
Effectiveness and safety of pembrolizumab as bridging to hematopoietic stem cell transplantation in relapsed and refractory classical Hodgkin’s lymphoma: a retrospective observational study
Introduction
Classical Hodgkin’s lymphoma (cHL) is a lymphoproliferative malignancy that originates from B-cells; it represents around 10% of all lymphomas (1). cHL affects mainly young adults between the age of 15 and 34 years and older adults as well who age around 60 years (2).
Most patients diagnosed with cHL are cured using standard first-line chemotherapy (2). Nevertheless, approximately 30% of patients experience primary refractory or relapsed disease with a 5-year overall survival (OS) rate around 53% (3). Salvage chemotherapy followed by high dose chemotherapy (HDC) and autologous stem cell transplant (ASCT) represents a curative approach for patients with chemo-sensitive relapsed and refractory (RRcHL) which results in a 5-year progression-free survival (PFS) rate of around 50% (2). However, up to 30% of RRcHL patients are chemo-resistant to standard salvage chemotherapies thus, they are not eligible to HDC/ASCT (2,4,5). Additionally, nearly 50% of RRcHL patients experience relapse eventually after HDC/ASCT (2,4). Therefore, newer agents with a novel mechanism of action as programmed cell death protein-1 (PD-1) inhibitors are developed to improve clinical outcomes for these patients (3).
PD-1 is an immune checkpoint receptor expressed on activated T cells and lymphoma B-cells (6). Programmed death ligand 1 (PD-L1) and PD-L2 are endogenous ligands identified for PD-1 receptors. Interactions between PD-1 receptor and PD-1 ligands on Reed-Sternberg cells; the hallmark malignant B-cells in cHL; promotes cancer development and progression by enhancing tumor cell survival (2,7). Therefore, blocking the PD-1 pathway represents an attractive target to terminate the growth of malignant cells in cHL through inhibiting the negative immune regulation caused by PD-1 receptor signaling.
The checkpoint inhibitor pembrolizumab is approved by the US Food and Drug Administration (FDA) for the treatment of adult and pediatric patients diagnosed with refractory cHL or who have relapsed after 3 or more previous lines of therapy until disease progression, experienced unacceptable toxicity or for up to 24 months whichever comes first (8).
The anti-tumor activity of pembrolizumab has been demonstrated in a single-arm phase II trial, KEYNOTE-087, which included 210 patients with RRcHL following progression after either (I) ASCT and subsequent brentuximab vedotin (BV), (II) salvage chemotherapy and BV, or (III) ASCT without subsequent BV. Despite the important information published in the KEYNOTE-087 trial regarding the overall response rate and safety associated with the use of pembrolizumab in the three different cohorts mentioned earlier, KEYNOTE-087 did not address the role of pembrolizumab in ASCT and BV naïve patients as this subgroup represents an important cohort of patients especially in countries where BV in not registered. Additionally, only few patients among responders in KEYNOTE-087 (7%) bridged to hematopoietic stem cell transplant (HSCT) (allogeneic: 10, autologous: 4) with limited follow-up data (8).
According to our knowledge, clinical studies that investigated the effect of pembrolizumab as bridging to ASCT in RRcHL patients is scarce, only one multicenter retrospective study conducted by Merryman et al. evaluated the outcome of ASCT after anti-PD-1 based salvage therapy (9). In our center, HDC/ASCT or allogeneic SCT (Allo-SCT) were used to consolidate the response to pembrolizumab in previously chemo-resistant patients. Therefore, we conducted this retrospective analysis to evaluate the effectiveness and safety outcomes of pembrolizumab as a bridge to HSCT in RRcHL patients.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/aol-20-21).
Methods
A retrospective observational cohort study was conducted at King Hussein Cancer Center; a comprehensive cancer center in Amman-Jordan. Adult patients (age ≥18 years old) diagnosed with chemo-resistant RRcHL who were treated with pembrolizumab with the aim of bridging to curative HSCT between January 2016 and December 2018 were included. This study was reviewed and approved by the institutional review board. A waiver of informed consent was obtained as this is a retrospective chart review.
