokine release, enhanced antibody dependent cellular cytotoxicity in a cereblon dependent pathway, promoted formation of NK mediated immunologic synapses to enhance cytolytic activity, and increased CD20 phagocytosis through stimulation of macrophages and monocytes (80,81).
In relapsed FL, lenalidomide and anti-CD20 antibody demonstrates significant activity (31,82,83) (Figure 1). In a randomized, double-blind, phase III study of 358 patients, rituximab and lenalidomide demonstrated a PFS of 39.4 months compared with 14.1 months for rituximab and placebo in patients with previously treated with FL (31). The clinical response as assess by an independent review committee was 78% and 53% in the rituximab-lenalidomide and rituximab-placebo arms, respectively. The most common grade 3 or more adverse event in the rituximab-lenalidomide arm was neutropenia (50%) (31). Preliminary data from the phase 3 MAGNIFY trial (NCT01996865) examining 12 cycles of rituximab and lenalidomide followed by maintenance of rituximab and lenalidomide versus rituximab monotherapy are promising. The induction phase of rituximab and lenalidomide reported a 1-year PFS of 70%, 66% for double refractory (refractory to CD20 and alkylating therapy); and 50% for early relapse patients (84).
Combinations of obinutuzumab and lenalidomide in the refractory setting also demonstrates promising overall responses even in patients with early relapse or other high-risk features (32,85). A phase I/II study of obinutuzumab and lenalidomide given for maximum of 12 cycles in relapsed indolent NHL enrolled 57 patients with FL. At a median follow up of 17.7 months, the estimated 2-year PFS was 74% in FL (85). The GALEN trial studied obinutuzumab and lenalidomide induction therapy followed by maintenance therapy initially with obinutuzumab and lenalidomide, then obinutuzumab alone (32). Patients on the trial had two median prior therapies, 27% of patients had early relapsed within 24 months, and 23% of patients were refractory to rituximab (32). At the end of induction, the overall response rate was 79% and complete response was 38% in 86 evaluable patients. Median PFS and duration of response were not reached. The 2-year PFS and duration of response was 65% and 70%, respectively (32). The most common grade 3 or higher adverse event was neutropenia (44%) and thrombocytopenia (14%).
Lenalidomide in treatment naïve FL demonstrated high overall and complete response rates when combined with anti-CD20 antibodies (86-89). The phase 3 RELEVANCE study demonstrated that the chemotherapy free combination of rituximab and lenalidomide had comparable efficacy to standard chemotherapy (87). No substantial new toxicity profile was identified. However, long term safety data need to be further delineated in lymphoma as secondary primary malignancies have been observed with maintenance lenalidomide in multiple myeloma (90).
Combination regimen including molecularly targeted therapy and future directions in FL
The diverse spectrum of therapies in FL facilitates many combinatorial strategies. These combinations have the benefit of targeting both the tumor and microenvironment to increase efficacy but may unveil new side effect profiles.
Combination of molecularly targeted agents with immunochemotherapy
Two phase 3 studies combining idelalisib with rituximab-bendamustine in both frontline CLL and relapsed NHL identified increased opportunistic infections with Pneumocystis jirovecii pneumonia (PJP) and cytomegalovirus (CMV) reactivation which led to recommendation of PJP prophylaxis and CMV monitoring with PI3K inhibitor therapy (91). Duvelisib was also combined with rituximab-bendamustine in NHL and CLL which demonstrated adverse events in 96% of patients considered related to duvelisib the most common being neutropenia (47.7%), fatigue (41.3%), and rash (41.3%). Signals for severe adverse events were CMV reactivation, colitis, and sepsis (92). Other PI3K inhibitors such as copanlisib is currently investigated in multiple phase 3 trials in combination with immunochemotherapy (CHRONOS-4, NCT02626455).
