
Minimal residual disease (MRD) in mantle cell lymphoma
Introduction
Mantle cell lymphoma (MCL) is a rare, aggressive non-Hodgkin lymphoma (NHL) characterized by considerable biological and clinical heterogeneity. It is usually associated to the t (11;14) translocation leading to an overexpression of cyclin D1. Though survival has improved, MCL is still considered incurable with frequent relapses and a shorter OS compared to most NHL entities. A number of baseline clinical histological and biological predictors have been identified including MIPI, proliferation index, blastoid histology and tp53 and KMT2D mutations (1). In recent years, minimal residual disease (MRD) detection has gained considerable interest as a post-treatment outcome predictor.
Several methods have proved useful for MRD monitoring in the context of indolent lymphomas. These will be described in the following section. Different techniques for MRD detection have been used in different hematological disorders, not only because of different performances in terms of applicability, accuracy, sensitivity and specificity but also with respect to disease specifications and the availability of diagnostic material with enough tumor cells to identify an MRD marker. Currently there is no single technique that could be considered optimal in any disease and any clinical context (2,3). However, at the present time, real time quantitative PCR (RQ-PCR) is considered the “gold standard” for follicular lymphoma (FL) and MCL.
A criticism to MRD detection in lymphoma has been the supposed “localized nature” of most lymphomas, which could hamper a successful detection of residual tumors in “liquid” tissues such as peripheral blood (PB) and/or bone marrow (BM). This hypothesis is only partly justified in MCL as this neoplasm substantially invades PB or BM in >90% of all cases (4,5). Moreover, a large bulk of data have demonstrated that even apparently localized relapses, are often heralded by signals of disease activity in PB or BM (Table 1). Of course, integration of imaging tools such as Positron Emission Tomography (PET) and MRD tools is a major field of interest, that could allow an even more complete characterization of these complex entities (26).
Table 1
| Study | Disease | Patients | Therapy | Tissue analyzed | Method | Marker | Clinical impact of MRD |
|---|---|---|---|---|---|---|---|
| Howard et al. 2002 (6) | MCL, untreated younger and elderly | 40 | R-CHOP | BM,PB | N-PCR | BCL1, IGH | MRD status doesn’t impact PFS (16.5 vs. 18.8 M, P=0.51). |
| Corradini et al. 2004 (7) | FL, MCL, untreated younger | 35 | R-HD + ASCT | BM, PB, Harvest | N-PCR | BLC2, IGH | 75 M Relapse incidence: 88% MRD+ vs. 8% MRD− |
| Pott et al. 2006 (8) | MCL, untreated younger | 29 | R-HD + TBI + ASCT | BM, PB, Harvest | RQ-PCR | IGH | Median PFS 92 M vs. 21 M (P<0.001) |
| Geisler et al. 2008 (9) | MCL, untreated younger | 79 | RmaxiCHOP/R-HDAraC + ASCT | BM, PB | N-PCR | BCL1, IGH | Median PFS: NR vs. 18 M (P<0.001) |
| Andersen et al. 2009 (10) | MCL, R/R younger | 78 | RmaxiCHOP/R-HDAraC + ASCT - + R pre-emptive | BM, PB | N-PCR, RQ-PCR | BCL1, IGH | Median RFS 43 M after pre-emptive treatment |
| Pott et al. 2010 (11) | MCL, untreated younger and elderly | 190 | R-CHOP + TBI + ASCT vs. R-CHOP/R-DHAP + R-HDAraC + TBI + ASCT (younger); R-CHOP vs. R-FC (elderly) | BM, PB, Harvest | RQ-PCR | BCL1, IGH | 24 M PFS 77% MRD− vs. 34% MRD+ (P<0.021) |
| Liu et al. 2012 (12) | MCL, untreated younger | 39 | R-HD-MTX + maxi-CHOP + ASCT + R maintenance | BM, PB | RQ-PCR | BCL1, IgH | 36 M TTP: 82% MRD+ vs. 48% MRD− (MRD at EOI) |
| Pott et al. 2011 (13) | MCL, untreated younger | 406 | R-CHOP + TBI + ASCT vs. R-CHOP/R-DHAP + R-HDAraC + TBI + ASCT (younger); R-CHOP vs. R-FC (elderly) | PB | RQ-PCR | BCL1, IGH | Median PFS 12 M: 5.8 Y MRD− vs. 3 Y MRD+; at 24 M: NR MRD− vs. 3.4 YMRD +; at 36 M: NR MRD− vs. 3.8 Y MRD+ (P<0.0001) |
| Visco et al. 2015 (14) | MCL, untreated elderly | 46 | R-BAC500 | BM,PB | N-PCR | BCL1, IgH | MRD status doesn’t impact PFS/OS but the number of events was still low |
| Callanan et al. 2015 (15) | MCL, untreated younger | 178 | R-DHAP + R-BEAM + ASCT + R maintenance | BM, PB | RQ-PCR | BCL1, IGH | 36 M PFS without R maintenance 61.6% MRD+ vs. 83.9% MRD− (P=0.011); With R maintenance 86.2% MRD+ vs. 91.8% MRD− (P=0.011) |
| Kolstad et al. 2017 (16) | MCL, untreated younger | 183 | RmaxiCHOP + HDAraC + /-Zevalin + ASCT | BM, PB | RQ-PCR, N-PCR | BCL1, IGH | Median PFS: 20 M MRD+ vs. 142 M MRD− post ASCT (P<0.0001) |
| Kaplan et al. 2018 (17) | MCL, untreated younger | 42 | CHOP + MTX + EAR + CBV-ASCT + bortezomib consolidation vs. maintenance | BM | PCR | BCL1, IGH | 8 PFS: 80% MRD− vs. 43.2% MRD+ (post induction) (P=0.009) |
| Ferrero et al. 2018 (18) | MCL, untreated younger and elderly | 163 | 3RCHOP + HDC + ASCT + /- Lenalidomide maintenance | BM, PB | N-PCR, RQ-PCR | BCL1, IGH | 36 M PFS: 25% MRD+ vs. 66% MRD− (after ASCT) (P=0.037) |
| Klener et al. 2018 (19) | MCL, untreated elderly | 67 | R-CHOP/R-ARAC + R maintenance | BM, PB | RQ-PCR | BCL1, IGH | MRD status doesn’t impact PFS/OS |
| Hermine et al. 2016 (20) | MCL, untreated younger | 497 | R-CHOP + TBI + HD CTX + ASCT vs. R-CHOP/R-DHAP + AraC + TBI + Mel + ASCT | BM, PB | RQ-PCR | Unspecified | EOI MRD neg: 4,796 vs. 7,996 (PB), 2,696 vs. 6,196 (BM) |
| Zaja et al. 2017 (21) | MCL, untreated younger and elderly | 42 | R2B + R2 consolidation + Len maintenance | BM, PB | N-PCR, RQ-PCR | BCL1, IGH | 36% of MRD negativization, predictive of PFS |
| Albertsson-Lindblad et al. 2016 (22) | MCL, untreated elderly | 51 | Len - BR x6 + Len maintenance | BM, PB | N-PCR | BCL1, IGH | EOI MRD neg: 32% |
| Gressin et al. 2019 (23) | MCL, untreated elderly | 76 | RiBVD | BM, PB | RQ-PCR | IGH | MDR neg: 83% (PB) and 74% (BM) at 6 months |
| Armand et al. 2016 (24) | MCL, untreated younger | 23 | BR + R-HD-ARA-C | PB, plasma | NGS | IGH | 93% MRD neg at EOT |
| Le Gouill et al. 2019 (25) | MCL, untreated younger | 83 | GA-DHAP + GA-BEAM + ASCT + GA maintenance | BM, PB | RQ-PCR, ddPCR | IGH | After the end of induction, qPCR showed that 75% of patients were MRD negative in BM, while the ddPCR showed it to be 85% of patients |
ASCT, autologous stem cell transplant; BEAM, carmustine, etoposide, cytarabine, melphalan; BM, bone marrow; BR, bendamustine, rituximab; CBV, cyclophosphamide, carmustine, etoposide; CR, complete remissione; ddPCR, digital droplet- PCR; EAR, etoposide, cytarabine, rituximab; EOI, end of induction; FFR, failure free survival; FL, follicular lymphoma; G, obinutuzumab; HDS, high dose scheme; M, month; Y, year; MCL, mantle cell lymphoma; MTX, methotrexate; N-PCR, nested-PCR; NGS, next generation sequencing; PFS, progression free survival; NR, not reached; PB, peripheral blood; PD, progression disease; PR, partial remission; R/R, relapse/refractory; R-CHOP, rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin, prednisone; R-HDAraC, rituximab, high dose cytarabine; R-DHAP, dexamethasone, high dose cytarabine, cisplatin; RTX, rituximab; RQ-PCR, quantitative real time polymerase chain reaction; SD, stable disease; TBI, total body irradiation.
