
PD-L1 expression and response to nivolumab in a case of relapsed/refractory lymphomatoid granulomatosis
Introduction
Epstein-Barr virus (EBV), a ubiquitous virus associated with both Hodgkin and non-Hodgkin lymphomas, persists in latently infected B-lymphocytes by exploiting multiple mechanisms to evade and suppress anti-viral immunity. Despite the variable expression of antigenic EBV latency associated proteins, the host immune response fails to restrain EBV-associated lymphomas (1). This may be explained, at least in part, by the expression of PD-L1, a T-cell inhibitory “checkpoint”, that is variably expressed in many B- and T-cell derived lymphomas, and promotes immune evasion (2-5). Therefore, EBV-associated lymphomas, given the expression of both PD-L1 and antigenic viral proteins, are potentially suitable candidates for PD-L1/PD-1 checkpoint blockade. This contention may be supported by the experience with checkpoint blockade in extranodal NK/T-cell lymphoma, an alternative EBV-associated lymphoma. In a retrospective series of seven patients with relapsed/refractory extranodal NK/T-cell lymphoma treated with pembrolizumab (6), combined radiographic and molecular (i.e., EBV viral load) assessments demonstrated a response in all patients, including five who achieved a complete response.
Lymphomatoid granulomatosis (LyG) is a rare EBV-driven lymphoproliferative disorder that almost uniformly involves the lungs, but may also involve extranodal sites in a minority of patients. LyG infiltrates, comprised of EBV-infected B cells in an inflammatory background, form angiocentric and angiodestructive lesions (7). The relative proportion of atypical, EBV-infected B cells to infiltrating reactive T cells, and the degree of necrosis, distinguishes low-grade (grades I-II) from high-grade (grade III) lesions (7,8) LyG may be associated with congenital or acquired immunodeficiencies, and spontaneous remissions have been observed with cessation of immunosuppression (9), thus highlighting the role of immune surveillance in this disorder. High-grade lesions are managed with immunochemotherapy, much like diffuse large B-cell lymphoma. In contrast, management of low-grade LyG may include observation and cessation of any immunosuppressive medications for those with non-bulky and asymptomatic disease, or immunomodulatory strategies, including interferon or single-agent rituximab, for those with more extensive, symptomatic disease. While the utility of checkpoint blockade in LyG is uncertain, this approach is supported by the available experience with checkpoint blockade in other EBV-driven lymphoproliferative disorders like extranodal NK/T cell lymphomas. We performed a PubMed search using the terms “lymphomatoid granulomatosis” and “PD-L1”, “B7-H1”, “CD274”, “nivolumab”, “pembrolizumab”, and “checkpoint blockade”, and found zero publications in the literature addressing this question. While we were aware of an ongoing clinical trial addressing the utility of checkpoint blockade in LyG (NCT03258567), and encourage clinical trial participation, we recently treated a patient who was unable to participate in this trial.
Case presentation
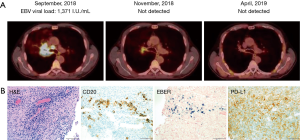
Our patient was a 54-year-old gentleman, who presented with nasal congestion, consistent with acute bacterial sinusitis. He received serial courses of antibiotics, but subsequently developed urticaria and arthralgias, which were attributed to a serum sickness-like reaction secondary to levofloxacin, for which he required several months of prednisone (up to 10 mg daily). In this setting, CT imaging of the chest was performed, and a spiculated nodule was appreciated in the right middle lobe in this former smoker. A pet scan was performed 1 month later and revealed two lung masses in the right lower lobe. A follow up bronchoscopy and biopsy were performed, revealing an extensively necrotic neoplasm, with angiocentric proliferation, and EBV positive cells consistent with LyG, grade II. Subsequent PET/CT imaging three months later demonstrated FDG avid lymphadenopathy above and below the diaphragm, and evidence of extranodal disease involving the adrenals (SUV max 17.7), thyroid, and soft tissue adjacent to the proximal right femur, in addition to multiple lung masses. Given concerns for high-grade LyG, he completed six cycles of R-CHOP and achieved a complete response. Surveillance imaging 17 months later was concerning for recurrent disease, as a right suprahilar mass was appreciated, and was associated with bronchial narrowing. Observation was continued given he was asymptomatic at this time. Repeat CT Chest showed multiple pulmonary nodules and a very narrow right bronchus. Bronchoscopy and biopsy of the lesion revealed lymphoid infiltration, necrosis, EBV positive cells consistent with LyG (grade II). Extrapulmonary disease was not appreciated and, he completed single-agent rituximab (weekly ×4), achieving a partial response, but with resolution of the previously noted bronchial narrowing. Six months later, single agent rituximab was repeated again in the setting of progressive dyspnea and CT findings demonstrating bronchial compression secondary to disease progression. PET/CT imaging was performed upon completion of rituximab, and FDG-avid lymphadenopathy was appreciated, in addition to the large, right suprahilar mass that surrounded and compressed the right main stem bronchus (Figure 1A). PD-L1 expression was observed in biopsy tissue, which confirmed low-grade LyG (Figure 1B), and treatment with nivolumab (480 mg IV every 4 weeks) was initiated. Two months later, EBV DNA was undetectable, and a partial response was noted on PET/CT imaging (Figure 1A). A complete response was documented 5 months later (Figure 1A). The patient has continued Nivolumab with no adverse events or toxicities for 12 months without clinical, laboratory, or radiographic evidence of recurrent disease.
