
Prognostic factors and risk of transformation in marginal zone lymphoma
Introduction
Marginal zone lymphoma (MZL) is included in the World Health Organization classification as a mature B-cell lymphoid neoplasm originating from memory B lymphocytes normally present in the marginal zone of the secondary lymphoid follicles (1,2). Marginal zone B cells are continuously exposed to exogenous antigens and have a physiologically reduced threshold for proliferation induction, which may predispose to malignant transformation (3). MZL is subclassified into extranodal MZL (EMZL) of mucosa-associated lymphoid tissue (MALT), splenic MZL (SMZL), and nodal MZL (NMZL) and all share similar immunophenotype but exhibit distinct presentation and behavior (4). EMZL usually remain confined to the site of origin at diagnosis. In contrast, SMZL commonly involves bone marrow and frequently found in peripheral blood as circulating villous or non-villous lymphocytes, but rarely infiltrates peripheral lymph nodes and extranodal tissue. In contrast, NMZL typically presents with enlarged lymph nodes and only occasionally involves bone marrow and peripheral blood (5).
MZL is a rare disease representing 10.5% to 11.8% of all non-Hodgkin lymphomas (NHLs) with significant variability in incidence by geographic region (1,6). EMZL is the most common subtype accounting for 7% to 8% of all B-cell lymphomas followed by SMZL (2%) and NMZL (1.5% to 1.8%) (1). MZL typically exhibit an indolent course associated with long survival (7-10). In an analysis of the Surveillance, Epidemiology, and End Results (SEER) database, Olszewski et al. evaluated the relative survival of MZL patients. The 5-year relative survival rates (adjusted to age, sex and race) for patients with EMZL, SMZL and NMZL were 88.7%, 79.7% and 76.5%, respectively (11). However, a subset of patients exhibits a more aggressive disease course and succumbs early to their disease (12,13). Different prognostic indexes can identify patients with more aggressive disease in individual MZL subtypes.
In this manuscript, we review recent advances in MZL prognostic factors used in stratifying patients and discuss MZL transformation to diffuse large B-cell lymphoma (DLBCL).
Prognosis factors
EMZL
EMZL has been described in virtually all anatomical locations and especially in organs that are normally devoid of lymphatic tissue (3). The stomach is the most common localization in EMZL followed by ocular adnexa, salivary glands, skin, lung, thyroid, upper airways, breast, other gastrointestinal (GI) sites, and liver (8). Gastric EMZL has been commonly associated with chronic gastritis induced by Helicobacter pylori (H. pylori) (3,14). H. pylori can contribute directly to EMZL pathogenesis by acting on normal and transformed B cells and indirectly through T-cell stimulation (15). Over last decade a declining incidence of H. pylori-associated gastric EMZL was observed in population-based studies most likely due to a generalized use of proton pump inhibitors (16). Importantly, lymphomas bearing the t(11;18) have a low probability of response to H. pylori eradication therapy (17).
Different inflammatory, autoimmune and pathogenic factors have been implicated in individual location sites. However, presence of underlying autoimmune processes was not associated with outcome determination (18). Prognostic scores have been developed in an effort to identify patients with more aggressive disease at the time of initial diagnosis. Thus, in patients with EMZL the MALT-international prognostic index (MALT-IPI), which is based on age ≥70 years, Ann Arbor stage III or IV and elevated lactate dehydrogenase (LDH) level, was designed to identify 3 risk-groups of patients with distinct survival: the low-risk (0 factors), intermediate-risk (1 factor) and high risk (≥2 factors) with 5-year event-free survival (EFS) of 70%, 56%, and 29%, respectively (19).
The prognostic significance of primary anatomical location of EMZL is controversial. Some studies suggested that initial EMZL localization may bear effect on patients’ outcomes. Zucca et al. evaluated 180 patients with nongastric EMZL and reported 5-year progression-free survival (PFS) at distinct presentation locations: orbit 23%, multiple sites 25%, breast 33%, skin 53%, bowel 63%, salivary gland 67%, and lung 75% (8). Similarly, Thieblemont et al. reported shorter freedom-from progression (FFP) in EMZL localized outside of the GI tract (20). Other factors associated with shorter FFP in this analysis were anemia, high β2-microglobulin, and failure to achieve complete response (CR) following initial therapy (20). Concordantly, better EFS, PFS and CR rate were observed in patients with gastric (72%, 95% CI: 65% to 79%) vs. primary nongastric EMZL (61%; 95% CI: 54% to 67%) in the IELSG-19 clinical trial (21). A tendency towards shorter EFS was observed in patients with disease primary localized in the lung and skin in this prospective study, (21) contradicting some of the previous observations (8,22,23). In contrast, PFS and overall survival (OS) were similar in patients with localized EMZL treated with radiation therapy (RT) irrespective of primary organ-specific location; however, patients with disease localized in the thyroid and stomach seemed to have a lower risk for disease relapse (7,22,24). Overall, these studies suggest better outcomes in patients with gastric EMZL.
In our study of EMZL patients with stage I disease treated with frontline RT, we did not observe differences in PFS and OS based on the primary anatomical localization of EMZL (7). The reported differences in outcomes in diverse lymphoma sites may be attributed to different therapies used in these patients and studies. Also, a controversial aspect in the treatment of patients with EMZL is the dose of recommended RT. Current international guidelines endorse a radiation dose between 20 and 30 Gy (25). However, this recommendation is based on randomized studies that mainly included patients with other indolent lymphomas with a primary endpoint of local disease control and not PFS or OS (26,27). In our previously mentioned retrospective analysis of patients with stage I EMZL treated with curative intent, RT dose <30 Gy was associated with a higher risk for disease relapse which was confirmed in various multivariable analyses models (7,28). The incidence of relapse based on radiation field was 17% inside, 17% inside & outside, and 8% outside of all patients treated with radiation for stage I EMZL. Nevertheless, only limited number of patients treated with lower doses of RT were included in this study and thus prospective randomized studies with long follow up are eagerly needed to address this question.
Disease dissemination at diagnosis is observed in 23% to 34% of patients with EMZL and may be associated with worse outcome (8,20). For example, several studies showed that presence of lymph nodes and/or bone marrow involvement at presentation is associated with worse prognosis (8,29). Advanced stage (III/IV) is also incorporated as an unfavorable prognostic factor in the MALT-IPI. However, the prognostic significant of multiple mucosal sites (MMSs) at the time of diagnosis is controversial (8,20,30,31). Few studies evaluated the prognostic significance of MMS and three studies suggested lack of association between the MMS at presentation and outcome (8,20,30), but worse prognosis was found in another study (31). However, these studies included small numbers of MMS patients (n=17 and 24) with a short follow up period precluding robustness of conclusions claimed by the authors. Further, MMS definition varied between these studies. In a large retrospective cohort of EMZL patients (n=405) followed up to 22.3 (range, 0.02–22.3) years we observed that patients presenting with MMS involvement at diagnosis, defined as EMZL in two or more different anatomical organs independent of spleen and BM involvement, exhibited shorter PFS (HR: 5.39, P<0.001) with a median PFS of 1.7 vs. 13.2 years in patients without MMS. Similarly, shorter OS (HR: 4.44, P<0.001) with 10-year OS of 40.5% (95% CI: 20.7% to 59.5%) vs. 81.1% (95% CI: 75.1% to 85.8%) was observed in patients with and without MMS, respectively. Greater incidence of higher grade transformation (HGT) with a 5-year cumulative incidence of 13.2% (95% CI: 4.7% to 26.1%) was also observed in patients with MMS (13).