The electronic medical charts of included patients were reviewed by study investigators to collect patients’ characteristics and disease’s baseline variables using a standardized data collection form. Patients’ age, gender, initial diagnosis date, number and type of previous treatments, pre-pembrolizumab disease characteristics; as disease stage, burden, site of involvement if any, and graft-versus-host disease (GVHD) prophylaxis for patients who bridged to Allo-SCT were collected.
Primary and secondary endpoints
Primary endpoints were effectiveness outcomes expressed as treatment response rate, HSCT rate; defined as the proportion of patients, out of the entire cohort, who bridged successfully to ASCT or Allo-SCT, 1-year PFS rate; and 1-year OS rate. Secondary endpoints were relapse rate post-HSCT, the incidence and severity of treatment-related adverse events (TRAEs) during pembrolizumab treatment in addition to the incidence and severity of GVHD as a post-HSCT complication.
Effectiveness and safety assessment
Response to pembrolizumab was assessed by the participating oncologist using end-of-treatment computed tomography (CT) or positron emission tomography-CT (PET-CT) scan results according to Lyric and Cheson revised response criteria in malignant lymphoma (10). Relapse post-HSCT was assessed using follow-up CT or PET-CT scan results according to the same criteria.
The safety of pembrolizumab was determined by reviewing the internal pharmacy adverse drug event documentation system and the electronic medical notes following the initiation of treatment. The severity of the reported TRAEs was assessed using the Hartwig severity assessment scale (11) and validated by the participating oncologist and the clinical pharmacist specialist.
For patients who bridged to Allo-SCT after pembrolizumab, the incidence of acute and chronic GVHD was determined by the participating oncologist based on related biopsies’ specimens or laboratory tests. Glucksberg score was used for grading acute GVHD (12) and the global scoring consensus criteria of the US National Institutes of Health was used to grade chronic GVHD (13).
Statistical analysis
The 1-year PFS and OS were estimated using Kaplan-Meier product-limit estimates. A descriptive statistical analysis was used to report treatment response rate, HSCT rate, relapse rate, patients and disease baseline characteristics, in addition to incidence of both TRAEs and acute/chronic GVHD. The analysis was conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
Patients’ characteristics
A total of 32 patients were included. Patients received pembrolizumab as 200 mg intravenously every 3 weeks. The median number of doses given was 5 (range, 1–8) and the median follow-up duration was 11.6 months (range, 6.7–27.4 months).
The baseline characteristics of the included patients are shown in Table 1. Among the entire cohort the median age was 29.5 years (range, 18.0–53.0 years), 19 patients (59%) were male. Before starting pembrolizumab, 27 patients (84.3%) had advanced-stage disease (stage III or IV), 2 (6%) had bulky disease. Patients were heavily pre-treated with a median of 4 (range, 1–10) lines of chemotherapies received prior pembrolizumab initiation.
Table 1
| Characteristic | Value |
|---|---|
| Nationality, n (%) | |
| Jordanian | 15 (46.9) |
| Non-Jordanian | 17 (53.1) |
| Gender, n [%] | |
| Female | 13 [41] |
| Male | 19 [59] |
| Age (years), median (range) | 29.5 (18.0–53.0) |
| Smoking status, n (%) | |
| Active | 6 (18.8) |
| Ex-smoker | 5 (15.6) |
| Non-smoker | 21 (65.6) |
| Comorbid conditions, n (%) | |
| No | 21 (65.6) |
| Yes | 11 (34.4) |
| Hodgkin’s lymphoma stage, n (%) | |
| IIA | 5 (15.6) |
| IIIA | 5 (15.6) |
| IVA | 16 (50.0) |
| IVB | 6 (18.8) |
| Disease involvement, n (%) | |
| No | 9 (28.1) |
| Yes | 23 (71.9) |
| Disease burden, n [%] | |
| Bulky | 2 [6] |
| Non-bulky | 30 [94] |
| Radiotherapy, n (%) | |
| No | 15 (46.9) |
| Yes | 17 (53.1) |
| Previous bone-marrow transplant, n [%] | |
| No | 22 [69] |
| Yes | 10 [31] |
Patients failed a median of 3 salvage chemotherapies indicating chemo-resistant disease. None of the included patients had previously been exposed to any PD-1 inhibitors or BV.