From a historical point of view, it should be noted that the first studies in the field date back to the last decade of the previous millennium (27-29). In the last two decades we had witnessed remarkable development in therapeutic strategies and progressive improvement in diagnostic techniques, which have acquired greater robustness, accuracy, applicability and standardization. These have been achieved thanks to both intrinsic technical progress and collaborative efforts for standardization (2,30,31).
This review will focus on the description of the methods available for MRD monitoring in MCL and will discuss their strengths and weaknesses. Some exhaustive manuscripts on similar subjects were written in the past (4,32). This review will integrate the recent availability of novel data with more established knowledge. Finally, we will examine the state of the art on the application of MRD in modern era and we will discuss its use in relation to new biological therapies.
Methods
Methods for MRD determination
Several approaches have been employed to detect the presence of lymphoma cells in BM or PB to assess lymphoma infiltration or to determine residual tumor burden during and after treatment (Table 2). These methods vary in terms of sensitivity, specificity, accuracy of target quantification, potential technical biases and level of standardization between different laboratories (30-33). This chapter will cover the most widely used methods i.e., flow cytometry (FC) and molecular-based tools, including polymerase chain reaction (PCR) based approaches, as well as the more recent next generation sequencing (HTS) based approaches. Both FC-based and molecular methods have considerably improved over the last decade with a substantial gain in their performance and sensitivity (30,31,34,35). In MCL, most clinical studies have used real-time quantitative PCR (RQ-PCR), which is currently the “gold standard” in this setting. Nevertheless, comparative studies are in progress and could lead to a paradigm shift with progressive implementation of novel next-generation PCR approaches, improved FC and HTS tools (4,30-32).
Table 2
| Method | MFC | Consensus PCR | Nested-PCR | RQ-PCR | Droplet digital PCR | HTS |
|---|---|---|---|---|---|---|
| Aim to study | Immunophenotype | IGH rearrangement or t(11;14) | IGH rearrangement or t(11;14) | IGH rearrangement or t(11;14) | IGH rearrangement or t(11;14) | IGH rearrangement |
| Method sensitivity limit | 10−3 to 10−4 (4-color MFC); 10−4 (8-color MFC) | IGH: 10−2 to 10−3; t(11;14): 10−4 | 10−5 | 10−5 | 10−5 | theoretically Up to 10−6 (dependent on DNA amount) |
| Information type | Quantitative | Qualitative | Qualitative | Quantitative (above limit of quantification) | Quantitative (above limit of quantification) | Quantitative (above limit of quantification) |
| Patient-specific PCR primer needed | Not applicable | No | Depending on approach | Yes | Yes | No |
| Method applicability for advanced stages of disease | >85% | >95% | >85% | >85% | At least as RQ-PCR possibly better | No data yet |
| Expertise | High for 6-8-color MFC | Lower | High | High | High | High |
| Method Standardization | No | No | No | Yes | Ongoing | No |
| Turnaround time | 3–4 h | 3–4 h | Dependent on method; mostly 3–4 h | 2 weeks | 2 weeks | 1 week |
| Advantages | Rapid quantification | Rapid | No establishment of serial dilution for quantification needed | High sensitivity | High sensitivity | High sensitivity |
| Greatest interlaboratory reproducibility | Absolute quantification method. Less “positive non-quantifiable” cases compared to RQ-PCR | Independent of patient specific primers | ||||
| Multilab standardization | Higher tolerance to different types of inhibitors | Additional information on background B-cell repertoire | ||||
| Disadvantages | Low sensitivity | Low sensitivity | Not standardized | ASO primer design necessary | ASO primer design necessary | Super multiplex PCR (disproportional target amplification) |
| Expertise needed for evaluation | Unspecific amplification possible | Not quantitative | Time consuming workflow | Time consuming workflow | Complex bioinformatic evaluation | |
| Not standardized | No quantification | need for a standard curve | Discrimination from normal cell background (requires about 5% tumor cell infiltration) |
MRD, minimal residual disease; MCL, mantle cell lymphoma.
MRD detection by FC
Flow cytometry is a method routinely used in the diagnosis of blood disorders. It is based on the determination of immunophenotypic aberrations and the detection of the restriction of the immunoglobulin light chain which is a key marker of clonality among B-cell populations. It is faster and more broadly available compared to PCR or sequencing methods. Therefore, it is an appealing method for MRD detection (5,36). Unfortunately, in MCL, to our knowledge, there are no validated panels for MRD detection which are standardized at the multilaboratory level. A major obstacle is its high immunophenotypic heterogeneity, requiring more widespread marker combinations for high sensitivity MRD detection. Large studies such as the European MCL Network MCL Younger and Elderly trials, have shown that more than 85% of patients with MCL with Ann Arbor in stages II to IV at diagnosis have FC disease detectable disease in PB or BM (20,37).