BCL2 inhibitors may synergize with other chemotherapy to improve responses. In the CONTRALTO clinical trial in relapsed/refractory FL, complete response were similar (69–75%) with the triplet venetoclax-rituximab-bendamustine and rituximab-bendamustine (30). The venetoclax-rituximab-bendamustine arm enrolled 51 patients and observed an overall response of 84% and complete response of 75% (Figure 1). At 1 year, the complete response decreased to 43%. Similarly, the rituximab-bendamustine arm enrolled 51 patients with near identical overall response of 84% and complete response of 69%. However, addition of venetoclax to rituximab-bendamustine increased the rate of adverse events which lead to treatment discontinuation in 40% in this group compared to 4–5% in the rituximab-venetoclax and rituximab-bendamustine arms (30). The high rate of treatment discontinuation in the triple combination arm likely clouded the analysis and apparent lack of benefit to this combination.
The tolerability profile and ubiquitous dependence of FL on germinal center development identify tazemetostat as a rationale candidate for combination therapy. EZH2 inhibitors may synergize with chemotherapy as shown in solid tumors (93) A phase I study of RCHOP and tazemetostat in newly diagnosed DLBCL demonstrated the FDA approved dose of 800 mg BID oral tazemetostat was well tolerated without substantial additive toxicity (94). Grade 3 or higher adverse events observed in >10% of the patients were constipation (24%), nausea (12%), and hypokalemia (12%). The most common grade 3 to 4 hematologic adverse events were neutropenia (47%), leukopenia (29%), anemia (17%), and thrombocytopenia (12%). Tazemetostat with RCHOP is also being studied in high-risk FL patients with FLIPI scores of 3–5 in patients meeting GELF treatment criteria in the frontline setting (NCT02889523).
A series of early phase clinical studies evaluated obinutuzumab-polatuzumab combinations with either lenalidomide or venetoclax (Figure 1). The two sister studies demonstrate that addition of a targeted agent improves the complete response but with additive toxicities. In a study of polatuzumab-obinutuzumab-lenalidomide, the overall response was 89% and complete response of 60% in 52 patients (33). The combination was also associated with grade 3 or higher adverse events in 75% of patients including neutropenia (46%), thrombocytopenia (17%), anemia (12%) and infections (12%). Lenalidomide adjustments were common with over 50% needing dose interupttions and 30% needing dose reductions. The polatuzumab-obinutuzumab-venetoclax combination showed an overall response of 87% and a complete response of 60% (34). Both studies suggest synergism of the triplet and improved disease control compared to the polatuzumab-obinutuzumab combination (35), however, the combinations led to additive toxicities. Lenalidomide added to RCHOP was also studied in frontline FL with no clear indication of improved efficacy but notable grade 4 neutropenia (95). An assessment of safety and clinical benefit ratio is warranted in future development of combination therapies.
Combinations of different classes of molecularly targeted therapies
The reliance of FL on multiple oncogenic pathways rationalizes combinatorial strategies using molecularly targeted agents targeting distinct separate pathways. Our experience in these strategies have mixed results with some combination showing clear synergistic toxicity while others are under investigation. Close monitoring of these studies to analyze safety profiles and clinical efficacy are of utmost importance.
Combinations of rituximab, lenalidomide and idelalisib identified new toxicities across three clinical studies. In two Alliance clinical trials for follicular and mantle cell lymphoma, four of the first eight patients given this combination experienced dose-limiting toxicities, including grade 4 sepsis syndrome, grade 4 hypotension with grade 3 rash and fevers, grade 4 transaminase elevation with fevers, and grade 3 pulmonary infection with grade 3 maculopapular rash (96). In a separate multi-arm phase I study of combination agents with idelalisib, the combination arm of rituximab, lenalidomide, and idelalisib demonstrated grade 3 or higher transaminitis in 6 of 7 patients including two deaths, one from hepatic failure and another from respiratory failure in the setting of gram-positive bacteremia (97). A phase 2 study combining the spleen tyrosine kinase inhibitor, entospletinib, and idelalisib was prematurely ended when grade 3 or higher pneumonitis was observed in 17% of patients (98).
Synergism are predicted between BTK and PI3K inhibitors based on high throughput strategies using unbiased small molecule combination screening (99). Duvelisib was observed to synergize with dexamethasone, ibrutinib, and the BCL-2 inhibitor venetoclax in multiple lymphoma cell lines using orthogonal studies such as inhibitory growth curves and xenograph mouse models (100). The Combinations of duvelisib are being studied and warrant close monitoring for unexpected toxicities.combination of buparlisib and ibrutinib in NHL enrolled FL had reassuring lack of new toxicities (101). A triple combination of umbralisib, ibrutinib and an anti-CD20 antibody, ublituximab, also had 7 evaluable FL patients, 5 (71%) responded with one complete response (36). This combination of PI3K, BTK inhibitors with CD20 MoAb also reveal no unexpected adverse events with infusion reactions being one of most common reasons for treatment interruptions (36).