In addition, ten patients (31%) had formerly failed HSCT (ASCT: 9, Allo-SCT: 1), and were eligible for a second HSCT if they achieved a sufficient response post pembrolizumab.
Figure 1 summarize the study profile.
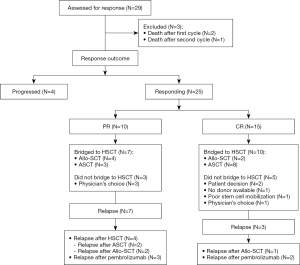
Effectiveness outcomes
Response rate
The response was assessable in 29 patients (91%), as two patients died after the first cycle (N=2) due to disease or pembrolizumab related respiratory failure and one died after the second cycle (N=1) due to disease related respiratory failure. Twenty-five patients (78%) responded to pembrolizumab, of the entire cohort, 15 (47%) achieved complete response (CR) and 10 (31%) achieved partial response (PR) (Table 2).
Table 2
| Response | N (%) |
|---|---|
| Overall response | 25 [78] |
| Type of response | |
| Complete | 15 (46.8) |
| Partial | 10 (31.3) |
| Progressive disease | 4 (12.5) |
| Not assessable | 3 (9.4) |
HSCT rate
Among the entire cohort, 17 patients (53%) bridged to HSCT after a median of 4 pembrolizumab cycles (range, 2–8). Eleven patients bridged successfully to ASCT and six to Allo-SCT. Out of the 15 patients who achieved CR, only 8 patients (53%) bridged to ASCT and 2 patients (13%) bridged to Allo-SCT, whereas, out of the 10 patients who experienced PR, 3 (30%) bridged to ASCT and 4 (40%) bridged to Allo-SCT. The median duration from the last cycle of pembrolizumab to HSCT was 1.4 months (range, 0.6–4.6 months).
Five patients out of the 17 (29%) who had bridged to Allo-SCT post pembrolizumab had previously received and failed ASCT.
On the other hand, eight responders (32%) did not bridge to HSCT due to poor stem cell mobilization, lack of a donor, or patient’s/physician’s decision. It is worth mentioning that 5 patients of those who did not bridged to HSCT, had achieved CR and 3 had PR after pembrolizumab.
PFS and OS for HSCT patients
Among patients who bridged to HSCT, the 1-year PFS and OS were 85% and 91%, respectively.
PFS and OS for ASCT patients
Eleven patients (34%) underwent ASCT after a median of 1.4 months (range, 0.6–4.3 months) from the last cycle of pembrolizumab with a 1-year PFS of 83% and a 1-year OS of 100% (Figure 2).
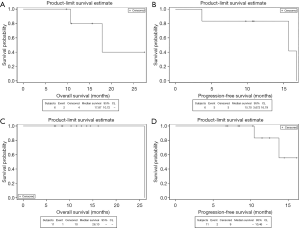
Carmustine-Etoposide-Cytarabine-Melphalan (BEAM) was the most common conditioning regimen used in ASCT (82%).
PFS and OS for Allo-SCT patients
Six patients (19%) underwent Allo-SCT after a median of 1.8 months (range, 1.4–4.6 months) from the last cycle of pembrolizumab with a 1-year PFS and OS of 83% and 80%, respectively (Figure 2).
All patient who bridged to Allo-SCT received reduced intensity conditioning regimens. Fludarabine-Melphalan, Fludarabine-Cyclophosphamide and Thiotepa-Busalfan-Melphalan were used equally.