In MCL the sensitivity of conventional 4-color-flow-MRD reaches 10−4 and is comparable to that of IGH-PCR methods at initial lymphoma staging but is less sensitive at follow-up after immunochemotherapy, with a substantial number of samples (18%) being positive by PCR and not by FC (36). A recent publication showed that a single, 8-colors 10-antibody MFC tube permits specific MRD evaluation with a robust sensibility of 0.01% in all patients (38); using the 0.01% cut-off level, MFC detected MRD in only 80% of patients who were MRD positive by real-time quantitative PCR (RQ-PCR) (4,38).
The Euro Flow consortium of the European Scientific foundation for Laboratory Hemato Oncology (ESLHO) currently develops standards for instrument set-up, panel composition and data interpretation (39,40) and a quality control program for MFC based MRD detection in various haematological malignancies (41,42). For MRD purposes it is become fundamental to be able to take advantage of very low levels of MRD. Therefore, optimized MFC strategies are required that use highly effective antibody panels and new bioinformatics tools to evaluate a greater number of cells to reach a sensitivity comparable or even higher than that of RQ-PCR. Moreover, these assays need to be validated in the context of clinical trials with respect to applicability and prognostic impact to prove their value as novel MRD tools for MCL patients.
A next generation flow (NGF) approach has been developed for highly sensitive and standardized detection of MRD in multiple myeloma (MM) by the Euro Flow consortium. This approach takes advantage of innovative tools and procedures recently developed by the consortium for sample preparation, antibody panel construction and automatic identification of plasma cells (43). This fully standardized approach for MRD detection in MM overcomes the major limitations of conventional flow-MRD methods and is ready for implementation in routine diagnostics. Comparable approaches for MRD detection in MCL are underway and might further improve the field of flow-MRD detection (44).
MRD detection by PCR-based methods (RQ-PCR)
Methods based on quantitative PCR are the methods of choice for MRD detection because they are sensitive, standardized and validated in large multicenter trials. They explore the persistence of residual tumor cells by amplifying the lymphoma unique genotype on a sensitive level (32,33).
There are two types of genetic hallmarks in lymphomas that can be used for MRD detection: tumor specific translocations and antigen receptor rearrangements.
The most broadly applicable marker for MRD studies in malignant B-cell lymphomas is the immunoglobulin heavy chain gene rearrangement (IGH) that is detectable in more than 80–95% of B-cell neoplasia. Consensus PCR strategies using consensus VH and JH region primers have a detection limit of about 1–2% lymphoma cells in a polyclonal background and by this are limited in their suitability for MRD detection. Sequencing of the junctional region of rearranged IGH genes allows the identification of tumor specific VH-DH-JH rearrangement and by this an allele-specific (ASO) primer design for a real-time quantitative (RQ)-PCR approach. In MCL, clonal IGHV rearrangements are detectable in >90% of MCL patients (4) and are therefore the most frequently used MRD target.
Structural chromosomal translocations are characteristic for histological subtypes of mature lymphoid malignancies as t(14;18) in FL and t(11;14) in MCL and can also serve as PCR targets for clonality assessment and MRD detection (Figure 1). In MCL, the characteristic chromosomal translocation t(11;14) involves a region of 360 kb 5’ of the cyclin D1 (CCND1) gene, in nearly 35% of these cases the breakpoints on chromosome 11 cluster within an 85 bp region referred to as major translocation cluster region (BCL1-MTC). Chromosomal translocations are ideal PCR targets because of a high stability and the lack of somatic mutations (45).
Despite being present in the vast majority of MCL patients and detectable by FISH, breakpoints in the BCL1-MTC region scatter up to 2 kb downstream of the MTC region resulting in only 35% of PCR detectable t(11;14) translocations. Recently, the application of the target Locus Amplification (TLA) methodology proved able to identify a suitable MRD target derived from the t(11;14) in almost 80% of patients holding promise for a wider application of this target in MCL patients (46).
One major problem for MRD quantification in lymphoma in contrast to acute leukemia is the non-universal presence of a substantial tumor infiltration of the sample used for target identification. For generating a highly sensitive and quantitative serial dilution, lymphoma cell infiltration is ideally >5% of MCL cells. First approaches to PCR-based detection of MRD were based on qualitative endpoint amplification approaches and particularly on nested-PCR (27,28,47). These approaches proved informative but had a number of technical biases, including high risk of contamination, and appeared less predictive when compared head to head with second generation approaches such as RQ-PCR (18).
One of the main technical advances in the detection of MRD in lymphoid tumors has been the development and standardization of RQ-PCR tools (Figure 2) (48,49). RQ-PCR is robust, accurate and reproducible and substantially minimizes the risk of contamination due to the allele-specific approach.
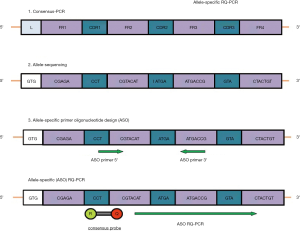
Qualitative nested-PCR or RQ-PCR using ASO primers for IGH rearrangement or t(11;14) achieve reproducible detection limits of 1 MCL cell among up to 100,000 white cells (10−5). Moreover, the value of RQ-PCR has been further increased by the development of multi-laboratory standardization efforts, which allowed to reach a very high level of reproducibility among different MRD laboratories. The standardization of MRD assessment and the conduction of regular quality controls is essential to ensure high interlaboratory comparability of MRD that could represent the basis for MRD-driven treatment. This effort was originally undertaken in Europe in the context of the Euro-MRD consortium (a subgroup of ESHLO) for patients with acute lymphoblastic leukemia (50). In the last decade, the standardization effort of Euro-MRD has also been applied mature lymphoid malignancies, specifically to MCL. Currently at least one major phase multicenter randomized phase III trial is running in Europe, with standardized MRD assessment performed in several different laboratory of the Euro-MRD consortium (EUDRACT-NR. 2014-001363-12).
Notwithstanding its benefits, RQ-PCR also has some limitations. At first, it is not an absolute quantification tool, as it is based on a standard curve obtained from samples with known amounts of target DNA. As allele-specific primers are used for each patient, each requires an individual standard curve, which is a laborious procedure. Second, for technical reasons, quantification is limited to 10−4, resulting specifically after treatment in numerous samples that cannot be fully quantified and are defined as “non-quantifiable positives” (PNQs) (2,51). RQ-PCR is also sensitive to PCR inhibitors that can influence amplification kinetics and target quantification. In recent years, digital droplet PCR (ddPCR) has been able to overcome some of these limitations (30). ddPCR is an absolute quantification method based on Poisson’s statistics and because is based on endpoint amplification it is less sensitive to PCR inhibitors. The levels of sensitivity of ddPCR are comparable to qPCR and has the potential to overcome and quantify a substantial part of the cases classified as PNQ by RQ-PCR (30). While very promising from a technical point of view, ddPCR still has to prove to be predictive as RQ-PCR in the framework of large multi-center studies in MCL (4). This is currently undertaken by the Euro-MRD consortium in cooperation with the EMCL study group.
