
A survey of the therapeutic landscape in peripheral T-cell lymphomas: the importance of expert hematopathology review in the era of targeted therapies and precision medicine
Sir William Osler, a “father of modern medicine”, and a towering figure in the late 19th and early 20th centuries, revolutionized post-graduate medical education, emphasizing the importance of patient-centered and bedside teaching. He famously quipped: “Each case has its lesson—a lesson that may be, but is not always, learnt, for clinical wisdom is not the equivalent of experience. A man who has seen 500 cases of pneumonia (or PTCL) may not have the understanding of the disease which comes with an intelligent study of a score of cases, so different are knowledge and wisdom.”
Osler’s long shadow lingers still, and so we will utilize a peripheral T-cell lymphoma (PTCL) case to not only survey the current therapeutic landscape, but also highlight the importance of both expert hematopathology review and clinical trial participation.
Case presentation
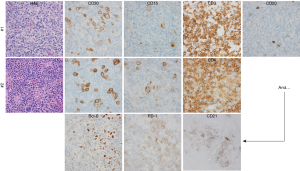
A 55-year-old gentleman with no significant past medical history presented with B symptoms, dyspnea, and diffuse pruritus. On physical exam, diffuse lymphadenopathy, splenomegaly and bilateral pleural effusions were noted. An excisional lymph node biopsy, performed locally, revealed large and morphologically atypical CD30+CD15+ cells with Hodgkin and Reed-Sternberg (HRS)-like morphology (Figure 1). These findings were interpreted as classical Hodgkin lymphoma (cHL). With stage IIIB disease, he completed 6 cycles of ABVD, achieving a complete response. However, recurrent lymphadenopathy was noted 8 months later, and biopsies obtained. A similarly aberrant population of CD30+CD15+ large cells was noted, but further immunohistochemical studies were performed, demonstrating aberrant co-expression of multiple T-cell associated antigens and absent PAX5 expression. An expanded follicular dendritic cell (FDC) network was observed, as was Bcl-6 and PD-1 expression. Flow cytometric immunophenotyping further identified an aberrant population of CD2+CD4+CD5+CD3− T cells. Concordant T-cell receptor gene rearrangements were observed in both biopsies. Collectively, these findings were consistent with angioimmunoblastic T-cell lymphoma (AITL) or TFH-derived PTCL (PTCL-TFH).
The importance of expert hematopathology review
The importance of expert pathologic review was recently demonstrated in a large, prospective study in which well over 30,000 lymphoma biopsies that had been interpreted locally were referred to a reference center within the French Lymphopath Network (1). The rate and clinical impact of pathologic reclassification following a second, centralized review by expert hematopathologist was reported (1). The vast majority (≈85%) of referral and expert diagnoses were concordant among the most common B-cell NHL (i.e., DLBCL, FL). In stark contrast, ≈65% of the most common PTCL diagnoses (PTCL, NOS and AITL) were concordant. Cases initially classified as cHL were among the approximately one in three PTCL cases that were reclassified, as HRS-like B-cells may be observed in AITL and PTCL-TFH (2,3). These HRS-like B cells are frequently EBV positive and may pose a diagnostic challenge, particularly when immunophenotyping is incomplete (4).
The role of HDT-ASCT: missed therapeutic opportunity?