Tazemetostat combinations are promising based on the favorable safety profile of tazemetostat. Tazemetostat in combination with lenalidomide demonstrated synergistic effects in a high throughout in vitro combination platform testing antiproliferative activity. This has led to the ongoing phase I/III clinical study combining tazemetostat with rituximab and lenalidomide (NCT04224493). The trial will enroll in up to three phases, an initial safety run-in followed by a cohort of EZH2 unselected FL patients, and possibly a third cohort of EZH2 mutated FL. A phase Ib/III study of rituximab, lenalidomide and tazemetostat is underway and plans to enroll >500 patients to evaluate this combination in the second line setting (NCT04224493). Other preclinical data support synergism between tazemetostat and the BCL2-inhibitor, venetoclax, based on cell line models and patient derived xenografts demonstrating tazemetostat can upregulate Bcl-2 family members and prime mitochondria mediated apoptosis (102) paving the rationale for testing this combination in the clinic.
Finally, new modalities of immunotherapies with bispecific antibodies, CAR T cells were shown to be highly promising in FL based on early response data. Combination of molecularly targeted drugs with immunotherapies are warranted and work is progress to identify rationale combination and maintenance strategies with immunotherapies to optimize duration of response and PFS in FL. One such study is the highly anticipated phase Ib study of bispecific antibody cominbations, mosenutuzumab-lenalidomide, glofitamab-lenalidomide, and glofitamab-obinutuzumab-lenalidomide, in relapsed/refractory FL in second line or later treatment (NCT04246086).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mark Roschewski, Carla Casulo) for the series “Follicular Lymphoma” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-45). The series “Follicular Lymphoma” was commissioned by the editorial office without any funding or sponsorship. Dr. CLB reports non-financial support from Janssen, non-financial support from Novartis, personal fees and non-financial support from Epizyme, non-financial support from Bayer, non-financial support from Autolus, non-financial support from Roche, personal fees from BMS/Celgene/Juno, personal fees from Kite/Gilead, personal fees from Karyopharm, personal fees from TG Therapeutics, outside the submitted work and Stock ownership in Pfizer, BMS, Regeneron, Viatris with less than $10,000 USD value. Dr. FM reports personal fees from Roche/Genetech, personal fees from Celgene/BMS, personal fees from Abbvie, personal fees from Gilead, personal fees from Epizyme, personal fees from Genmab, outside the submitted work.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lamason RL, McCully RR, Lew SM, et al. Oncogenic CARD11 mutations induce hyperactive signaling by disrupting autoinhibition by the PKC-responsive inhibitory domain. Biochemistry 2010;49:8240-50. [Crossref] [PubMed]
- Krysiak K, Gomez F, White BS, et al. Recurrent somatic mutations affecting B-cell receptor signaling pathway genes in follicular lymphoma. Blood 2017;129:473-83. [Crossref] [PubMed]
- Okosun J, Bodor C, Wang J, et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet 2014;46:176-81. [Crossref] [PubMed]
- Morin RD, Mendez-Lago M, Mungall AJ, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 2011;476:298-303. [Crossref] [PubMed]
- Radcliffe CM, Arnold JN, Suter DM, et al. Human follicular lymphoma cells contain oligomannose glycans in the antigen-binding site of the B-cell receptor. J Biol Chem 2007;282:7405-15. [Crossref] [PubMed]