MRD detection by next generation sequencing
New high-throughput molecular biology technologies may provide a new approach to MRD detection that may outweigh some of the disadvantages of classical ASO-RQ-PCR-based MRD approaches (31). The new methodologies are based on a high-throughput (HTS) sequencing of the clonal IGHV rearrangement (Figure 3). This step bypasses the time-consuming laboratory steps of designing and testing patient-specific assays and is more specific than the RQ-PCR reading. A comparative analysis of our groups addressing the potential of to overcome some of the limitations of ASO-RQ-PCR have shown that both methods have comparable sensitivity and HTS has potential for further increasing sensitivity and specificity (31).
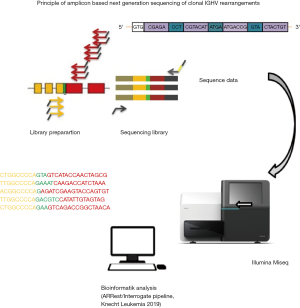
The first step of HTS-based MRD-detection is a multiplex PCR for amplification of V-D-J rearrangements of IG or TR genes. This is followed by a second-round PCR with barcoded primers for library preparation and subsequent high-throughput sequencing. The crucial step is then the correct identification of the index sequence identifying the tumour specific IG/TR rearrangement. In contrast to RQ-PCR, the laborious design and testing of patient-specific assays is avoided as the same multiplex approach is employed in follow-up samples, with re-identification of the index sequence, allowing for MRD quantification. However, this requires a well-established bioinformatics approach. As shown in ALL, 5% frequency cut-off is used to allocate a clone as coming from the tumour (31,52). This threshold may be difficult to achieve in BM or PB samples of lymphoma patients due to lower infiltration and to unrelated B and T cell clones which can contribute to a significant background of non-clonal B- or T-cell sequences. Therefore, HTS does not overcome the problem of marker identification in case of low-level lymphoma cell infiltration.
A further issue in amplicon-based sequencing strategies are somatic mutations in primer binding sites hampering proper primer binding. This is particularly important in mature B-cell malignancies where the clonal IG index sequence might harbor considerable rates of somatic hyper mutation (SHM) (e.g., MM or FL and diffuse large B-cell lymphoma), but probably is of less relevance in the majority of MCL patients (53).
This is shown in a series of Martinez-Lopez and colleagues (54) in MM patients, where a clonal IGH gene rearrangement was identified by HTS in only 63% of the diagnostic BM samples, most probably due to somatic mutations of the IGH gene locus leading to mismatches at the primer binding sites. In these cases, the addition of IGK and IGH DH-JH increases the overall identification rate of an index marker to 93%. Furthermore, ongoing SHM of the IG loci may lead to IgH clonal heterogeneity (51) resulting in a decrease of amplification efficacy and thereby to false negative/low MRD result.
A further aspect that has not been sufficiently addressed in recent publications is the correct MRD quantification particularly in the situation of low numbers of polyclonal background B-cells. MRD quantification by counting number of index sequences and dividing them by the total number of sequenced amplicons is error prone, as IG/TR multiplex PCR only amplifies rearranged IG/TR genes, i.e., cells with the respective gene in germline configuration are not targeted. This might lead to false results particularly in situations with a low number of polyclonal background B-cells, because preferential sequencing of IGH rearranged B-cells might lead to a considerable overestimation of MRD. Therefore, standardized internal controls must be included in each sequencing reaction for correct MRD quantification. Currently different approaches are proposed like different plasmids containing known IGH gene rearrangements (55), or synthetic control templates spiked at limiting dilution into each sample and computed the average number of reads for each sequenced spiked synthetic template (56).
To address all these problems, the EuroClonality-HTS consortium (www.EuroClonality.org) was formed under the umbrella of ESHLO, with the principal aims of developing, standardizing and validating the entire workflow of the IG/TR HTS tests for (I) evaluation of clonality, (II) MRD detection and (III) analysis of the IG/TR gene repertoire. Leading papers focusing on an amplicon based HTS approach for MRD marker identification and clonality detection in lymphoid malignancies have been published recently by the consortium (57,58). An important section of this consortium is the development of a bioinformatics platform for standardized input elaboration, data selection and filtration, immunogenic annotation of sequences and comparative calculations and visualization. A bioinformatic pipeline (ARResT/Interrogate pipeline) has also recently been published by the group and is used for standardized evaluation of MRD by HTS (55,58). Alternative bioinformatic pipelines have also been reported (59,60). In conclusion, validated procedures, standardized execution, regular quality controls and guidelines for interpretation of results are preconditions for MRD-directed treatment in lymphoid neoplasms. The most important aspect of any MRD assessment will require a rapid, reliable and repeatable test that is sensitive enough to detect the disease before the clinical relapse; HTS holds remarkable promises in this respect, but extensive standardization and clinical validation is still required.
Clinical significance of MRD in MCL
Determination of MRD is emerging as a safe and practical method of predicting the risk of therapeutic failure as documented in several independent studies. Several different time-points have been investigated as well as different tissue sources. Both early and late time-points proved to be informative in the majority of settings. Some of the earlier studies were based on nested-PCR, while more recent studies mainly employed RQ-PCR (6,7,10). Only one study systematically addressed both, indicating a better predictive value of RQ-PCR in most time points (18). Both PB and BM have been investigated. Both proved generally informative but contradictory results on which source is more predictive emerged from different trials. Unfortunately, many relevant studies have still not been published as full papers for the MRD results and straightforward comparison is currently difficult. At diagnosis, tumor infiltration was comparable in BM and PB, but it has been observed that during and after treatment tumor clearance is more rapid in PB compared to BM. On the other hand, PB is clearly a more accessible source compared to BM (8,15,18,21,61-63). In addition some preliminary results indicate that also cell-free DNA might represent an informative tissue source for MRD detection as reported by Lakhotia (64). Currently there is no well-defined consensus on which is the most informative source to investigate, and most ongoing trials include testing of both sources at least at same time-points. The experience so far accumulated demonstrated that MRD is a strong and independent outcome predictor and might provide reliable tool to tailor treatment according to the presence of residual tumor burden and the kinetics of disease.
The impact of MRD monitoring was assessed since the end of the previous millennium in MCL. Main results arising from this long experience can be summarized as follows:
- CHOP-like chemotherapy without rituximab does not lead to a meaningful reduction of tumour burden suggesting that monotherapy with CHOP is not an ideal treatment for MCL (8). In the European MCL Younger and Elderly Trials, where combined immuno-chemotherapy was used with or without ASCT in younger patients and anti-CD20 maintenance in patients unfit for transplantation, it could be shown relevant MRD response rates of 40% could be achieved after R-CHOP treatment (20).
- The use of rituximab combined with high-dose Ara-C chemotherapy represents a very effective induction approach to reduce the tumor burden. This was originally observed using the R-HDS regimen (8,9,65) and then demonstrated on larger series in the context of EU-MCL network trials (20,66). Intensification by high-dose cytarabine (HA) and rituximab demonstrated unprecedented MRD response rates, and became a new target for younger and fit patients (9,11,66).