Relapse rate
Post HSCT, 5 patients (29%) relapsed after a median of 7.8 months (range, 1.6–13 months), three of them were Allo-SCT (50%) and two were ASCT patients (18%).
Median time to relapse from day 0 of HSCT
Only two patients relapsed after a median of 6.4 months (range, 4.8–7.9 months) post-ASCT, both had achieved PR prior to ASCT. Whereas, three Allo-SCT patients relapsed after a median of 10.8 months (range, 1.6–13 months), two had achieved PR and one had achieved CR prior to Allo-SCT.
Safety
TRAEs were reported in 21 patients (66%), most of them were mild and moderate (86%). Only three patients (14%) experienced severe TRAEs, 2 of them reported severe immune-mediated pneumonitis and one had severe infection. The most common reported TRAEs were dermatologic reactions, infections, diarrhea, hypothyroidism and pneumonitis (Table 3). Two patients (9.5%) discontinued pembrolizumab secondary to immune-mediated pneumonitis (N=1) and hepatitis (N=1). One patient died as a result of severe infection 19 days after receiving one cycle of pembrolizumab. No unusual inflammatory symptoms were observed after HSCT.
Table 3
| Adverse event | N (%) |
|---|---|
| Infection | 5 (23.8) |
| Diarrhea | 3 (14.3) |
| Nausea and vomiting | 2 (9.5) |
| Rash | 5 (23.8) |
| Itching | 5 (23.8) |
| Pneumonitis | 5 (23.8) |
| Hypothyroidism | 3 (14.3) |
| Colitis | 1 (4.8) |
| Hepatitis | 1 (4.8) |
| Pain | 3 (14.3) |
GVHD after Allo-SCT
GVHD prophylaxis given to Allo-SCT patients was a combination of mycophenolate with either cyclosporine or tacrolimus. GVHD occurred in 5 patients (83%). Acute GVHD was reported in all five patients; three of them also developed chronic GVHD in addition to the acute episodes. The median duration from the last pembrolizumab cycle to first acute GVHD episode was 4.4 months (range, 2.1–5.8 months) and 5.5 months (range, 4.4–14.6 months) to the first chronic GVHD episode.
Acute GVHD grades range from 2 to 4 in all five patients, whereas, grades 3–4 acute GVHD were reported in 3 patients (60%). The only reported grade 4 acute gut GVHD was fatal. Global score for chronic GVHD was moderate in 2 patients (67%) and severe in 1 (33%). Gut and skin were the most common sites of acute GVHD. Nevertheless, chronic GVHD was detected equally in lungs, eyes, mouth, and skin. (Tables 4,5).
Table 4
| Acute GVHD grade | N (%) | Organs involved |
|---|---|---|
| 2 | 2 [40] | Skin |
| 3 | 2 [40] | Skin, gut |
| 4 | 1 [20] | Gut |
GVHD, graft-versus-host disease.
Table 5
| Chronic GVHD grade | N (%) | Organs involved |
|---|---|---|
| Moderate | 2 [67] | Mouth, eyes, liver |
| Severe | 1 [33] | Skin, mouth, lung |
GVHD, graft-versus-host disease.
Discussion
With around 1-year follow-up, pembrolizumab induced a substantial overall response among the majority of patients (overall response: 78%; PR: 31%, CR: 47%), following a median of 5 cycles. Our study demonstrated the feasibility of using pembrolizumab as bridging to ASCT in around one-third of chemo-resistant RRcHL. Our patients had chemo-resistant disease, which necessitated the use of pembrolizumab to restore chemo-sensitivity followed by ASCT to maintain disease remission, as substantial proportion of patients will relapse post pembrolizumab with a median PFS of 13.8 months (14). Bridging patients to HSCT post-pembrolizumab in our center, was based on results of a retrospective study conducted by Carreau et al. which demonstrated clearly the positive impact of using anti-PD-1 to sensitize RRcHL chemo-resistant patients toward subsequent treatment-including HSCT after anti-PD-1 (15). The rationale behind bridging 65% of our patients to ASCT and not to Allo-SCT is that 69% of them were transplant-naïve.