In the same study we also observed shorter PFS (HR: 1.82, P=0.016) and OS (HR: 2.25, P=0.033) in patients presenting with monoclonal gammopathy (MG) at diagnosis. MG was present in 10.7% of the patients and was characterized by predominance of immunoglobulin G (IgG) as the most common paraprotein. In other series IgM has been described as the most common MG in EMZL (29,32), but this difference could be secondary to small number of patients incorporated, difference in primary location of EMZL at presentation, and the potential inclusion of some patients with lymphoplasmacytic lymphomas, which may mimic EMZL presentation. We observed a higher incidence of MG in patients with GI-non gastric (12.5%) and MMS (11.6%) presentations. Other studies support this observation, associating presence of MG at diagnosis with worse outcome of patients with EMZL (33,34). Additional reported clinical factors associated with worse outcome are age >60 years, elevated LDH, β2 microglobulin, anemia (hemoglobin <12 g/dL), thrombocytopenia, lymphopenia, low serum albumin, poor performance status, systemic symptoms, failure to achieve CR following initial treatment, follicular lymphoma IPI (FLIPI) >2, and IPI >2 (6,13,35).
There is a limited data on prognostic biomarkers in EMZL. EMZL is typically a CD5 negative neoplasm, however, expression of CD5 in EMZL was associated with nongastric involvement and disease dissemination (36). Similarly, CXCR4 expression was associated with bone marrow infiltration in gastric EMZL (37). Presence of t(11;18) was associated with a longer median time to disease relapse compared to patients without this translocation (76 vs. 29 months; P=0.012) (38). However the presence of t(11;18) in patients with gastric EMZL was associated with resistance to oral alkylating agents and shorter remission duration (39). FOXP1 expression was associated with a higher relapse rate and shorter disease-free survival, (40) while BCL10 expression was associated with advanced EMZL (41).
Progression of disease within 24 months (POD24) was associated with a shorter survival in patients with follicular lymphoma (FL) (42,43). Most recently, three independent studies demonstrated that POD24 is also an important marker for shorter survival in patients with MZL (13,35,44). Our group demonstrated that POD24 was associated with shorter survival in patients with EMZL (5-year survival post progression 46.8% vs. 91.1% in the reference group) (13). Luminari et al. examined the impact of POD24 in patients enrolled in the NF10 prospective international registry. This group reported a 3-year OS for patients with POD24 of 53% (95% CI: 37% to 67%) with a HR of 19.5 (95% CI: 8.4–45.4) compared to 88% (95% CI: 89% to 98%) in patients without POD24. Conconi et al. also demonstrated worse outcomes in patients with POD24 enrolled in the IELSG19 clinical trial (n=401) with a 10-year OS of 64% in POD24 group vs. 85% in reference group (HR: 2.42, 95% CI: 1.5–4.34, P=0.002). Importantly, this is the only study that validated their results in an independent cohort of patients (n=287) (44). However, all three studies indicate prognostic significance of POD24 in EMZL.
The prognostic significance of positron-emission tomography (PET)/CT in MZL remains debatable. Albano et al. attempted to evaluate prognostic impact of qualitative and semi quantitative baseline PET/CT variables such as maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) in EMZL. One hundred and sixty-one patients were retrospectively evaluated and (18F) fluorodeoxyglucose (FDG)-avidity correlated with Ki67 expression and tumor size, however, no correlation between survival and PET/CT parameters was observed (45). A study carried out by investigators from Memorial Sloan Kettering Cancer Center evaluated the role of PET/CT at the time of EMZL diagnosis in 123 patients. They found that SUV ≥10 was an independent factor associated with significantly shorter 5-year OS (78% vs. 92%, P=0.008) and higher rate of subsequent HGT (20% vs. 5%, P=0.035) (46). However, 54% (n=314) of the initial EMZL cohort (n=582) was excluded from this analysis because they did not have staging PET/CT rising a concern that patients included in this study might have a more aggressive disease behavior and/or suspicion for HGT that prompted treating physician to request PET/CT and thus may not be representative of the general EMZL population. Only 20 (16%) patients presented with SUV ≥10 and thus this study warrants further validation before reaching any conclusion on prognostic significance of PET/CT is EMZL. In a retrospective study including all three MZL subtypes (n=110), a positive end of treatment PET/CT was associated with shorter PFS with a HR of 3.4 (95% CI: 1.27–9.14, P=0.02). Importantly, this finding did not correlate with OS (47). Another smaller study (n=32) also found better PFS in patients achieving complete metabolic response at the end of treatment PET/CT (48). These findings are consistent with results obtained in FL in the GALLIUM and PRIMA studies (49,50). In these studies, patients achieving PET/CT complete metabolic response attained a 2.5-year PFS of 87.4% (95% CI: 83.7–90.2%) vs. 54.9% (40.5% to 67.3%) in non-responders (P<0.0001), and 42 months PFS of 70.7% (95% CI: 59.3% to 79.4%) vs. 32.9% (95% CI: 17.2% to 49.5%) (P<0.001), respectively (49,50) (Tables 1,2).
Table 1
| EMZL | SMZL | NMZL |
|---|---|---|
| MALT-IPI (19) | IIL (51) | Poor performance status (52) |
| POD24 (13,35,44) | HPLL (53) | MALT-IPI (6) |
| Radiation dose <30 Gy (7) | MALT-IPI (6) | FLIPI (52) |
| MMS (13) | POD24 (35) | CD5 positive (54) |
| MG (13) | ||
| CD5 positive (36) | ||
| Elevated β2 microglobulin (20,35) | ||
| Failure to achieve CR following initial treatment (20) | ||
| FLIPI >2 (6,35) | ||
| IPI >2 (6) |
MZL, marginal zone lymphoma; EMZL, extranodal marginal zone lymphoma; SMZL, splenic marginal zone lymphoma; NMZL, nodal marginal zone lymphoma; MALT-IPI, mucosa-associated lymphoid tissue international prognostic index; IIL, Intergruppo Italiano Linfomi; POD24, progression of disease within 24 months; LDH, lactate dehydrogenase; HPLL, hemoglobin concentration, platelet count, elevated LDH, and extrahilar lymphadenopathy; FLIPI, follicular lymphoma international prognostic index; MMS, multiple mucosal site; MG, monoclonal gammopathy; CR, complete remission; IPI, international prognostic index.