- ASCT improves outcome of patients with MCL. In MCL, the impact of high doses chemotherapy and subsequent autologous stem cell transplantation (ASCT) has improved clinical response and long-term survival (67,68) and is currently the standard of care in younger patients. In the European MCL studies, ASCT increases molecular remission rates after R-CHOP from 47% to 68% in PB and from 26% to 59% in BM (20). In the Nordic MCL3 study, ASCT raised the percentage of MRD-negative patients in PB and/or BM from 53% after alternating R-CHOP/R-HA to 83% (65). In an interim analysis of the LYSA-LYMA trial, ASCT increased MRD-negative rates among patients in clinical remission after 4 cycles R-DHAP from 80% to 95% in PB and from 66% to 82% in BM (15).
- The efficiency of induction treatment prior to ASCT also preserves its influence on prognosis, which can be assessed by the MRD state prior to ASCT (15,65). In the LYMA study of LYSA (15) and by the Italian study MCL0208 (18) some pre-ASCT and all post ASCT landmarks were highly predictive of the outcome (15,18).
- Long-term follow-up of patients in clinical remission by MRD is of clinical importance, as data of the European MCL network demonstrate that reappearance of MRD in clinical remission is associated with clinical relapse (11,13,16,18,20,65). Post-ASCT MRD status is highly prognostic for PFS, with PFS at 4 years of about 38% for MRD positive patients (median PFS about 3 years) (20) and is independent of MIPI score, Ki-67 index, CT-ASCT status and pre-ASCT PET status. In MCL0208 trial the MRD positivity was linked to higher risk of relapse or death and the presence of at least two consecutive MRD-negative results conferred a significantly reduced risk of relapse (18). Similar evidence was found in elderly MCL (13,16,22,23,62,68). In EU-MCL elderly trial MRD is a predictor of clinical outcome and identifies patients with long lasting remissions (13,23).
- For long-term disease control, not only achievement of MRD response but also its maintenance is a prerequisite. The role of maintenance therapy after induction regimens to prevent disease relapse is a matter of debate (17,19,22,25,62). The Lyma 101 study which found that Obinutuzumab plus DHAP (O-DHAP) followed by ASCT plus Obinutuzumab maintenance, provided a high MRD response rate in untreated patients with MCL, is currently investigating the role of MRD-driven maintenance in this population (25).
- Since MRD positivity, even at low levels, predicts an imminent clinical relapse, this approach can lead to the tailoring of treatment, with the aim of preventing or delaying the clear progression of the disease. In a number of prospective and retrospective reports, a preventive treatment with rituximab of MRD positive patients has been able to convert them back to MRD negativity, with the possibility of prolonging their PFS (16,69,70). In a retrospective series of Italian FL and MCL patients after ASCT,18 patients with MRD reappearance (n=12) or MRD persistence (n=6) received 4 courses of rituximab and 2 additional rituximab infusions in case of persisting PCR positivity inducing MRD negativity and stable clinical response (70).
- Allogenic BM transplantation can induce MRD negativity in patients whom other therapies had failed (71-73). After alloSCT, few MRD positive patients (with or without clinical relapse) received modulation of immunosuppression or donor lymphocyte infusions with positive results in terms of molecular response (74,75).
- In the last decades several news drugs have been evaluated for the treatment landscape MCL (76). Lenalidomide is one of the first biological treatment adopted. Even when given outside a chemotherapy backbone, lenalidomide plus rituximab was able to induce eight molecular response (MR) in a very small series of 10 evaluable patients at diagnosis (77). When combined with Bendamustine, Lenalidomide induced a significant number of MRs, both when used frontline and at relapse (21,22). In the study of Zaja et al. MRD-negativity was associated to a superior outcome, while Albertsson-Lindblad et al. do not report the impact of MRD on outcome (21,22). The FIL MCL0208 trial has tested the value of lenalidomide maintenance. However, MRD data splitted by treatment arm have not been reported yet.
- Other new drugs active in MCL include BTK inhibitors and Bcl-2 antagonists (76,78,79). Data on MRD evaluation are still scant. Currently, the “TRIANGLE” trial, a randomized, three-arm, parallel-group, open label international phase 3 Trial aims is investigating whether the addition of ibrutinib to current standard treatment could improve outcomes (EudraCT number: 2014-001363-12). This study will include standardized multi-timepoint MRD detection on the whole trial population, and will therefore allow to establish the impact of Bruton’s Tyrosine kinase inhibitors (BTKi) on MRD kinetics in MCL.
In summary, available data demonstrate the major predictive role of MRD in MCL. Most ongoing MCL clinical trials include MRD-negativity as secondary endpoint and some studies such as LYMA-101 are further exploring the value of MRD-tailored treatment in this setting.
Integrating MRD and imaging tools
Fluorodeoxyglucose-PET (18F-FDG-PET)/computed tomography is recommended by international guidelines for initial staging in all histological subtypes of FDG-avid lymphomas, including MCL (80,81). PET response proved to be an independent outcome predictor in several lymphoma subtypes including MCL (23). Given the high potential of both tools it seems reasonable to combine clinical response assessment by PET and by MRD assessment. This approach has been already tested in FL (26), indicating that the two approaches are able to identify different subgroups of high-risk patients, and therefore should be regarded as complementary response assessment tools. Studies addressing the value of PET response vs. MRD are ongoing f.e. in the context of MCL0208 and other FIL trials. These efforts will test if FL findings can be reproduced also in MCL.