- McCann KJ, Ottensmeier CH, Callard A, et al. Remarkable selective glycosylation of the immunoglobulin variable region in follicular lymphoma. Mol Immunol 2008;45:1567-72. [Crossref] [PubMed]
- Zhu D, McCarthy H, Ottensmeier CH, et al. Acquisition of potential N-glycosylation sites in the immunoglobulin variable region by somatic mutation is a distinctive feature of follicular lymphoma. Blood 2002;99:2562-8. [Crossref] [PubMed]
- Irish JM, Myklebust JH, Alizadeh AA, et al. B-cell signaling networks reveal a negative prognostic human lymphoma cell subset that emerges during tumor progression. Proc Natl Acad Sci U S A 2010;107:12747-54. [Crossref] [PubMed]
- Phelan JD, Young RM, Webster DE, et al. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature 2018;560:387-91. [Crossref] [PubMed]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2002;2:489-501. [Crossref] [PubMed]
- Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 2011;117:591-4. [Crossref] [PubMed]
- Srinivasan L, Sasaki Y, Calado DP, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell 2009;139:573-86. [Crossref] [PubMed]
- Del Prete A, Vermi W, Dander E, et al. Defective dendritic cell migration and activation of adaptive immunity in PI3Kgamma-deficient mice. EMBO J 2004;23:3505-15. [Crossref] [PubMed]
- Hirsch E, Katanaev VL, Garlanda C, et al. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 2000;287:1049-53. [Crossref] [PubMed]
- Erdmann T, Klener P, Lynch JT, et al. Sensitivity to PI3K and AKT inhibitors is mediated by divergent molecular mechanisms in subtypes of DLBCL. Blood 2017;130:310-22. [Crossref] [PubMed]
- Paul J, Soujon M, Wengner AM, et al. Simultaneous inhibition of PI3Kdelta and PI3Kalpha induces ABC-DLBCL regression by blocking BCR-dependent and -independent activation of NF-kappaB and AKT. Cancer Cell 2017;31:64-78. [Crossref] [PubMed]
- Gopal AK, Schuster SJ, Fowler NH, et al. Ibrutinib as treatment for patients with relapsed/refractory follicular lymphoma: results from the open-label, multicenter, phase II DAWN study. J Clin Oncol 2018;36:2405-12. [Crossref] [PubMed]
- Fowler NH, Coleman M, Stevens DA, et al. Acalabrutinib alone or in combination with rituximab (R) in follicular lymphoma (FL). J Clin Oncol 2018;36:abstr 7549.
- Gopal AK, Kahl BS, de Vos S, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 2014;370:1008-18. [Crossref] [PubMed]
- Flinn IW, Miller CB, Ardeshna KM, et al. DYNAMO: a phase II study of duvelisib (IPI-145) in patients with refractory indolent non-Hodgkin lymphoma. J Clin Oncol 2019;37:912-22. [Crossref] [PubMed]
- Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol 2017;35:3898-905. [Crossref] [PubMed]
- Zinzani PL, Samaniego F, Jurczak W, et al. Umbralisib, the once daily dual inhibitor of PI3Kδ and casein kinase-1ε demonstrates clinical activity in patients with relapsed or refractory indolent non-Hodgkin lymphoma: results from the phase 2 global unity-NHL trial. Blood 2020;136:abstr 34-5.
- Zelenetz AD, Reddy N, Jagadeesh D, et al. Tolerability and durable respones of the PI3Kδ inhibitor ME-401 administered on an intermittent schedule in relapsed/refractory (R/R) follicular lymphoma (FL) and other B-cell malignancies. J Clin Oncol 2020;38:abstr 8016.
- Smith SD, Munoz J, Stevens D, et al. Rapid and durable responses with the SYK/JAK inhibitor cerdulatinib in a phase 2 study in relapsed/refractory follicular lymphoma-alone or in combination with rituximab. Blood 2019;134:abstr 3981.