Table 2
| EMZL | SMZL | NMZL |
|---|---|---|
| MALT-IPI (19), 5-y EFS | IIL (51), 5-y CSS | FLIPI (52), 4-y OS |
| Low-risk: 70% | Low-risk: 88% | Low-risk: 90% |
| Intermediate-risk: 56% | Intermediate-risk: 73% | Intermediate-risk: 70% |
| High-risk: 29% | High-risk: 50% | Low-risk: 88% |
| FLIPI (55), 5-y PFS | HPLL (53), 5-y LSS | |
| Low/intermediate risk: 92% | Low-risk: 94% | |
| Poor risk: 62% | Intermediate-risk: 78% | |
| POD24 (13), 5-y OS | High-risk: 69% | |
| No POD24: 91.1% | POD24 (35), 3-y OS | |
| POD24: 46.8% | No POD: 95% | |
| POD24: 44% |
MZL, marginal zone lymphoma; EMZL, extranodal marginal zone lymphoma; SMZL, splenic marginal zone lymphoma; NMZL, nodal marginal zone lymphoma; MALT-IPI, mucosa-associated lymphoid tissue international prognostic index; EFS, event-free survival; IIL, Intergruppo Italiano Linfomi; CSS, cause-specific survival; FLIPI, follicular lymphoma international prognostic index; OS, overall survival; PFS, progression-free survival; HPLL, hemoglobin concentration, platelet count, elevated LDH, and extrahilar lymphadenopathy; LSS, lymphoma-specific survival; POD24, progression of disease within 24 months.
SMZL
SMZL is a rear and indolent NHL with a median OS of more than 10 years (11,56). The survival rates have improved with the incorporation of rituximab in the treatment of SMZL (57). A recent SEER database analysis found that age >60 years, Hispanic ethnicity, presence of B symptoms, HGT, and treatment with non-rituximab containing chemotherapy are associated with shorter lymphoma-specific survival (LSS) (58). In another analysis, none of the components of the age-adjusted IPI (aaIPI) scoring (performance status, stage and LDH levels) were associated with shorter OS or PFS (59). Two scores have been proposed to identify SMZL patients at risk for shorter survival (51,53). The first scoring system was developed by the Intergruppo Italiano Linfomi (IIL) (n=309) which identified hemoglobin <12 g/dL, elevated LDH, and albumin <3.5 g/dL as independent variables associated with poor outcome in SMZL (51). The 5-year cause-specific survival (CSS) rates were 88% for the low-risk group, 73% for the intermediate-risk group, and 50% for the high-risk group. The latter group accounted for 54% of all lymphoma-related deaths (9,51). The HPLL (hemoglobin concentration, platelet count, elevated LDH and extrahilar lymphadenopathy) score was developed based on international analysis of 593 patients. In this index, hemoglobin <9.5 g/dL, splenic extrahilar lymphadenopathy, platelets <80.000/mL and elevated LDH directly correlated with LSS in SMZL and three risk groups with different outcome were identified (5-year LSS of 94%, 78% and 69%, respectively) (53,60). MALT-IPI >1 was also shown to predict shorter PFS but not OS in SMZL (6). FDG-avidity of splenic uptake (homogeneous vs. focal) and semi quantitative determinations in PET/CT do not affect survival in SMZL patients (61).
Like EMZL, only limited data on prognostic biomarkers in SMZL is available. The prognosis of NOTCH2 mutations in SMZL is controversial. Rossi et al. reported better PFS and OS in patients harboring NOTCH2 mutations compared to wild type in a cohort of 94 SMZL patients (5-year PFS 83% vs. 44.1%, P=0.020 and OS 93% vs. 74.3%, P=0.048, respectively) (62). However, inferior outcomes in patients harboring NOTCH2 mutation were reported by other investigators and this event seems to be required before lymphoma dissemination to MMSs (63-65). The NOTCH pathway is affected in up to 30% of SMZL and among other indolent B-cell lymphoproliferative disorders, NOTCH2 mutations are more frequently seen in SMZL (62).
Other cytogenetic abnormalities associated with poor prognosis are complex karyotype, 14q aberrations, TP53 deletions and KLF2 mutations (63,64,66). Methylation of CACNB2, HTRA1 and KLF4 identified a group of patients with poor outcome (65). Importantly, the prognostic significance of most of these biological alterations was not confirmed in independent studies.
NMZL
Very few studies evaluated prognostic factors in NMZL. Poor performance status was the only variable associated with significantly worse outcome, while advanced stage showed a trend toward shorter survival but this did not reach statistical significance in a study of 56 patients (67). Starr et al. reported age >60 years and elevated LDH associated with inferior OS in NMZL treated in the rituximab era (68). No specific prognostic score has been developed for NMZL, however, some studies have shown the utility of MALT-IPI and FLIPI to predict outcome in NMZL (6,52,55,68). CD5 positive NMZL was associated with disseminated disease, similarly to EMZL (54).
In summary, clinical prognostic indexes were developed to predict outcome of patients with EMZL and SMZL. However, whether these indices can guide treatment selection was not analyzed. Further, the differences in the outcomes between patients in individual risk groups are generally smaller than outcome differences between FL patients with low and high FLIPI. Consequently, there is a place for improvement and development of novel clinical indices that can better identify patients with short survival that may need different therapeutic approaches. Biologic prognostic biomarkers are largely unknown and further studies of biology of these tumors are needed to identify prognostic and predictive biomarkers.
HGT in MZL
Incidence of MZL transformation
The natural history and clinical course of patients with MZL is characterized by increased risk of transformation to aggressive lymphoma which is an independent risk factor for shorter survival (6,8,69). This phenomenon has been extensively studied in other low-grade lymphomas and the vast majority of clinical data in the literature refers to transformed FL (70). However, only limited data on HGT in MZL is available in the literature (Table 3). HGT of MZL, usually to DLBCL, is a rare event occurring in 3.8% to 13% of patients, though, some series in SMZL report transformation in up to 19% (6,8,59,69,71-76). The incidence of HGT in MZL is markedly lower than in patients with FL (77,78). Meyer et al. reported an annual frequency of 2.4% (69). In a large study performed by Conconi et al. (n=340), HGT was observed in 5% of SMZLs, 4% of EMZLs, and 3% of NMZLs, which suggests a similar incidence across all MZL subtypes (74). However, in our study of 453 patients, a higher incidence of HGT was observed in patients with NMZL and SMZL compared to patients with EMZL (6). Similar to FL patients, the incidence of HGT reaches a plateau after 10 years and HGT is a harbinger of a change in lymphoma course associated with markedly shortened survival (6,59,74,78-80). The median time to HGT was 29 (range, 1.3 to 135) months after MZL diagnosis. The cumulative incidence rate of HGT was 6.6% and 8.4% at 5 and 10 years, respectively, and a 10.1% plateau at 12 years. The corresponding estimated annual incidence rate of HGT was 1.1 (95% CI: 0.7 to 1.5) events per 100 patients per year. Patients experiencing HGT had significantly shorter OS with a 2 and 5-year rate of 57% and 65%, respectively (6,74). Thus, HGT is associated with decreased survival, regardless if it is observed in the context of NMZL, EMZL or SMZL (81). Importantly, patients who presented with HGT within 12 months from MZL diagnosis had shorter OS than those with HGT at MZL diagnosis and HGT more than 12 months later (4-year rate, 43% vs. 84%, P<0.001) (6).