Discussion
Future perspectives
MRD analysis has become an increasingly important tool for assessing treatment performance in clinical trials and evaluating prognosis in MCL. Currently MRD-guided decision making is still not considered suitable for routine clinical practice and therefore MRD monitoring of individual patients is not recommended (4). However given its major clinical relevance, it is currently implemented in most clinical trials aiming at maximal cytoreduction in MCL allowing more precise evaluation of new treatment modalities. Consequently, standardized MRD diagnostics should be available for assessment of treatment response in the broadest possible population, for personalized medicine and accurate risk group assessment (82). A major disadvantage of the currently used methods for MRD in MCL is the inability of obtaining a molecular marker in approximately 15% of patients due to technical reasons. HTS might bridge this gap and might raise the number of patients with a sensitive MRD marker in clinical trials. However, validation of HTS as clinical endpoint is currently lacking for most mature B-cell malignancies. In addition, standardized technical procedures must be defined for multi-center operations, including sensitivity definition, MRD cutoff levels for risk group identification, practical conditions of application, and notification of results. An international effort and a comparison with currently used methods as well regular quality controls are already ongoing within the Euro-Clonality/Euro-MRD HTS consortium, though further development and broader diffusion will be required. The landscape is made more complex also by the numerous novel treatment options, where the evaluation of MRD will be of major importance for response evaluation. Finally, careful integration of MRD results (including assessment of cell-free DNA in the near future) with PET response data, as well as baseline clinical, genetic, and microenvironmental parameters will be needed through the development of dedicated sophisticated data collection and interpretation tools (83). This will allow exploiting the full potential of biological and clinical knowledge for the purpose of optimal risk assessment of MCL patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Martin Dreyling) for the series “Future Directions for Mantle Cell Lymphoma” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-2018-mcl-009). The series “Future Directions for Mantle Cell Lymphoma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferrero S, Rossi D, Rinaldi A, et al. KMT2D mutations and TP53 disruptions are poor prognostic biomarkers in mantle cell lymphoma receiving high-dose therapy: a FIL study. Haematologica 2020;105:1604-12. [PubMed]
- Pott C. Minimal Residual Disease Detection in Mantle Cell Lymphoma: Technical Aspects and Clinical Relevance. Semin Hematol 2011;48:172-84. [Crossref] [PubMed]
- Galimberti S, Luminari S, Ciabatti E, et al. Minimal Residual Disease after Conventional Treatment Significantly Impacts on Progression-Free Survival of Patients with Follicular Lymphoma: The FIL FOLL05 Trial. Clin Cancer Res 2014;20:6398-405. [Crossref] [PubMed]
- Hoster E, Pott C. Minimal residual disease in mantle cell lymphoma: insights into biology and impact on treatment. Hematology Am Soc Hematol Educ Program 2016;2016:437-45. [Crossref] [PubMed]
- Cheminant M, Derrieux C, Touzart A, et al. Minimal residual disease monitoring by 8-color flow cytometry in mantle cell lymphoma: An EU-MCL and LYSA study. Haematologica 2016;101:336-45. [Crossref] [PubMed]
- Howard OM, Gribben JG, Neuberg DS, et al. Rituximab and CHOP Induction Therapy for Newly Diagnosed Mantle-Cell Lymphoma: Molecular Complete Responses Are Not Predictive of Progression-Free Survival. J Clin Oncol 2002;20:1288-94. [Crossref] [PubMed]
- Corradini P, Ladetto M, Zallio F, et al. Long-Term Follow-Up of Indolent Lymphoma Patients Treated with High-Dose Sequential Chemotherapy and Autografting: Evidence That Durable Molecular and Clinical Remission Frequently Can Be Attained Only in Follicular Subtypes. J Clin Oncol 2004;22:1460-8. [Crossref] [PubMed]
- Pott C, Schrader C, Gesk S, et al. Quantitative assessment of molecular remission after high-dose therapy with autologous stem cell transplantation predicts long-term remission in mantle cell lymphoma. Blood 2006;107:2271-8. [Crossref] [PubMed]
- Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a non-randomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood 2008;112:2687-93. [Crossref] [PubMed]
- Andersen NS, Pedersen LB, Laurell A, et al. Pre-emptive treatment with rituximab of molecular relapse after autologous stem cell transplantation in mantle cell lymphoma. J Clin Oncol 2009;27:4365-70. [Crossref] [PubMed]
- Pott C, Hoster E, Delfau-Larue MH, et al. Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: a European MCL intergroup study. Blood 2010;115:3215-23. [Crossref] [PubMed]
- Liu H, Johnson JL, Koval G, et al. Detection of minimal residual disease following induction immunochemotherapy predicts progression free survival in mantle cell lymphoma: final results of CALGB 59909. Haematologica 2012;97:579-85. [Crossref] [PubMed]
- Pott C, Delfau-Larue M, Beldjord K, et al. R-CHOP VS R-FC followed by maintenance with rituximab or-INF: first results of MRD assessment within the randomized trial for elderly patients with MCL. Ann Oncol 2011;22:abstr 234.
- Visco C, Chiappella A, Nassi L, et al. Rituximab, bendamustine, and low-dose cytarabine as induction therapy in elderly patients with mantle cell lymphoma: a multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet Haematol 2017;4:e15-e23. [Crossref] [PubMed]
- Callanan M, Delfau MH, Thieblemont ME, et al. Predictive Power of Early, Sequential MRD Monitoring in Peripheral Blood and Bone Marrow in Patients with Mantle Cell Lymphoma Following Autologous Stem Cell Transplantation with or without Rituximab Maintenance ;Interim Results from the LyMa-MRD Project. Blood 2015;126:abstr 338.
- Kolstad A, Pedersen LB, Eskelund CWNordic Lymphoma Group, et al. Molecular monitoring after autologous stem cell transplantation and preemptive rituximab treatment of molecular relapse; Results from the nordic mantle cell lymphoma studies (MCL2 and MCL3) with median follow-up of 8.5 years. Biol Blood Marrow Transplant 2017;23:428-35. [Crossref] [PubMed]
- Kaplan LD, Maurer MJ, Stock W, et al. Bortezomib maintenance (BM) or consolidation (BC) following aggressive immunochemotherapy and autologous stem cell transplant (ASCT) for untreated mantle cell lymphoma (MCL): 8 year follow up of CALGB 50403 (alliance). Blood 2018;132:146. [Crossref]
- Ferrero S, Barbero S, Lo Schirico M, et al. Comprehensive Minimal Residual Disease (MRD) Analysis of the Fondazione Italiana Linfomi (FIL) MCL0208 Clinical Trial for Younger Patients with Mantle Cell Lymphoma: A Kinetic Model Ensures a More Refined Risk Stratification. Blood 2018;132:abstr 920.
- Klener P, Fronkova E, Kalinova M, et al. Potential loss of prognostic significance of minimal residual disease assessment after R-CHOP-based induction in elderly patients with mantle cell lymphoma in the era of rituximab maintenance. Hematol Oncol 2018;36:773-8. [Crossref] [PubMed]
- Hermine O, Hoster E, Walewski J, et al. Alternating courses of 3x CHOP and 3x DHAP plus rituximab followed by a high-dose cytarabine containing myeloablative regimen and autologous stem cell transplantation versus 6 courses of CHOP plus rituximab followed by myeloablative radiochemotherapy and autologous stem cell transplantation in patients <65 years (MCL Younger): a randomised, open-label, phase 3 trial of the European MCL Network. Lancet 2016;388:565-75. [Crossref] [PubMed]
- Zaja F, Ferrero S, Stelitano C, et al. Second-line rituximab, lenalidomide, and bendamustine in mantle cell lymphoma: a phase II clinical trial of the Fondazione Italiana Linfomi. Haematologica 2017;102:e203-e206. [Crossref] [PubMed]
- Albertsson-Lindblad A, Kolstad A, Laurell A, et al. Lenalidomide-bendamustine-rituximab in untreated mantle cell lymphoma >65 years, the Nordic Lymphoma Group phase I+II trial NLG-MCL4. Blood 2016;128:1814-20. [Crossref] [PubMed]
- Gressin R, Daguindau N, Tempescul A, et al. A Phase 2 Study of Rituximab, Bendamustine, Bortezomib And Dexamethasone for First-Line Treatment of Older Patients with Mantle Cell Lymphoma. Haematologica 2019;104:138-46. [Crossref] [PubMed]
- Armand P, Redd R, Bsat J, et al. A phase 2 study of Rituximab-Bendamustine and Rituximab-Cytarabine for transplant-eligible patients with mantle cell lymphoma. Br J Haematol 2016;173:89-95. [Crossref] [PubMed]
- Le Gouill S, Beldi-Ferchiou A, Cacheux V, et al. Obinutuzumab plus DHAP followed by ASCT plus Obinutuzumab maintenance provides a high MRD response rate in untreated MCL patients, results of the LYMA-101 trial, a LYSA group study. Abstract S103. 24th European Hematology Association Congress, Amsterdam, NL 2019 Jun 14.