- Morschhauser F, Tilly H, Chaidos A, et al. Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol 2020;21:1433-42. [Crossref] [PubMed]
- Batlevi CL, Crump M, Andreadis C, et al. A phase 2 study of mocetinostat, a histone deacetylase inhibitor, in relapsed or refractory lymphoma. Br J Haematol 2017;178:434-41. [Crossref] [PubMed]
- Ogura M, Ando K, Suzuki T, et al. A multicentre phase II study of vorinostat in patients with relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Br J Haematol 2014;165:768-76. [Crossref] [PubMed]
- Ribrag V, Kim WS, Bouabdallah R, et al. Safety and efficacy of abexinostat, a pan-histone deacetylase inhibitor, in non-Hodgkin lymphoma and chronic lymphocytic leukemia: results of a phase II study. Haematologica 2017;102:903-9. [Crossref] [PubMed]
- Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol 2017;35:826-33. [Crossref] [PubMed]
- Zinzani PL, Flinn IW, Yuen SLS, et al. Venetoclax-rituximab with or without bendamustine vs bendamustine-rituximab in relapsed/refractory follicular lymphoma. Blood 2020;136:2628-37. [PubMed]
- Leonard JP, Trneny M, Izutsu K, et al. AUGMENT: a phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol 2019;37:1188-99. [Crossref] [PubMed]
- Morschhauser F, Le Gouill S, Feugier P, et al. Obinutuzumab combined with lenalidomide for relapsed or refractory follicular B-cell lymphoma (GALEN): a multicentre, single-arm, phase 2 study. Lancet Haematol 2019;6:e429-37. [Crossref] [PubMed]
- Diefenbach C, Kahl B, Banerjee L, et al. Polatuzumab vedotin (pola) + obinutuzumab (G) + lenalidomide (LEN) in patients (PTS) with relapsed/refractory (R/R) follicular lymphoma (FL): phase Ib/II interim analysis. Hematol Oncol 2019;37:175-6. [Crossref]
- Yuen S, Arthur C, Phillips T, et al. Polatuzumab vedotin (POLA) + obinutuzumab (G) + venetoclax (VEN) in patients (PTS) with relapsed/refractory (R/R) follicular lymphoma (FL): interim analysis of a phase Ib/II trial. HemaSphere 2020;4:1-1168.
- Phillips T, Brunvand M, Chen A, et al. Polatuzumab vedotin combined with obinutuzumab for patients with relapsed or refractory non-Hodgkin lymphoma: preliminary safety and clinical activity of a phase Ib/II study. Blood 2016;128:abstr 622.
- Nastoupil LJ, Lunning MA, Vose JM, et al. Tolerability and activity of ublituximab, umbralisib, and ibrutinib in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: a phase 1 dose escalation and expansion trial. Lancet Haematol 2019;6:e100-9. [Crossref] [PubMed]
- Bartlett NL, Costello BA, LaPlant BR, et al. Single-agent ibrutinib in relapsed or refractory follicular lymphoma: a phase 2 consortium trial. Blood 2018;131:182-90. [Crossref] [PubMed]
- Coutré SE, Barrientos JC, Brown JR, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma 2015;56:2779-86. [Crossref] [PubMed]
- Pasqualucci L, Dominguez-Sola D, Chiarenza A, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature 2011;471:189-95. [Crossref] [PubMed]
- Casulo C, Sancho J-M, Van Eygen K, et al. Contempo: preliminary results in first-line treatment of follicular lymphoma with the oral dual PI3K-δ,γ inhibitor, duvelisib, in combination with rituximab or obinutuzumab. Blood 2016;128:abstr 2979.
- Gerecitano J, Santoro A, Leppä S, et al. Safety run-in of copanlisib in combination with rituximab plus bendamustine in patients with relapsed indolent non-Hodgkin’s lymphoma. Hematol Oncol 2017;35:408-10. [Crossref]
- Burris HA 3rd, Flinn IW, Patel MR, et al. Umbralisib, a novel PI3Kdelta and casein kinase-1epsilon inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol 2018;19:486-96. [Crossref] [PubMed]
- Moreno O, Butler T, Zann V, et al. Safety, Pharmacokinetics, and pharmacodynamics of ME-401, an oral, potent, and selective inhibitor of phosphatidylinositol 3-kinase P110delta, Following single ascending dose administration to healthy volunteers. Clin Ther 2018;40:1855-67. [Crossref] [PubMed]
- Zelenetz AD, Jagadeesh D, Reddy NM, et al. Results of the PI3Kδ inhibitor ME-401 alone or with rituximab in relapsed/refractory (R/R) follicular lymphoma (FL). J Clin Oncol 2019;37:abstr 7512.
- Lynch RC, Paneesha S, Avigdor A, et al. Phase 2 study evaluating the efficacy and safety of parsaclisib in patients with relapsed or refractory follicular lymphoma (CITADEL-203). In: 623. Mantle Cell, Follicular, and Other Indolent B-Cell Lymphoma—Clinical Studies: Poster III, 2020:abstr 2935.