Table 3
| Author | N of patients | Type of MZL | Diagnosis of HGT | Frequency | Median time to HGT |
|---|---|---|---|---|---|
| Alderuccio et al., 2018, (6) | 453 | All MZL | Pathologic | 7.5% | 2.4 years |
| Starr et al., 2016, (71) | 211 | EMZL | N/A | 6.6% | N/A |
| Maeshima et al., 2016, (72) | 467 | EMZL | Pathologic | 8% | 4 years |
| Xing et al., 2015, (73) | 107 | SMZL | Clinical/pathologic | 9% at 5 years; 18% at 10 years | N/A |
| Conconi et al., 2015, (74) | 340 | All MZL | Pathologic | 3.8%; 5% at 5 years; 10% at 12 years | 2.8 years |
| Lenglet et al., 2014, (59) | 100 | SMZL | Pathologic | 11% | 1.9 years |
| Meyer et al., 2014, (69) | 197 | All MZL | Pathologic | 11.6%; 2.4% per year | N/A |
| Dungarwalla et al., 2008, (75) | 9 | SMZL | Pathologic | 19% | 3.75 years |
| Zucca et al., 2003, (8) | 180 | EMZL (nongastric) | Pathologic | 3% | N/A |
| Camacho et al., 2001, (76) | 12 | SMZL | Pathologic | 13% | N/A |
HGT, higher grade transformation; MZL, marginal zone lymphoma; N, number; EMZL, extranodal marginal zone lymphoma; SMZL, splenic marginal zone lymphoma; N/A, not available.
Risks factors associated with MZL transformation
Clinical variables associated with HGT in MZL are elevated LDH, more than 4 nodal sites at diagnosis and failure to achieve CR after initial treatment (6,74). Advanced stage disease was also described as a risk factor for HGT in EMZL (72). Implementing competing risk analysis neither IPI, FLIPI nor MALT-IPI were significant predictors of HGT (6). Elevated LDH and B symptoms are frequently observed at the time of HGT (74). However, clinical and laboratory features at transformation are variable and tissue biopsy is always required. In SMZL with circulating villous lymphocytes presence of peripheral lymphadenopathies has also been associated with a risk of HGT (75). Initial treatment strategy (chemotherapy vs. splenectomy) does not seem to affect the incidence of HGT in SMZL (76).
Biology of MZL transformation
The biology of HGT in MZL is largely unknown with only few studies addressing pathogenesis of this event. In EMZL, strong FOXP1 expression, polymorphic morphology and presence of trisomy 3 and 18 were reported to be associated with a higher risk of transformation to nongerminal center DLBCL (40). In patients with gastric EMZL, transformation to DLBCL was accompanied by upregulation of chemokine receptors CCR1, CCR5, CCR8, CCR9, CXCR7 and XCR1 (37). Presence of t(14;18) IGH/BCL2 in primary cutaneous EMZL was reported to increase the risk of HGT (82) but was not confirmed in independent cohorts.
TP53 deletion was described in 40% of patients experiencing HGT in SMZL (83). However, in a cohort of 12 patients with transformed SMZL most patients presented wild type TP53 and only one patient had p16 deletion. 7q deletion was observed in 42% of cases and a nonsignificant trend towards higher risk for HGT was observed in patients presenting with this abnormality (30% vs. 18.2%, P=0.45) (76). Risk for HGT associated with 7q deletion is questionable. Parry et al. found higher risk in patients harboring 7q31-32 deletion (64), however, 7q deletion, including chromosome regions 31 and 32, is the most common genetic abnormality found in SMZL and this finding may not represent a true risk factor for transformation (84,85). Mutations of TNFAIP3 and epigenetic changes including higher-promoter methylation which is associated with IGHV1-02 usage, NOTCH2 mutations, were also associated with HGT in SMZL (64).
Biology of transformation in NMZL is largely unknown secondary to rarity of this disease. Qian and collaborators evaluated 6 cases of transformed NMZL and found significantly higher incidence of del(20q12) compared to nontransformed NMZL. These cases were significantly enriched in extracellular matrix proteins COL1A1 and FN1, growth factor receptor PDGFRβ, DNA repair protein RAD51, and signaling protein WNT11 (86).
Diagnosis of MZL transformation
Patients usually present with a rise in LDH level, sudden decline in performance status, rapid localized nodal growth, new extranodal sites of disease, presence of new B symptoms or hypercalcemia (12,74,87). The diagnosis of HGT should wherever possible be based on a biopsy sample rather than relying on cytological samples or on clinical/laboratory criteria (70). Transformed DLBCL usually presents non-germinal center immunophenotype (63%) and double expression of BCL2 and MYC is rarely observed (10%) (72). PET/CT has shown to be an important tool pointing to potential HGT in FL and biopsy should aim to sample a lymph node/extranodal area with highest FDG-avidity on PET/CT. Noy et al. analyzed 40 patients with transformed indolent lymphomas including 11 MZL. The authors reported that the observed SUV in biopsy-proven site of transformation ranged from 3 to 38, with a mean of 14; SUV >10 predicted an aggressive lymphoma with >80% certainty and SUV >13 with >90% certainty (88). Other authors confirmed that SUV values ≥10 correlate with more aggressive histology (46,89).
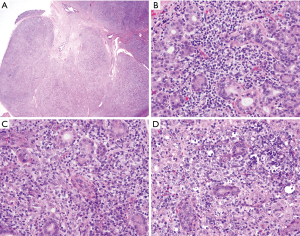
The diagnosis of transformation requires visualization of sheets of large cells (Figure 1) (1). An unequivocal definition of transformation requires demonstration of a clonal relationship between the original MZL and subsequent neoplasm. This can be established by molecular techniques demonstrating use of the same immunoglobulin gene comprised of variable (VH), diversity (D) and joining (JH) segments that shares a backbone of common somatic mutations, thus allowing inference of a common progenitor cell. Molecular studies in Richter’s transformation of chronic lymphocytic leukemia to DLBCL demonstrated that while some of the paired tumors indeed demonstrate identical or related IG sequences, confirming a clonal relationship, others showed presence of different molecular B-cell clones, suggesting presence of molecularly unrelated second malignancy (90). However, since in daily clinical practice IG cloning and sequencing are not routinely performed, demonstration of at least light chain restriction by flow cytometry or immunohistochemistry may be sufficient to suggest transformation and rule the appearance of an unrelated second malignancy (91).