- Luminari S, Galimberti S, Versari A, et al. Positron emission tomography response and minimal residual disease impact on progression-free survival in patients with follicular lymphoma. A subset analysis from the FOLL05 trial of the Fondazione Italiana Linfomi. Haematologica 2016;101:e66-8. [Crossref] [PubMed]
- Gribben JG, Freedman A, Woo SD, et al. All advanced stage non-Hodgkin’s lymphomas with a polymerase chain reaction amplifiable breakpoint of bcl-2 have residual cells containing the bcl-2 rearrangement at evaluation and after treatment. Blood 1991;78:3275-80. [Crossref] [PubMed]
- Gribben JG, Freedman AS. Neuberg D at al. Immunologic purging of marrow assessed by PCR before autologous bone marrow transplantation for B-cell lymphoma. N Engl J Med 1991;325:1525-33. [Crossref] [PubMed]
- Gribben JG, Neuberg D, Freedman AS, et al. Detection by polymerase chain reaction of residual cells with the bcl-2 translocation is associated with increased risk of relapse after autologous bone marrow transplantation for B-cell lymphoma. Blood 1993;81:3449-57. [Crossref] [PubMed]
- Drandi D, Kubiczkova-Besse L, Ferrero S, et al. Minimal Residual Disease Detection by Droplet Digital PCR in Multiple Myeloma, Mantle Cell Lymphoma, and Follicular Lymphoma: A Comparison with Real-Time PCR. J Mol Diagn 2015;17:652-60. [Crossref] [PubMed]
- Ladetto M, Brüggemann M, Monitillo L, et al. Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia 2014;28:1299-307. [Crossref] [PubMed]
- Ferrero S, Drandi D, Mantoan B, et al. Minimal residual disease detection in lymphoma and multiple myeloma: impact on therapeutic paradigms. Hematol Oncol 2011;29:167-76. [Crossref] [PubMed]
- Pott C, Brüggemann M, Ritgen M, et al. MRD detection in B-cell non-hodgkin lymphomas using Ig gene rearrangements and chromosomal translocations as targets for real-time quantitative PCR. Methods Mol Biol 2013;971:175-200. [Crossref] [PubMed]
- Brüggemann M, Kotrová M, Knecht H, et al. Standardized next-generation sequencing of immunoglobulin and T-cell receptor gene recombinations for MRD marker identification in acute lymphoblastic leukaemia; a EuroClonality-NGS validation study. Leukemia 2019;33:2241-53. [Crossref] [PubMed]
- Böttcher S, Ritgen M, Kneba M. Flow cytometric MRD detection in selected mature B-cell malignancies. Methods Mol Biol 2013;971:149-74. [Crossref] [PubMed]
- Böttcher S, Ritgen M, Buske S, et al. Minimal residual disease detection in mantle cell lymphoma: methods and significance of four-color flow cytometry compared to consensus IGH-polymerase chain reaction at initial staging and for follow-up examinations. Haematologica 2008;93:551-9. [Crossref] [PubMed]
- Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med 2012;367:520-31. [Crossref] [PubMed]
- Chovancová J, Bernard T, Stehlíková O, et al. Detection of minimal residual disease in mantle cell lymphoma-establishment of novel eight-color flow cytometry approach. Cytometry B Clin Cytom 2015;88:92-100. [Crossref] [PubMed]
- van Dongen JJ, Lhermitte L, Böttcher S, et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia 2012;26:1908-75. [Crossref] [PubMed]
- Kalina T, Flores-Montero J, Van der Velden VHJ, et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia 2012;26:1986-2010. [Crossref] [PubMed]
- Theunissen P, Mejstrikova E, Sedek L, et al. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood 2017;129:347-57. [Crossref] [PubMed]
- Kalina T, Flores-Montero J, Lecrevisse Q, et al. Quality assessment program for EuroFlow protocols: Summary results of four-year (2010-2013) quality assurance rounds. Cytometry A 2015;87:145-56. [Crossref] [PubMed]
- Flores-Montero J, Sanoja-Flores L, Paiva B, et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 2017;31:2094-103. [Crossref] [PubMed]
- Böttcher S. Flow Cytometric MRD Detection in Selected Mature B-Cell Malignancies. Methods Mol Biol 2019;1956:157-97. [Crossref] [PubMed]
- Rimokh R, Berger F, Delsol G, et al. Detection of the chromosomal translocation t(11;14) by polymerase chain reaction in mantle cell lymphomas. Blood 1994;83:1871-5. [Crossref] [PubMed]
- Genuardi E, Klous P, Drandi D, et al. Targeted Locus Amplification (TLA): A Novel Next Generation Sequencing (NGS) Technology to Detect New Molecular Markers and Monitoring Minimal Residual Disease (MRD) in Mantle Cell and Follicular Lymphoma. Blood 2017;30:abstr 2742.