- Karube K, Martinez D, Royo C, et al. Recurrent mutations of NOTCH genes in follicular lymphoma identify a distinctive subset of tumours. J Pathol 2014;234:423-30. [Crossref] [PubMed]
- Bouska A, Zhang W, Gong Q, et al. Combined copy number and mutation analysis identifies oncogenic pathways associated with transformation of follicular lymphoma. Leukemia 2017;31:83-91. [Crossref] [PubMed]
- Ying ZX, Jin M, Peterson LF, et al. Recurrent mutations in the MTOR regulator RRAGC in follicular lymphoma. Clin Cancer Res 2016;22:5383-93. [Crossref] [PubMed]
- Okosun J, Wolfson RL, Wang J, et al. Recurrent mTORC1-activating RRAGC mutations in follicular lymphoma. Nat Genet 2016;48:183-8. [Crossref] [PubMed]
- Witzig TE, Reeder CB, LaPlant BR, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia 2011;25:341-7. [Crossref] [PubMed]
- Smith SM, van Besien K, Karrison T, et al. Temsirolimus has activity in non-mantle cell non-Hodgkin's lymphoma subtypes: The University of Chicago phase II consortium. J Clin Oncol 2010;28:4740-6. [Crossref] [PubMed]
- Green MR, Kihira S, Liu CL, et al. Mutations in early follicular lymphoma progenitors are associated with suppressed antigen presentation. Proc Natl Acad Sci U S A 2015;112:E1116-25. [Crossref] [PubMed]
- Morin RD, Johnson NA, Severson TM, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet 2010;42:181-5. [Crossref] [PubMed]
- Zhang J, Dominguez-Sola D, Hussein S, et al. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat Med 2015;21:1190-8. [Crossref] [PubMed]
- Zhang J, Vlasevska S, Wells VA, et al. The CREBBP acetyltransferase is a haploinsufficient tumor suppressor in B-cell lymphoma. Cancer Discov 2017;7:322-37. [Crossref] [PubMed]
- Béguelin W, Popovic R, Teater M, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 2013;23:677-92. [Crossref] [PubMed]
- Cao R, Wang L, Wang H, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 2002;298:1039-43. [Crossref] [PubMed]
- Su IH, Basavaraj A, Krutchinsky AN, et al. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat Immunol 2003;4:124-31. [Crossref] [PubMed]
- Ott HM, Graves AP, Pappalardi MB, et al. A687V EZH2 is a driver of histone H3 lysine 27 (H3K27) hypertrimethylation. Mol Cancer Ther 2014;13:3062-73. [Crossref] [PubMed]
- Peng D, Kryczek I, Nagarsheth N, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015;527:249-53. [Crossref] [PubMed]
- Italiano A, Soria JC, Toulmonde M, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol 2018;19:649-59. [Crossref] [PubMed]
- Proudman D, Nellesen D, Gupta D, et al. Tazemetostat is associated with lower risk for safety outcomes versus the PI3-kinases idelalisib, duvelisib and copanlisib, in patients with relapsed/refractory follicular lymphoma who have received at least 2 prior systemic treatments: a matching-adjusted indirect comparison of single-arm trials. Blood 2020;136:16. [Crossref]
- Morishima S, Ishitsuka K, Izutsu K, et al. First-in-human study of the EZH1/2 dual inhibitor valemetostat in relapsed or refractory non-Hodgkin lymphoma (NHL) - updated results focusing on adult T-cell leukemia-lymphoma (ATL). Blood 2019;134:abstr 4025.