Treatment of MZL transformation
There are no prospective studies specifically evaluating treatment strategies in patients with transformed MZL. Efficacy of different therapeutic regimens is reported mostly in small, retrospective cohorts and most approaches to manage transformed MZL are extrapolated from other indolent NHLs (81). In transformed FL anthracycline-containing regimens like rituximab, cyclophosphamide, Adriamycin, vincristine and prednisone (R-CHOP) are recommended, leading to an overall response rate (ORR) and CR of 60% and 40%, respectively (78). Because responses achieved with conventional chemotherapy in patients with transformed FL are frequently of short duration and historically patients with transformed disease exhibit inferior outcomes with conventional therapy, high-dose therapy with autologous stem cell transplant (ASCT) should be considered in young fit patients (12,91). In a cohort of 172 transformed FL patients from the Canadian Blood and Marrow Transplant Group, improved outcomes were reported in patients undergoing consolidation with ASCT compared to those treated with rituximab-containing regimens only. Importantly, 85% of the patients presented chemosensitive disease before transplantation and in a multivariable analysis there was no difference in OS between patients treated with allogeneic stem-cell transplant and ASCT (HR: 1.5, 95% CI: 0.65–3.47, P=0.35) (92). Nevertheless, younger patients who were not exposed to chemotherapy prior to HGT seem to have long survival even without transplantation (2-year OS in chemotherapy naïve vs. exposed to chemotherapy prior to HGT was 100% vs. 35%, P=0.03, respectively) (93).
Compared to FL, there is only limited information on the best treatment strategy in patients with transformed MZL. In the largest study evaluating the role of ASCT in MZL, patients with transformed disease were excluded (94). Response to treatment and long-term outcome seems to be better in previously untreated patients with HGT, similar to findings in transformed FL (6,95). In our analysis of HGT we observed that most previously untreated patients (either diagnosed with HGT at the time of MZL or after active surveillance) achieved a CR rate of 91% with standard frontline DLBCL therapy. Although none of these patients underwent ASCT, we did not observe DLBCL recurrence in any of them during a median follow up of 22.5 (range, 2 to 130) months. Similarly, good results were obtained in another large cohort of transformed EMZL (n=37) patients where the ORR was 97% and 5-year PFS rate of 80% after R-CHOP/CHOP with no patients undergoing ASCT (72). Therefore, untreated patients with HGT may respond to standard DLBCL therapy and may not need consolidation with ASCT. In contrast, the CR rate was less frequent in previously treated patients (52.6%), and eight patients died as a result of DLBCL, even after ASCT (6).
Based on the currently available data which is limited in scope and mostly derived from small retrospective studies we suggest the following treatment algorithm (Figure 2):
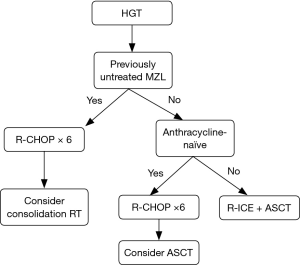
- Previously untreated MZL patients with HGT or HGT diagnosed at the time of initial MZL diagnosis should be treated with anthracycline-containing regimen such as R-CHOP and considered for consolidation with RT if disease is limited. One exception to this recommendation would be the presence of double hit DLBCL (MYC and BCL2 and/or BCL6 rearrangements) where more intensive regimen is required and ASCT after frontline therapy should be strongly considered.
- Patients not achieving CR following anthracycline-containing regimen should receive salvage DLBCL treatment and, if chemosensitive disease is demonstrated, proceed with ASCT.
- Patients achieving CR following anthracycline-containing regimen can be observed if they did not receive prior therapy for MZL. ASCT may be considered in selected cases but may not be necessary in majority of these patients.
- Previously treated MZL presenting with HGT should receive anthracycline-containing regimen or DLBCL salvage regimens followed by ASCT.
- Patients treated with anthracycline-containing regimen for MZL prior to HGT should receive standard DLBCL salvage regimen followed by ASCT.
In conclusion, poor risk clinical features in EMZL have been elucidated in recent years. Similarly, characteristics influencing prognosis in SMZL have also been described, however, high risk features in NMZL remain scarce. Compared to FL, MZL biology associated with adverse outcomes and pathogenesis of transformation are largely unknown. Current data attempting to address treatment strategies in high risk patients and in those experiencing HGT is almost inexistent, representing an unmet need in the field. Given the infrequency of MZL and rarity of HGT, it is unlikely that randomized and prospective studies will ever be conducted, thus highlighting the importance of well design multi-institutional studies attempting to address gaps in the understanding of biology and treatment of high risk/transformed patients with MZL.
Acknowledgments
The authors would like to thank Dr. Jennifer R. Chapman for providing pathologic imaging.
Funding: JPA is a K12 Scholar supported by the National Cancer Institute of the National Institutes of Health under Award Number K12CA226330. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. ISL is supported by grant 1R01CA233945 from the National Cancer Institute, the Dwoskin, Recio, and Anthony Rizzo Families Foundations and Jaime Erin Follicular Lymphoma Research Consortium.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francesco Bertoni, Thomas Habermann, Davide Rossi, and Emanuele Zucca) for the series “Marginal Zone Lymphomas” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-8). The series “Marginal Zone Lymphomas” was commissioned by the editorial office without any funding or sponsorship. JPA has received honoraria from OncLive and Oncinfo, and immediate family member has served on advisory boards from Puma Biotechnology, Inovio Pharmaceuticals, Agios Pharmaceuticals, Forma Therapeutics and Foundation Medicine. ISL has served on advisory boards from Seattle Genetics, Janssen Scientific and Verastem. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press, 2017.