- Voena C, Malnati M, Majolino I, et al. Detection of minimal residual disease by real-time PCR can be used as a surrogate marker to evaluate the graft-versus-myeloma effect after allogeneic stem cell transplantation. Bone Marrow Transplant 2003;32:791-3. [Crossref] [PubMed]
- Ladetto M, Sametti S, Donovan JW, et al. A validated real-time quantitative PCR approach shows a correlation between tumor burden and successful ex vivo purging in follicular lymphoma patients. Exp.Hematol 2001;29:183-93. [Crossref] [PubMed]
- Brüggemann M, Droese J, Bolz I, et al. Improved assessment of minimal residual disease in B cell malignancies using fluorogenic consensus probes for real-time quantitative PCR. Leukemia 2000;14:1419-25. [Crossref] [PubMed]
- van der Velden VH, Cazzaniga G, Schrauder A, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia 2007;21:604-11. [Crossref] [PubMed]
- Andersen NS, Donovan JW, Zuckerman A, et al. Real-time polymerase chain reaction estimation of bone marrow tumor burden using clonal immunoglobulin heavy chain gene and bcl-1/JH rearrangements in mantle cell lymphoma. Exp Hematol 2002;30:703-10. [Crossref] [PubMed]
- Faham M, Zheng J, Moorhead M, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood 2012;120:5173-80. [Crossref] [PubMed]
- Hadzidimitriou A, Agathangelidis A, Darzentas N, et al. Is there a role for antigen selection in mantle cell lymphoma? Immunogenetic support from a series of 807 cases. Blood 2011;118:3088-95. [Crossref] [PubMed]
- Martinez-Lopez J, Lahuerta JJ, Pepin F, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood 2014;123:3073-9. [Crossref] [PubMed]
- Wu D, Emerson RO, Sherwood A, et al. Detection of minimal residual disease in B lymphoblastic leukemia by high-throughput sequencing of IGH. Clin Cancer Res 2014;20:4540-8. [Crossref] [PubMed]
- Brüggemann M, Kotrová M, Knecht H, et al. on behalf of the EuroClonality-NGS working group. Standardized next-generation sequencing of immunoglobulin and T-cell receptor gene recombinations for MRD marker identification in acute lymphoblastic leukaemia;a EuroClonality-NGS validation study. Leukemia 2019;33:2241-53. [Crossref] [PubMed]
- Scheijen B, Meijers RWJ, Rijntjes J, et al. on behalf of the EuroClonality-NGS Working Group. Next-generation sequencing of immunoglobulin gene rearrangements for clonality assessment: a technical feasibility study by EuroClonality-NGS. Leukemia 2019;33:2227-40. [Crossref] [PubMed]
- Knecht H, Reigl T, Kotrovà M, et al. Quality control and quantification in IG/TR next-generation sequencing marker identification: protocols and bioinformatic functionalities by EuroClonality-NGS. Leukemia 2019;33:2254-65. Abstract. [Crossref] [PubMed]
- Duez M, Giraud M, Herbert R, et al. Correction: Vidjil: A Web Platform for Analysis of High-Throughput Repertoire Sequencing. PLoS One 2017;12:e0172249 [Crossref] [PubMed]
- Avram O, Vaisman-Mentesh A, Yehezkel D, et al. ASAP - A Webserver for Immunoglobulin-Sequencing Analysis Pipeline. Front Immunol 2018;9:1686. [Crossref] [PubMed]
- Ferrero S, Dreyling MEuropean Mantle Cell Lymphoma Network. Minimal residual disease in mantle cell lymphoma: are we ready for a personalized treatment approach? Haematologica 2017;102:1133-6. [Crossref] [PubMed]
- Gressin R, Callanan M, Daguindau N, et al. Frontline Therapy with the Ribvd Regimen Elicits High Clinical and Molecular Response Rates and Long PFS in Elderly Patients Mantle Cell Lymphoma (MCL);Final Results of a Prospective Phase II Trial by the Lysa Group. Blood 2014;124:148. [Crossref]
- Di Martino S, Catapano O, Siesto SR, et al. Quantitative detection of t (11;14) bcl-1/JH in mantle cell lymphoma patients: comparison of peripheral blood and bone marrow aspirate samples. Eur Rev Med Pharmacol Sci 2015;19:4801-10. [PubMed]
- Lakhotia R, Melani C, Pittaluga S, et al. Circulating tumor DNA dynamics during therapy predict outcomes in mantle cell lymphoma. Blood 2018;132:abstr 147.
- Kolstad A, Laurell A, Jerkeman M, et al. Nordic MCL3 study: 90Y-ibritumomab-tiuxetan added to BEAM/C in non-CR patients before transplant in mantle cell lymphoma. Blood 2014;123:2953-9. [Crossref] [PubMed]
- Pott C, Hoster E, Beldjord K, et al. R-CHOP/R-DHAP Compared to R-CHOP Induction Followed by High Dose Therapy with Autologous Stem Cell Transplantation Induces Higher Rates of Molecular Remission In MCL: Results of the MCL Younger Intergroup Trial of the European MCL Network. Blood 2010;116:965. [Crossref]
- Lenz G, Dreyling M, Schiegnitz E, et al. Myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission prolongs progression-free survival in follicular lymphoma: Results of a prospective, randomized trial of the German Low-Grade Lymphoma Study Group. Blood 2004;104:2667-74. [Crossref] [PubMed]
- Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: Results of a prospective randomized trial of the European. Blood 2005;105:2677-84. [Crossref] [PubMed]
- Ferrero S, Monitillo L, Mantoan B, et al. Rituximab-based pre-emptive treatment of molecular relapse in follicular and mantle cell lymphoma. Ann Hematol 2013;92:1503-11. [Crossref] [PubMed]
- Ladetto M, Magni M, Pagliano G, et al. Rituximab induces effective clearance of minimal residual disease in molecular relapses of mantle cell lymphoma. Biol Blood Marrow Transplant 2006;12:1270-6. [Crossref] [PubMed]
- Kobrinski DA, Smith SE, Al-Mansour Z, et al. Allogeneic hematopoietic stem cell transplantation for mantle cell lymphoma in a heavily pretreated patient population. J Clin Oncol 2017;35:abstr 7558.
- Magnusson EA, Cao Q, Linden LA, et al. Autologous and Allogeneic Donor Transplantation for Mantle Cell Lymphoma in Rituximab Era: Impact of Pre-Transplant Burden on Survival. Clinical allogenic and autologous transplantation -Poster II. Blood 2012;120:3092. [Crossref]
- Gerson JN, Barta SK. Mantle Cell Lymphoma: Which Patients Should We Transplant? Curr Hematol Malig Rep 2019;14:239-46. [Crossref] [PubMed]
- Dreyling M, Campo E, Hermine O, et al. Newly diagnosed and relapsed Mantle Cell Lymphoma: ESMO clinical practice guidelines. Ann Oncol 2017;28:iv62-iv71. [Crossref]
- Krüger WH, Hirt C, Basara N, Sayer HG, et al. Allogeneic stem cell transplantation for mantle cell lymphoma--final report from the prospective trials of the East German Study Group Haematology/Oncology (OSHO). Ann Hematol 2014;93:1587-97. [Crossref] [PubMed]
- Jain P, Wang M. Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am J Hematol 2019;94:710-25. [Crossref] [PubMed]
- Ruan J, Martin P, Christos P, et al. Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood 2018;132:2016-25. [Crossref] [PubMed]
- Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood 2015;126:739-45. [Crossref] [PubMed]
- Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus Venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med 2018;378:1211-23. [Crossref] [PubMed]
- Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol 2014;32:3048-58. [Crossref] [PubMed]
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059-68. [Crossref] [PubMed]
- Pott C, Macintyre E, Delfau-Larue MH, et al. MRD Eradication Should be the Therapeutic Goal in Mantle Cell Lymphoma and May Enable Tailored Treatment Approaches: Results of the Intergroup Trials of the European MCL Network. Blood 2014;124:147. [Crossref]
- Zaccaria GM, Ferrero S, Rosati S, et al. Applying Data Warehousing to a Phase III Clinical Trial from the Fondazione Italiana Linfomi Ensures Superior Data Quality and Improved Assessment of Clinical Outcomes. JCO Clin Cancer Inform 2019;3:1-15. [Crossref] [PubMed]
Cite this article as: Ladetto M, Tavarozzi R, Pott C. Minimal residual disease (MRD) in mantle cell lymphoma. Ann Lymphoma 2020;4:4.