Interestingly, few studies addressed the role of anti-PD-1 in RRcHL patients as bridging to HSCT. The results of our study are comparable to previously published studies (9,16). In our study, pembrolizumab bridged around 53% of the included patients to HSCT (ASCT: 11, Allo-SCT: 6) with a 1-year PFS and OS of 85% and 91%, respectively, indicating a durable response.
In our study, ASCT patients had a 1-year PFS rate of 83% and a 1-year OS rate of 100%. On the other hand, Allo-SCT patients had 1-year PFS and OS of 83% and 80%, respectively.
Nevertheless, approximately 30% of HSCT patients relapsed after a median of 8 months from transplant. It is worth mentioning that 80% of the relapsed patients (4 out of 5) bridged to HSCT on top of PR following pembrolizumab. More than half of them relapsed post Allo-SCT (N=3, 60%). The relapse rate in ASCT and Allo-SCT patients was 18% and 50%, respectively. In general, the post-HSCT relapse rate reported in our study was comparable with what is reported in Crump (17) for ASCT and in Castagna et al. (18) for Allo-SCT.
The difference in relapse rate between ASCT and Allo-ASCT may be explained by the fact that the majority of ASCT patients bridged after CR (73%) compared to 33% of Allo-SCT patients. Moreover, the KEYNOTE-087 showed that durable responses were mainly seen in the subgroup of patients who achieved CR (8). These results clearly show the value of achieving CR following pembrolizumab and prior HSCT to minimize relapse risk.
All of the observed 1-year PFS, OS and relapse rates for Allo-SCT patients in our study are comparable to those reported in Merryman et al. (19).
Shah and Moskowitz (20) recommend the use of anti-PD-1 in patients who relapsed following ASCT or those who are ineligible for ASCT as bridging to Allo-SCT only after achieving sufficient response. However, in our study more than 69% of the included patients were transplant-naïve and around 40% of responders bridged after achieving PR.
Although the use of anti-PD-1 before Allo-SCT yields important response rates, different clinical evidence suggests that bridging to Allo-SCT using anti-PD-1 may be associated with increased risk in both the incidence and severity of GVHD due to modulation of antigen-specific T-cell responses (21). Moreover, Merryman et al. (19,21) concluded that prior exposure of anti-PD-1 lead to a significantly increased risk of GVHD related morbidity and mortality post-Allo-SCT. Similar to Merryman et al., the increased risk of GVHD post anti-PD-1 was clearly noted in our study. Despite, the small number of our patients who underwent Allo-SCT, the reported GVHD incidence is high.
Therefore, Shah and Moskowitz (20) recommend bridging to Allo-SCT after anti-PD-1 but with increasing the time between the last dose of anti-PD-1 and Allo-SCT, if there is disease stability, to minimize the risk of GVHD. Our study met the recommended 6-week anti-PD-1 treatment-free period prior Allo-SCT (4), with a median time from the last anti-PD-1 dose to Allo-SCT of 52 days.
The role of cyclophosphamide as additional GVHD prophylaxis post-Allo-SCT was previously evaluated in two retrospective studies performed by Schoch et al. (22) and Castagna et al. (23). Both studies demonstrated that cyclophosphamide post-Allo-SCT has a beneficial effect in reducing GVHD incidence following anti-PD-1 in different diseases including Hodgkin’s lymphoma. Cyclophosphamide was associated with lower grades of acute and chronic GVHD (4,22,23). It is noteworthy that cyclophosphamide post-Allo-SCT as GVHD prophylaxis was not used in our study because it is not one of the standard GVHD prophylaxis agents at our institution and in the international guidelines (24). However, the high incidence of GVHD observed in our patients necessitates the need of using additional GVHD prophylaxis. Post-transplant cyclophosphamide could be recommended for this purpose, though, this recommendation requires further evaluation in the future.