Discussion
Expression of inhibitory ligands, including PD-L1, among others, is a dominant mechanism utilized by many cancers to create a tumor microenvironment that is hostile for tumor-specific effector T cells and effectively promotes the evasion and suppression of host anti-tumor immunity (10). Among non-Hodgkin (NHL) and classical Hodgkin lymphomas (cHL), both endogenous and exogenous factors, including the genetic landscape and the availability of PD-L1-inducing cytokines, promote PD-L1 expression and immune escape. Cytokines, including interferon-γ and IL-10, promote PD-L1 transcription in a JAK/STAT-dependent manner. Therefore, PD-L1 is highly expressed in many lymphomas harboring JAK2 amplifications (2), gain-of-function STAT3 mutations (11), antigen-receptor signaling (12), or oncogenic drivers (e.g., NPM-ALK) that culminate in STAT3 activation (13,14). PD-L1 copy gains, amplifications, and rarely translocations (15), are recurrently observed, most commonly in cHL (2), but also in subsets of diffuse large B-cell lymphomas (16). Structural variants that enhance the stability of PD-L1 transcripts have also been observed (17). Of course, many of these mechanisms are not mutually exclusive, and may cooperate with EBV-dependent mechanism in upregulating PD-L1. Furthermore, PD-L1 expression may identify biologically distinct lymphoma subsets characterized by a robust immune response (i.e., “hot” lymphomas), and for which PD-L1/PD-1 blockade is particularly attractive (16). Collectively, the available data would suggest that PD-L1 should be highly expressed in LyG, although to the best of our knowledge this has not been systematically explored. PD-L1 expression, in conjunction with the expression of EBV antigens in an inflammatory lesion with abundant, “reactive” T cells, would suggest that LyG, particularly low-grade lesions, may be well suited for checkpoint blockade. Our experience not only supports that contention, but further supports an ongoing clinical trial addressing this question in LyG and other EBV-associated lymphomas and lymphoproliferative disorders (ClinicalTrials.gov Identifier: NCT03258567). To the best of our knowledge, LyG patients have not yet been enrolled in this trial (M. Roschewski, personal communication). Our experience, which to the best of our knowledge is the first reported LyG patient treated with checkpoint blockade, further supports PD-L1/PD-1 blockade and clinical trial participation in LyG.
Acknowledgments
Funding: This work was supported by the University of Michigan Rogel Cancer Center (RA Wilcox).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol.2019.11.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Young LS, Dawson WC, Eliopoulos GA. The expression and function of Epstein-Barr virus encoded latent genes. Mol Pathol 2000;53:238-47. [Crossref] [PubMed]
- Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 2010;116:3268-77. [Crossref] [PubMed]
- Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 2013;19:3462-73. [Crossref] [PubMed]
- Wilcox RA, Feldman AL, Wada DA, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood 2009;114:2149-58. [Crossref] [PubMed]
- Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res 2012;18:1611-8. [Crossref] [PubMed]
- Kwong YL, Chan TSY, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 2017;129:2437-42. [Crossref] [PubMed]
- Song JY, Pittaluga S, Dunleavy K, et al. Lymphomatoid granulomatosis—a single institute experience. Pathologic findings and clinical correlations. Am J Surg Pathol 2015;39:141-56. [Crossref] [PubMed]
- Dunleavy K, Roschewski M, Wilson WH. Lymphomatoid granulomatosis and other Epstein-Barr virus associated lymphoproliferative processes. Curr Hematol Malig Rep 2012;7:208-15. [Crossref] [PubMed]
- Aiko N, Sekine A, Umeda S, et al. The Spontaneous Regression of Grade 3 Methotrexate-related Lymphomatoid Granulomatosis: A Case Report and Literature Review. Intern Med 2018;57:3163-7. [Crossref] [PubMed]
- Wilcox RA, Ansell SM, Lim MS, et al. The B7 homologues and their receptors in hematologic malignancies. Eur J Haematol 2012;88:465-75. [Crossref] [PubMed]
- Song TL, Nairismagi ML, Laurensia Y, et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood 2018;132:1146-58. [Crossref] [PubMed]
- Li L, Zhang J, Chen J, et al. B-cell receptor-mediated NFATc1 activation induces IL-10/STAT3/PD-L1 signaling in diffuse large B-cell lymphoma. Blood 2018;132:1805-17. [Crossref] [PubMed]
- Marzec M, Zhang Q, Goradia A, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci U S A 2008;105:20852-7. [Crossref] [PubMed]
- Zhang JP, Song Z, Wang HB, et al. A novel model of controlling PD-L1 expression in ALK+ Anaplastic Large Cell Lymphoma revealed by CRISPR screening. Blood 2019;134:171-85. [Crossref] [PubMed]
- Georgiou K, Chen L, Berglund M, et al. Genetic basis of PD-L1 overexpression in diffuse large B-cell lymphomas. Blood 2016;127:3026-34. [Crossref] [PubMed]
- Godfrey J, Tumuluru S, Bao R, et al. PD-L1 gene alterations identify a subset of diffuse large B-cell lymphoma harboring a T-cell-inflamed phenotype. Blood 2019;133:2279-90. [Crossref] [PubMed]
- Kataoka K, Shiraishi Y, Takeda Y, et al. Aberrant PD-L1 expression through 3'-UTR disruption in multiple cancers. Nature 2016;534:402-6. [Crossref] [PubMed]
Cite this article as: Weiss J, Mayer T, Brown N, Wilcox RA. PD-L1 expression and response to nivolumab in a case of relapsed/refractory lymphomatoid granulomatosis. Ann Lymphoma 2019;3:12.