- Jiang Y, Ortega-Molina A, Geng H, et al. CREBBP inactivation promotes the development of HDAC3-dependent lymphomas. Cancer Discov 2017;7:38-53. [Crossref] [PubMed]
- Watanabe T, Kato H, Kobayashi Y, et al. Potential efficacy of the oral histone deacetylase inhibitor vorinostat in a phase I trial in follicular and mantle cell lymphoma. Cancer Sci 2010;101:196-200. [Crossref] [PubMed]
- Chen R, Frankel P, Popplewell L, et al. A phase II study of vorinostat and rituximab for treatment of newly diagnosed and relapsed/refractory indolent non-Hodgkin lymphoma. Haematologica 2015;100:357-62. [Crossref] [PubMed]
- Kirschbaum M, Frankel P, Popplewell L, et al. Phase II study of vorinostat for treatment of relapsed or refractory indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol 2011;29:1198-203. [Crossref] [PubMed]
- Morschhauser F, Terriou L, Coiffier B, et al. Phase 1 study of the oral histone deacetylase inhibitor abexinostat in patients with Hodgkin lymphoma, non-Hodgkin lymphoma, or chronic lymphocytic leukaemia. Invest New Drugs 2015;33:423-31. [Crossref] [PubMed]
- Evens AM, Balasubramanian S, Vose JM, et al. A phase I/II multicenter, open-label study of the oral histone deacetylase inhibitor abexinostat in relapsed/refractory lymphoma. Clin Cancer Res 2016;22:1059-66. [Crossref] [PubMed]
- Chalkiadaki A, Guarente L. The multifaceted functions of sirtuins in cancer. Nat Rev Cancer 2015;15:608-24. [Crossref] [PubMed]
- Frazzi R, Zanetti E, Pistoni M, et al. Methylation changes of SIRT1, KLF4, DAPK1 and SPG20 in B-lymphocytes derived from follicular and diffuse large B-cell lymphoma. Leuk Res 2017;57:89-96. [Crossref] [PubMed]
- Li M, Chiang YL, Lyssiotis CA, et al. Non-oncogene addiction to SIRT3 plays a critical role in lymphomagenesis. Cancer Cell 2019;35:916-31 e9.
- Yunis JJ, Oken MM, Kaplan ME, et al. Distinctive chromosomal abnormalities in histologic subtypes of non-Hodgkin's lymphoma. N Engl J Med 1982;307:1231-6. [Crossref] [PubMed]
- Tsujimoto Y, Cossman J, Jaffe E, et al. Involvement of the bcl-2 gene in human follicular lymphoma. Science 1985;228:1440-3. [Crossref] [PubMed]
- Tsujimoto Y, Gorham J, Cossman J, et al. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science 1985;229:1390-3. [Crossref] [PubMed]
- Huet S, Szafer-Glusman E, Tesson B, et al. BCL2 mutations do not confer adverse prognosis in follicular lymphoma patients treated with rituximab. Am J Hematol 2017;92:515-9. [Crossref] [PubMed]
- Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science 2010;327:1345-50. [Crossref] [PubMed]
- Gribben JG, Fowler N, Morschhauser F. Mechanisms of action of lenalidomide in B-cell non-Hodgkin lymphoma. J Clin Oncol 2015;33:2803-11. [Crossref] [PubMed]
- Zhu YX, Braggio E, Shi CX, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood 2011;118:4771-9. [Crossref] [PubMed]
- Chiu H, Trisal P, Bjorklund C, et al. Combination lenalidomide-rituximab immunotherapy activates anti-tumour immunity and induces tumour cell death by complementary mechanisms of action in follicular lymphoma. Br J Haematol 2019;185:240-53. [Crossref] [PubMed]
- Menard C, Dulong J, Nguyen TT, et al. Lenalidomide treatment restores in vivo T cell activity in relapsed/refractory FL and DLBCL. Blood 2017;130:abstr 729.
- Morschhauser F, Salles G, Le Gouill S, et al. An open-label phase 1b study of obinutuzumab plus lenalidomide in relapsed/refractory follicular B-cell lymphoma. Blood 2018;132:1486-94. [Crossref] [PubMed]
- Leonard JP, Jung SH, Johnson J, et al. Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (alliance). J Clin Oncol 2015;33:3635-40. [Crossref] [PubMed]
- Andorsky DJ, Yacoub A, Melear JM, et al. Phase IIIb randomized study of lenalidomide plus rituximab (R2) followed by maintenance in relapsed/refractory NHL: Analysis of patients with double-refractory or early relapsed follicular lymphoma (FL). J Clin Oncol 2017;35:abstr 7502.