Bryan et al. (25) showed that pre-treatment with an anti-PD-1 prior ASCT appears safe and does not delay engraftment. Furthermore, two studies conducted by Herrera et al. showed that nivolumab as monotherapy or in combination with Ifosfamide, Carboplatin, Etoposide (ICE) chemotherapy (26) or BV (27) was well-tolerated and effective as salvage therapy in the majority of RRcHL patients. Despite the difference between our study and previous studies (25-27) in term of treatment regimens, all of them provide promising results for using anti-PD-1 as pembrolizumab or nivolumab for bridging to ASCT.
Additionally, previous studies showed that bridging with BV resulted in rates of “immediate” ASCT that ranged from 34% to 47%, which means that BV can overcome chemo-resistance and permits ASCT in patients who were ineligible for ASCT due to having chemo-resistant disease (28-33). Furthermore, in our study pembrolizumab provided similar results with an ASCT bridging rate of 34%.
In Jordan, BV is not registered. Therefore, pembrolizumab provides a treatment opportunity to RRcHL patients. The US-FDA does not restrict the use of pembrolizumab in patients who have formerly bridged to HSCT or used BV. However, the KEYNOTE-087 did not include any information about the outcomes of ASCT and BV naïve patients. Therefore, this study extends what has been provided by previous evidence to include outcomes about different cohort that is expected to be common among RRcHL patients particularly in countries where BV is not registered, especially after the promising results of the ongoing KEYNOTE-204; a randomized phase III trial; which demonstrated the superiority of pembrolizumab over BV in terms of PFS in RRcHL patients with a tolerable safety profile (34).
Regarding safety, our study showed that pembrolizumab was tolerable. The most common TRAEs reported in this study were infection (24%), rash (24%), itching (24%), pneumonitis (24%), hypothyroidism (14%) and pain (14%), while in the KEYNOTE-087 the most common TRAEs were hypothyroidism (12.4%) and pyrexia (10.5%) (8). Higher treatment discontinuation rate was noted in our study (9.5%) compared to KEYNOTE-087 (4.3%). The higher TRAEs incidence and discontinuation rate observed in this study could be attributed to (I) the design of the study; as the observational design reflects the real-world TRAEs incidence in clinical practice compared to reported incidences in interventional studies under controlled conditions, (II) genetic factors of our patients and other disease or patients’ characteristics that must be evaluated in future larger studies.
We acknowledge that our study has limitations. First, being a non-comparative study increasing the risk of bias and confounders. Second, the relatively small sample size, however, this sample represented all RRcHL patients who were treated with pembrolizumab at our center during the study’s duration. Third, assessing safety through a retrospective observational study was challenging because we relied on electronic systems to identify the reported TRAEs, with the potential of under-reporting, thus the results may not reflect the true incidence of TRAEs. Therefore, having a targeted pharmacovigilance system for immunotherapies is highly recommended to monitor their safety profile.
Irrespective of these limitations, we believe that this study has provided promising information regarding the effectiveness and safety of using pembrolizumab as bridging to HSCT in BV and HSCT naïve patients who have chemo-resistant disease. Furthermore, our study uncovered several research questions that require to be answered such as, (I) the optimum timing of transplant after pembrolizumab or any other anti-PD-1, (II) the role of Allo-SCT after anti-PD-1 within the context of the increased GVHD risk, (III) prognostic factors of the transplant rate and GVHD incidence when bridging to HSCT using pembrolizumab.
Conclusions
The distinctive feature of our study is that it included a unique group of chemo-resistant patients who are both HSCT and BV naïve. As the treatment paradigm of RRcHL is evolving, the results of our study suggest that pembrolizumab is considered to be an effective and safe treatment option to bridge RRcHL patients to ASCT rather than to Allo-SCT after achieving CR. Nevertheless, larger prospective investigational trials are required to be conducted to confirm these results.