- Sriskandarajah P, Dearden CE. Epidemiology and environmental aspects of marginal zone lymphomas. Best Pract Res Clin Haematol 2017;30:84-91. [Crossref] [PubMed]
- Zucca E, Bertoni F. The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance. Blood 2016;127:2082-92. [Crossref] [PubMed]
- Bertoni F, Rossi D, Zucca E. Recent advances in understanding the biology of marginal zone lymphoma. F1000Res 2018;7:406. [Crossref] [PubMed]
- Zucca E, Polliack A, Cavalli F. Marginal zone lymphomas: reconsidering similarities and differences while moving towards personalized treatment. Best Pract Res Clin Haematol 2017;30:1-4. [Crossref] [PubMed]
- Alderuccio JP, Zhao W, Desai A, et al. Risk factors for transformation to higher-grade lymphoma and its impact on survival in a large cohort of patients with marginal zone lymphoma from a single institution. J Clin Oncol 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Alderuccio JP, Isrow D, Reis IM, et al. Diagnostic bone marrow biopsy in patients with stage I EMZL treated with radiation therapy: needed or not? Blood 2020;135:1299-302. [Crossref] [PubMed]
- Zucca E, Conconi A, Pedrinis E, et al. Nongastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Blood 2003;101:2489-95. [Crossref] [PubMed]
- Arcaini L, Rossi D, Paulli M. Splenic marginal zone lymphoma: from genetics to management. Blood 2016;127:2072-81. [Crossref] [PubMed]
- Thieblemont C, Molina T, Davi F. Optimizing therapy for nodal marginal zone lymphoma. Blood 2016;127:2064-71. [Crossref] [PubMed]
- Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma. Cancer 2013;119:629-38. [Crossref] [PubMed]
- Casulo C, Burack WR, Friedberg JW. Transformed follicular non-Hodgkin lymphoma. Blood 2015;125:40-7. [Crossref] [PubMed]
- Alderuccio JP, Zhao W, Desai A, et al. Short survival and frequent transformation in extranodal marginal zone lymphoma with multiple mucosal sites presentation. Am J Hematol 2019;94:585-96. [Crossref] [PubMed]
- Zucca E, Bertoni F, Roggero E, et al. Molecular analysis of the progression from Helicobacter pylori-associated chronic gastritis to mucosa-associated lymphoid-tissue lymphoma of the stomach. N Engl J Med 1998;338:804-10. [Crossref] [PubMed]
- Kuo SH, Cheng AL. Helicobacter pylori and mucosa-associated lymphoid tissue: what's new. Hematology Am Soc Hematol Educ Program 2013;2013:109-17. [Crossref] [PubMed]
- Luminari S, Cesaretti M, Marcheselli L, et al. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol 2010;21:855-9. [Crossref] [PubMed]
- Liu H, Ye H, Ruskone-Fourmestraux A, et al. T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology 2002;122:1286-94. [Crossref] [PubMed]
- Wöhrer S, Troch M, Streubel B, et al. MALT lymphoma in patients with autoimmune diseases: a comparative analysis of characteristics and clinical course. Leukemia 2007;21:1812-8. [Crossref] [PubMed]
- Thieblemont C, Cascione L, Conconi A, et al. A MALT lymphoma prognostic index. Blood 2017;130:1409-17. [Crossref] [PubMed]
- Thieblemont C, Berger F, Dumontet C, et al. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood 2000;95:802-6. [Crossref] [PubMed]
- Zucca E, Conconi A, Martinelli G, et al. Final Results of the IELSG-19 randomized trial of mucosa-associated lymphoid tissue lymphoma: improved event-free and progression-free survival with rituximab plus chlorambucil versus either chlorambucil or rituximab monotherapy. J Clin Oncol 2017;35:1905-12. [Crossref] [PubMed]
- Tsang RW, Gospodarowicz MK, Pintilie M, et al. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol 2003;21:4157-64. [Crossref] [PubMed]
- Zinzani PL, Stefoni V, Musuraca G, et al. Fludarabine-containing chemotherapy as frontline treatment of nongastrointestinal mucosa-associated lymphoid tissue lymphoma. Cancer 2004;100:2190-4. [Crossref] [PubMed]
- Goda JS, Gospodarowicz M, Pintilie M, et al. Long-term outcome in localized extranodal mucosa-associated lymphoid tissue lymphomas treated with radiotherapy. Cancer 2010;116:3815-24. [Crossref] [PubMed]
- Yahalom J, Illidge T, Specht L, et al. Modern radiation therapy for extranodal lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 2015;92:11-31. [Crossref] [PubMed]
- Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol 2011;100:86-92. [Crossref] [PubMed]
- Hoskin PJ, Kirkwood AA, Popova B, et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): a randomised phase 3 non-inferiority trial. Lancet Oncol 2014;15:457-63. [Crossref] [PubMed]
- Desai A, Joag MG, Lekakis L, et al. Long-term course of patients with primary ocular adnexal MALT lymphoma: a large single-institution cohort study. Blood 2017;129:324-32. [Crossref] [PubMed]
- Arcaini L, Burcheri S, Rossi A, Passamonti F, et al. Nongastric marginal-zone B-cell MALT lymphoma: prognostic value of disease dissemination. Oncologist 2006;11:285-91. [Crossref] [PubMed]
- Raderer M, Wöhrer S, Streubel B, et al. Assessment of disease dissemination in gastric compared with extragastric mucosa-associated lymphoid tissue lymphoma using extensive staging: a single-center experience. J Clin Oncol 2006;24:3136-41. [Crossref] [PubMed]
- de Boer JP, Hiddink RF, Raderer M, et al. Dissemination patterns in non-gastric MALT lymphoma. Haematologica 2008;93:201-6. [Crossref] [PubMed]
- Wöhrer S, Streubel B, Bartsch R, et al. Monoclonal immunoglobulin production is a frequent event in patients with mucosa-associated lymphoid tissue lymphoma. Clin Cancer Res 2004;10:7179-81. [Crossref] [PubMed]
- Economopoulos T, Papageorgiou S, Pappa V, et al. Monoclonal gammopathies in B-cell non-Hodgkin's lymphomas. Leuk Res 2003;27:505-8. [Crossref] [PubMed]
- Asatiani E, Cohen P, Ozdemirli M, et al. Monoclonal gammopathy in extranodal marginal zone lymphoma (ENMZL) correlates with advanced disease and bone marrow involvement. Am J Hematol 2004;77:144-6. [Crossref] [PubMed]
- Luminari S, Merli M, Rattotti S, et al. Early progression as a predictor of survival in marginal zone lymphomas: an analysis from the FIL-NF10 study. Blood 2019;134:798-801. [Crossref] [PubMed]
- Jaso J, Chen L, Li S, et al. CD5-positive mucosa-associated lymphoid tissue (MALT) lymphoma: a clinicopathologic study of 14 cases. Hum Pathol 2012;43:1436-43. [Crossref] [PubMed]