- Fowler NH, Nastoupil LJ, Chin C, et al. A phase I/II study of lenalidomide plus obinutuzumab in relapsed indolent lymphoma. Blood 2019;134:abstr 348.
- Nastoupil LJ, Westin JR, Hagemeister FB, et al. Results of a phase II study of obinutuzumab in combination with lenalidomide in previously untreated, high tumor burden follicular lymphoma (FL). Blood 2019;134:abstr 125.
- Morschhauser F, Fowler NH, Feugier P, et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med 2018;379:934-47. [Crossref] [PubMed]
- Martin P, Jung SH, Pitcher B, et al. A phase II trial of lenalidomide plus rituximab in previously untreated follicular non-Hodgkin's lymphoma (NHL): CALGB 50803 (Alliance). Ann Oncol 2017;28:2806-12. [Crossref] [PubMed]
- Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol 2014;15:1311-8. [Crossref] [PubMed]
- Wang Y, Yang F, Shen Y, et al. Maintenance therapy with immunomodulatory drugs in multiple myeloma: a meta-analysis and systematic review. J Natl Cancer Inst 2015;108:djv342 [PubMed]
- Zelenetz AD, Barrientos JC, Brown JR, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2017;18:297-311. [Crossref] [PubMed]
- Flinn IW, Cherry MA, Maris MB, et al. Combination trial of duvelisib (IPI-145) with rituximab or bendamustine/rituximab in patients with non-Hodgkin lymphoma or chronic lymphocytic leukemia. Am J Hematol 2019;94:1325-34. [Crossref] [PubMed]
- Rugo HS, Jacobs I, Sharma S, et al. The promise for histone methyltransferase inhibitors for epigenetic therapy in clinical oncology: a narrative review. Adv Ther 2020;37:3059-82. [Crossref] [PubMed]
- Sarkozy C, Morschhauser F, Dubois S, et al. A LYSA phase Ib study of tazemetostat (EPZ-6438) plus R-CHOP in patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL) with poor prognosis features. Clin Cancer Res 2020;26:3145-53. [Crossref] [PubMed]
- Tilly H, Morschhauser F, Casasnovas O, et al. Lenalidomide in combination with R-CHOP (R2-CHOP) as first-line treatment of patients with high tumour burden follicular lymphoma: a single-arm, open-label, phase 2 study. Lancet Haematol 2018;5:e403-10. [Crossref] [PubMed]
- Smith SM, Pitcher BN, Jung SH, et al. Safety and tolerability of idelalisib, lenalidomide, and rituximab in relapsed and refractory lymphoma: the Alliance for Clinical Trials in Oncology A051201 and A051202 phase 1 trials. Lancet Haematol 2017;4:e176-82. [Crossref] [PubMed]
- Cheah CY, Nastoupil LJ, Neelapu SS, et al. Lenalidomide, idelalisib, and rituximab are unacceptably toxic in patients with relapsed/refractory indolent lymphoma. Blood 2015;125:3357-9. [Crossref] [PubMed]
- Barr PM, Saylors GB, Spurgeon SE, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood 2016;127:2411-5. [Crossref] [PubMed]
- Mathews Griner LA, Guha R, Shinn P, et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci U S A 2014;111:2349-54. [Crossref] [PubMed]
- Faia K, White K, Murphy E, et al. The phosphoinositide-3 kinase (PI3K)-delta,gamma inhibitor, duvelisib shows preclinical synergy with multiple targeted therapies in hematologic malignancies. PLoS One 2018;13:e0200725 [Crossref] [PubMed]
- Batlevi C, Hamlin P, Matasar M, et al. Phase I/Ib dose escalation and expansion of ibrutinib and buparlisib in relapsed/refractory diffuse large b-cell lymphoma, mantle cell lymphoma, and follicular lymphoma. Hematol Oncol 2017;35:abstr 54.
- Scholze H, Stephenson RE, Reynolds R, et al. Combined EZH2 and Bcl-2 inhibitors as precision therapy for genetically defined DLBCL subtypes. Blood Adv 2020;4:5226-31. [Crossref] [PubMed]
Cite this article as: Batlevi CL, Morschhauser F. Novel targeted agents for follicular lymphoma. Ann Lymphoma 2021;5:3.