Acknowledgments
We would like to acknowledge Ayat Taqash, from biostatistics unit-KHCC, whose statistical programming helped in the statistical analysis for our study and we would like to thank reviewers who reviewed our manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/aol-20-21
Data Sharing Statement: Available at http://dx.doi.org/10.21037/aol-20-21
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-21). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the institutional review board (No. 19-KHCC-78). A waiver of informed consent was obtained as this is a retrospective chart review.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Shindiapina P, Alinari L. Pembrolizumab and its role in relapsed/refractory classical Hodgkin’s lymphoma: evidence to date and clinical utility. Ther Adv Hematol 2018;9:89-105. [Crossref] [PubMed]
- Shanbhag S, Ambinder RF. Hodgkin lymphoma: a review and update on recent progress. CA Cancer J Clin 2018;68:116-32. [Crossref] [PubMed]
- Vassilakopoulos TP, Asimakopoulos JV, Konstantopoulos K, et al. Optimizing outcomes in relapsed/refractory Hodgkin lymphoma: a review of current and forthcoming therapeutic strategies. Ther Adv Hematol 2020;11:2040620720902911 [Crossref] [PubMed]
- Gerrie AS, Power MM, Shepherd JD, et al. Chemoresistance can be overcome with high-dose chemotherapy and autologous stem-cell transplantation for relapsed and refractory Hodgkin lymphoma. Ann Oncol 2014;25:2218-23. [Crossref] [PubMed]
- Hawkes EA, Grigg A, Chong G. Programmed cell death-1 inhibition in lymphoma. Lancet Oncol 2015;16:e234-45. [Crossref] [PubMed]
- Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol 2019;12:92. [Crossref] [PubMed]
- Chen R, Zinzani PL, Fanale MA, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 2017;35:2125. [Crossref] [PubMed]
- Merryman RW, Redd RA, Nieto Y, et al. Outcome of Autologous Stem Cell Transplantation Following PD-(L) 1 Based Salvage Therapy for Multiply Relapsed Patients with Classic Hodgkin Lymphoma. Blood 2019;134:abstr 4571.
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579-86. [Crossref] [PubMed]
- Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 1992;49:2229-32. [Crossref] [PubMed]
- Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donor, S. Transplantation 1974;18:295-304. [Crossref] [PubMed]
- Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant 2015;21:389-401.e1. [Crossref] [PubMed]
- Zinzani PL, Fanale MA, Chen R, et al. Pembrolizumab monotherapy in patients with primary refractory classical Hodgkin lymphoma: Subgroup analysis of the phase 2 Keynote-087 study. Hematol Oncol 2017;35:136-7. [Crossref]
- Carreau NA, Pail O, Armand P, et al. Checkpoint blockade therapy may sensitize Hodgkin lymphoma to subsequent therapy. Oncologist 2020;10:1-13. [Crossref] [PubMed]
- El Cheikh J, Massoud R, Abudalle I, et al. Nivolumab salvage therapy before or after allogeneic stem cell transplantation in Hodgkin lymphoma. Bone Marrow Transplant 2017;52:1074-7. [Crossref] [PubMed]
- Crump M. Management of Hodgkin lymphoma in relapse after autologous stem cell transplant. Hematology Am Soc Hematol Educ Program 2008;326-33. [Crossref] [PubMed]
- Castagna L, Sarina B, Crocchiolo R, et al. Outcomes of Hodgkin lymphoma patients who relapse after allogeneic stem cell transplantation. Bone Marrow Transplant 2016;51:1644-6. [Crossref] [PubMed]