- Deutsch AJA, Steinbauer E, Hofmann NA, et al. Chemokine receptors in gastric MALT lymphoma: loss of CXCR4 and upregulation of CXCR7 is associated with progression to diffuse large B-cell lymphoma. Mod Pathol 2013;26:182-94. [Crossref] [PubMed]
- Raderer M, Streubel B, Woehrer S, et al. High relapse rate in patients with MALT lymphoma warrants lifelong follow-up. Clin Cancer Res 2005;11:3349-52. [Crossref] [PubMed]
- Lévy M, Copie-Bergman C, Gameiro C, et al. Prognostic value of translocation t(11;18) in tumoral response of low-grade gastric lymphoma of mucosa-associated lymphoid tissue type to oral chemotherapy. J Clin Oncol 2005;23:5061-6. [Crossref] [PubMed]
- Sagaert X, de Paepe P, Libbrecht L, et al. Forkhead box protein P1 expression in mucosa-associated lymphoid tissue lymphomas predicts poor prognosis and transformation to diffuse large B-cell lymphoma. J Clin Oncol 2006;24:2490-7. [Crossref] [PubMed]
- Liu H, Ye H, Dogan A, et al. T(11;18)(q21;q21) is associated with advanced mucosa-associated lymphoid tissue lymphoma that expresses nuclear BCL10. Blood 2001;98:1182-7. [Crossref] [PubMed]
- Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol 2015;33:2516-22. [Crossref] [PubMed]
- Seymour JF, Marcus R, Davies A, et al. Association of early disease progression and very poor survival in the GALLIUM study in follicular lymphoma: benefit of obinutuzumab in reducing the rate of early progression. Haematologica 2019;104:1202-8. [Crossref] [PubMed]
- Conconi A, Thieblemont C, Cascione L, et al. Early progression of disease predicts shorter survival in MALT lymphoma patients receiving systemic treatment. Haematologica 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Albano D, Bosio G, Camoni L, et al. Prognostic role of baseline 18 F-FDG PET/CT parameters in MALT lymphoma. Hematol Oncol 2019;37:39-46. [Crossref] [PubMed]
- Qi S, Huang MY, Yang Y, et al. Uptake of [(18)F]fluorodeoxyglucose in initial positron-emission tomography predicts survival in MALT lymphoma. Blood Adv 2018;2:649-55. [Crossref] [PubMed]
- Vaxman I, Bernstine H, Kleinstern G, et al. FDG PET/CT as a diagnostic and prognostic tool for the evaluation of marginal zone lymphoma. Hematol Oncol 2019;37:168-75. [Crossref] [PubMed]
- Park JH, Kim S, Ryu JS, et al. Complete metabolic response (CMR) in positron emission tomography-computed tomography (PET-CT) scans may have prognostic significance in patients with marginal zone lymphomas (MZL). Hematol Oncol 2018;36:56-61. [Crossref] [PubMed]
- Trotman J, Barrington SF, Belada D, et al. Prognostic value of end-of-induction PET response after first-line immunochemotherapy for follicular lymphoma (GALLIUM): secondary analysis of a randomised, phase 3 trial. Lancet Oncol 2018;19:1530-42. [Crossref] [PubMed]
- Trotman J, Fournier M, Lamy T, et al. Positron emission tomography-computed tomography (PET-CT) after induction therapy is highly predictive of patient outcome in follicular lymphoma: analysis of PET-CT in a subset of PRIMA trial participants. J Clin Oncol 2011;29:3194-200. [Crossref] [PubMed]
- Arcaini L, Lazzarino M, Colombo N, et al. Splenic marginal zone lymphoma: a prognostic model for clinical use. Blood 2006;107:4643-9. [Crossref] [PubMed]
- Arcaini L, Paulli M, Burcheri S, et al. Primary nodal marginal zone B-cell lymphoma: clinical features and prognostic assessment of a rare disease. Br J Haematol 2007;136:301-4. [Crossref] [PubMed]
- Montalbán C, Abraira V, Arcaini L, et al. Risk stratification for Splenic Marginal Zone Lymphoma based on haemoglobin concentration, platelet count, high lactate dehydrogenase level and extrahilar lymphadenopathy: development and validation on 593 cases. Br J Haematol 2012;159:164-71. [Crossref] [PubMed]
- Jaso JM, Yin CC, Wang SA, et al. Clinicopathologic features of CD5-positive nodal marginal zone lymphoma. Am J Clin Pathol 2013;140:693-700. [Crossref] [PubMed]
- Heilgeist A, McClanahan F, Ho AD, et al. Prognostic value of the Follicular Lymphoma International Prognostic Index score in marginal zone lymphoma: an analysis of clinical presentation and outcome in 144 patients. Cancer 2013;119:99-106. [Crossref] [PubMed]
- Perrone S, D'Elia GM, Annechini G, et al. Splenic marginal zone lymphoma: Prognostic factors, role of watch and wait policy, and other therapeutic approaches in the rituximab era. Leuk Res 2016;44:53-60. [Crossref] [PubMed]
- Kalpadakis C, Pangalis GA, Sachanas S, et al. Rituximab monotherapy in splenic marginal zone lymphoma: prolonged responses and potential benefit from maintenance. Blood 2018;132:666-70. [Crossref] [PubMed]
- Florindez J, Alderuccio JP, Lossos IS. Splenic marginal zone lymphoma: a U.S population-based survival analysis (1997-2016). Blood 2019;134:4014. [Crossref]
- Lenglet J, Traullé C, Mounier N, et al. Long-term follow-up analysis of 100 patients with splenic marginal zone lymphoma treated with splenectomy as first-line treatment. Leuk Lymphoma 2014;55:1854-60. [Crossref] [PubMed]
- Montalban C, Abraira V, Arcaini L, et al. Simplification of risk stratification for splenic marginal zone lymphoma: a point-based score for practical use. Leuk Lymphoma 2014;55:929-31. [Crossref] [PubMed]
- Albano D, Giubbini R, Bertagna F. 18F-FDG PET/CT in splenic marginal zone lymphoma. Abdom Radiol (NY) 2018;43:2721-7. [Crossref] [PubMed]
- Rossi D, Trifonov V, Fangazio M, et al. The coding genome of splenic marginal zone lymphoma: activation of NOTCH2 and other pathways regulating marginal zone development. J Exp Med 2012;209:1537-51. [Crossref] [PubMed]
- Kiel MJ, Velusamy T, Betz BL, et al. Whole-genome sequencing identifies recurrent somatic NOTCH2 mutations in splenic marginal zone lymphoma. J Exp Med 2012;209:1553-65. [Crossref] [PubMed]
- Parry M, Rose-Zerilli MJ, Ljungström V, et al. Genetics and prognostication in splenic marginal zone lymphoma: revelations from deep sequencing. Clin Cancer Res 2015;21:4174-83. [Crossref] [PubMed]
- Arribas AJ, Rinaldi A, Mensah AA, et al. DNA methylation profiling identifies two splenic marginal zone lymphoma subgroups with different clinical and genetic features. Blood 2015;125:1922-31. [Crossref] [PubMed]
- Salido M, Baró C, Oscier D, et al. Cytogenetic aberrations and their prognostic value in a series of 330 splenic marginal zone B-cell lymphomas: a multicenter study of the Splenic B-Cell Lymphoma Group. Blood 2010;116:1479-88. [Crossref] [PubMed]
- van den Brand M, van der Velden WJFM, Diets IJ, et al. Clinical features of patients with nodal marginal zone lymphoma compared to follicular lymphoma: similar presentation, but differences in prognostic factors and rate of transformation. Leuk Lymphoma 2016;57:1649-56. [Crossref] [PubMed]
- Starr AG, Caimi PF, Fu P, et al. Dual institution experience of nodal marginal zone lymphoma reveals excellent long-term outcomes in the rituximab era. Br J Haematol 2016;175:275-80. [Crossref] [PubMed]
- Meyer AH, Stroux A, Lerch K, et al. Transformation and additional malignancies are leading risk factors for an adverse course of disease in marginal zone lymphoma. Ann Oncol 2014;25:210-5. [Crossref] [PubMed]
- Montoto S, Fitzgibbon J. Transformation of indolent B-cell lymphomas. J Clin Oncol 2011;29:1827-34. [Crossref] [PubMed]
- Starr AG, Caimi PF, Fu P, et al. Dual institution experience of extranodal marginal zone lymphoma reveals excellent long-term outcomes. Br J Haematol 2016;173:404-12. [Crossref] [PubMed]
- Maeshima AM, Taniguchi H, Toyoda K, et al. Clinicopathological features of histological transformation from extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue to diffuse large B-cell lymphoma: an analysis of 467 patients. Br J Haematol 2016;174:923-31. [Crossref] [PubMed]
- Xing KH, Kahlon A, Skinnider BF, et al. Outcomes in splenic marginal zone lymphoma: analysis of 107 patients treated in British Columbia. Br J Haematol 2015;169:520-7. [Crossref] [PubMed]
- Conconi A, Franceschetti S, Aprile von Hohenstaufen K, et al. Histologic transformation in marginal zone lymphomas†. Ann Oncol 2015;26:2329-35. [Crossref] [PubMed]
- Dungarwalla M, Appiah-Cubi S, Kulkarni S, et al. High-grade transformation in splenic marginal zone lymphoma with circulating villous lymphocytes: the site of transformation influences response to therapy and prognosis. Br J Haematol 2008;143:71-4. [Crossref] [PubMed]
- Camacho FI, Mollejo M, Mateo MS, et al. Progression to large B-cell lymphoma in splenic marginal zone lymphoma: a description of a series of 12 cases. Am J Surg Pathol 2001;25:1268-76. [Crossref] [PubMed]
- Wagner-Johnston ND, Link BK, Byrtek M, et al. Outcomes of transformed follicular lymphoma in the modern era: a report from the National LymphoCare Study (NLCS). Blood 2015;126:851-7. [Crossref] [PubMed]
- Montoto S, Davies AJ, Matthews J, et al. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol 2007;25:2426-33. [Crossref] [PubMed]
- Conconi A, Ponzio C, Lobetti-Bodoni C, et al. Incidence, risk factors and outcome of histological transformation in follicular lymphoma. Br J Haematol 2012;157:188-96. [Crossref] [PubMed]
- Sarkozy C, Trneny M, Xerri L, et al. Risk factors and outcomes for patients with follicular lymphoma who had histologic transformation after response to first-line immunochemotherapy in the PRIMA trial. J Clin Oncol 2016;34:2575-82. [Crossref] [PubMed]
- Casulo C, Friedberg J. Transformation of marginal zone lymphoma (and association with other lymphomas). Best Pract Res Clin Haematol 2017;30:131-8. [Crossref] [PubMed]
- Palmedo G, Hantschke M, Rütten A, et al. Primary cutaneous marginal zone B-cell lymphoma may exhibit both the t(14;18)(q32;q21) IGH/BCL2 and the t(14;18)(q32;q21) IGH/MALT1 translocation: an indicator for clonal transformation towards higher-grade B-cell lymphoma? Am J Dermatopathol 2007;29:231-6. [Crossref] [PubMed]
- Else M, Marín-Niebla A, de la Cruz F, et al. Rituximab, used alone or in combination, is superior to other treatment modalities in splenic marginal zone lymphoma. Br J Haematol 2012;159:322-8. [Crossref] [PubMed]
- Mateo M, Mollejo M, Villuendas R, et al. 7q31-32 allelic loss is a frequent finding in splenic marginal zone lymphoma. Am J Pathol 1999;154:1583-9. [Crossref] [PubMed]
- Vega F, Cho-Vega JH, Lennon PA, et al. Splenic marginal zone lymphomas are characterized by loss of interstitial regions of chromosome 7q, 7q31.32 and 7q36.2 that include the protection of telomere 1 (POT1) and sonic hedgehog (SHH) genes. Br J Haematol 2008;142:216-26. [Crossref] [PubMed]
- Qian L, Soderquist C, Schrank-Hacker A, et al. Deletion 20q12 is associated with histological transformation of nodal marginal zone lymphoma to diffuse large B-cell lymphoma. Am J Hematol 2020;95:238-44. [Crossref] [PubMed]
- Al-Tourah AJ, Gill KK, Chhanabhai M, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin's lymphoma. J Clin Oncol 2008;26:5165-9. [Crossref] [PubMed]
- Noy A, Schöder H, Gönen M, et al. The majority of transformed lymphomas have high standardized uptake values (SUVs) on positron emission tomography (PET) scanning similar to diffuse large B-cell lymphoma (DLBCL). Ann Oncol 2009;20:508-12. [Crossref] [PubMed]
- Ngeow JYY, Quek RHH, Ng DCE, et al. High SUV uptake on FDG–PET/CT predicts for an aggressive B-cell lymphoma in a prospective study of primary FDG–PET/CT staging in lymphoma. Ann Oncol 2009;20:1543-7. [Crossref] [PubMed]
- Matolcsy A, Inghirami G, Knowles DM. Molecular genetic demonstration of the diverse evolution of Richter's syndrome (chronic lymphocytic leukemia and subsequent large cell lymphoma). Blood 1994;83:1363-72. [Crossref] [PubMed]
- Lossos IS, Gascoyne RD. Transformation of follicular lymphoma. Best Pract Res Clin Haematol 2011;24:147-63. [Crossref] [PubMed]
- Villa D, Crump M, Panzarella T, et al. Autologous and allogeneic stem-cell transplantation for transformed follicular lymphoma: a report of the Canadian blood and marrow transplant group. J Clin Oncol 2013;31:1164-71. [Crossref] [PubMed]
- Ban-Hoefen M, Vanderplas A, Crosby-Thompson AL, et al. Transformed non-Hodgkin lymphoma in the rituximab era: analysis of the NCCN outcomes database. Br J Haematol 2013;163:487-95. [Crossref] [PubMed]
- Avivi I, Arcaini L, Ferretti VV, et al. High-dose therapy and autologous stem cell transplantation in marginal zone lymphomas: a retrospective study by the EBMT Lymphoma Working Party and FIL-GITMO. Br J Haematol 2018;182:807-15. [Crossref] [PubMed]
- Yuen AR, Kamel OW, Halpern J, et al. Long-term survival after histologic transformation of low-grade follicular lymphoma. J Clin Oncol 1995;13:1726-33. [Crossref] [PubMed]
Cite this article as: Alderuccio JP, Lossos IS. Prognostic factors and risk of transformation in marginal zone lymphoma. Ann Lymphoma 2020;4:6.