- Merryman RW, Kim HT, Zinzani PL, et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood 2017;129:1380-8. [Crossref] [PubMed]
- Shah GL, Moskowitz CH. Transplant strategies in relapsed/refractory Hodgkin lymphoma. Blood 2018;131:1689-97. [Crossref] [PubMed]
- Shah GL, Moskowitz CH. Checkpoint inhibition in lymphoma. Clin Adv Hematol Oncol 2018;16:45-55. [PubMed]
- Schoch LK, Borrello I, Fuchs EJ, et al. Checkpoint Inhibitor Therapy and Graft Versus Host Disease in Allogeneic Bone Marrow Transplant Recipients of Haploidentical and Matched Products with Post-Transplant Cyclophosphamide. Blood 2016;128:4571. [Crossref]
- Castagna L, Bramanti S, Devillier R, et al. Haploidentical transplantation with post-infusion cyclophosphamide in advanced Hodgkin lymphoma. Bone Marrow Transplant 2017;52:683-8. [Crossref] [PubMed]
- Penack O, Marchetti M, Ruutu T, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol 2020;7:e157-67. [Crossref] [PubMed]
- Bryan LJ, Smith SE, Allen P, et al. Safety and Toxicity Profile of Pembrolizumab (PEM) in Combination with ICE Chemotherapy Followed By Autologous Stem Cell Transplantation for Relapsed/Refractory Classical Hodgkin Lymphoma: No Impairment in Stem Cell Mobilization or Engraftment. Blood 2019;134:abstr 4029.
- Herrera AF, Chen RW, Palmer J, et al. PET-adapted nivolumab or nivolumab plus ICE as first salvage therapy in relapsed or refractory hodgkin lymphoma. Blood 2019;134:239. [Crossref]
- Herrera AF, Moskowitz AJ, Bartlett NL, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 2018;131:1183-94. [Crossref] [PubMed]
- Angelopoulou MK, Vassilakopoulos TP, Batsis I, et al. Brentuximab vedotin in relapsed/refractory Hodgkin lymphoma. The Hellenic experience. Hematol Oncol 2018;36:174-81. [Crossref] [PubMed]
- Eyre TA, Phillips EH, Linton KM, et al. Results of a multicentre UK-wide retrospective study evaluating the efficacy of brentuximab vedotin in relapsed, refractory classical Hodgkin lymphoma in the transplant naive setting. Br J Haematol 2017;179:471-9. [Crossref] [PubMed]
- Pellegrini C, Broccoli A, Pulsoni A, et al. Italian real life experience with brentuximab vedotin: results of a large observational study on 234 relapsed/refractory Hodgkin’s lymphoma. Oncotarget 2017;8:91703. [Crossref] [PubMed]
- Onishi M, Graf SA, Holmberg L, et al. Brentuximab vedotin administered to platinum-refractory, transplant-naive Hodgkin lymphoma patients can increase the proportion achieving FDG PET negative status. Hematol Oncol 2015;33:187-91. [Crossref] [PubMed]
- Sasse S, Rothe A, Goergen H, et al. Brentuximab vedotin (SGN-35) in patients with transplant-naive relapsed/refractory Hodgkin lymphoma. Leuk Lymphoma 2013;54:2144-8. [Crossref] [PubMed]
- Zinzani PL, Pellegrini C, Cantonetti M, et al. Brentuximab vedotin in transplant-naïve relapsed/refractory Hodgkin lymphoma: experience in 30 patients. Oncologist 2015;20:1413. [Crossref] [PubMed]
- Kuruvilla J, Ramchandren R, Santoro A, at al. KEYNOTE-204: Randomized, open-label, phase III study of pembrolizumab (pembro) versus brentuximab vedotin (BV) in relapsed or refractory classic Hodgkin lymphoma (R/R cHL). J Clin Oncol 2020;38:abstr 8005.
Cite this article as: Al Froukh RF, Abd Al-Jalil N, Faqeer N, Ma’koseh M, Al-Rabayah A. Effectiveness and safety of pembrolizumab as bridging to hematopoietic stem cell transplantation in relapsed and refractory classical Hodgkin’s lymphoma: a retrospective observational study. Ann Lymphoma 2020;4:9.