Few prospective studies have addressed the role of high-dose therapy (HDT) and autologous stem-cell transplantation (ASCT) as a consolidation strategy in first remission or as a salvage strategy in second remission (5,6). The largest prospective and PTCL specific study to address the role of HDT-ASCT in the frontline setting was conducted by the Nordic Lymphoma Group (7). Out of 166 enrolled patients, 115 achieved a partial or complete remission following CHOP or CHOEP induction and underwent HDT-ASCT. Treatment-related mortality was low (4%), and despite early treatment failures in ≈25% of patients, long-term progression-free survival (PFS) was 44%. Despite the acknowledged limitations of the available retrospective and prospective data, most notably the absence of a randomized-controlled trial addressing the absolute benefit of HDT-ASCT in first remission, this approach is endorsed by NCCN guidelines and by many experts in the field for the most common PTCL subtypes (8). Nonetheless, HDT-ASCT in first remission is likely underutilized, as fewer than 25% of patients underwent HDT-ASCT in a large, prospective cohort study examining practice patterns in the United States (9). Consequently, many patients with recurrent disease will not have undergone HDT-ASCT. In this setting, outcomes comparable to those achieved following HDT-ASCT in relapsed diffuse large B-cell lymphoma may be anticipated with this approach for PTCL, NOS/AITL patients achieving a complete remission following salvage therapy. The largest study to specifically address the role of HDT-ASCT in AITL was retrospectively conducted by the European Group for Blood and Marrow Transplantation (10). Approximately 75% of the 146 patients examined underwent HDT-ASCT after ≥2 lines of prior therapy, and 48% were transplanted in first or second complete remission. The remaining patients were transplanted with chemotherapy-sensitive (38%) or refractory (14%) disease. For patients achieving a complete remission prior to HDT-ASCT, 4-year PFS was 56%, and was significantly higher compared with the 4-year PFS observed in patients with chemotherapy-sensitive (30%) or refractory disease (23%) at the time of transplant. However, it is worth noting that 70% of those transplanted in complete remission underwent transplant following their first complete remission. Therefore, achieving a complete response (CR) prior to HDT-ASCT is an important goal.
While the therapeutic goal—achieving a CR—in the setting of relapsed PTCL, NOS or AITL is clear, the optimal salvage regimen is not, and clinical trial participation should be encouraged. Nonetheless, at the time of relapse, our patient was treated locally with CHOP, and achieved a partial response after 4 cycles of therapy. Given his suboptimal response and complicated course (i.e., neutropenia, sepsis, and atrial fibrillation), he was referred to our center for evaluation and treatment recommendations, at which time allogeneic stem cell transplantation was considered, but a suitable related or matched unrelated donor was not available.
What is the optimal salvage therapy in relapsed/refractory PTCL: does the “cell-of-origin” matter?
The cell-of-origin is best appreciated for AITL and PTCL-TFH (11). Follicular helper T cells (TFH cells) regulate humoral immunity via the production of cytokines (e.g., IL-4, IL-21) and expression of cell-surface ligands (e.g., CD40L) that regulate somatic hypermutation and isotype switching. Immunophenotyping and gene-expression profiling studies demonstrated that malignant T cells in AITL/PTCL-TFH are TFH cell derived (12-22). Understanding AITL ontogeny explains the classic histologic findings (e.g., expansion of germinal-center B cells and an expanded meshwork of FDCs) characteristic of this PTCL. Similarly, the differentiation of conventional CD4+ T-cell subsets, from which PTCL, NOS subsets are derived, is controlled by “master” transcription factors, including GATA-3 and T-bet (12,13). For example, the zinc-finger transcription factor GATA-3, while regulating the growth and survival of post-thymic T cells, is classically associated with Th2 differentiation (14). Interrogation of these transcription factors and their respective gene targets led to the observation that a majority of morphologically classified PTCL, NOS cases are characterized by the expression of the transcription factors T-bet or GATA-3 and their respective gene targets (12). GATA-3 expression was associated with dismal survival, and may be attributed, at least in part, to GATA-3-dependent cell-autonomous and non-cell-autonomous effects (12,15). These adverse prognostic implications associated with GATA-3 expression were independently validated in two subsequent studies (16,17). A more robust gene expression profiling study similarly identified these distinct subtypes, demonstrating that PTCL, NOS cases highly expressed either GATA-3 and its gene targets or TBX21 (T-bet) and its gene targets (13). Collectively, these findings demonstrate that PTCL, NOS, when excluding PTCL-TFH or morphologically misclassified cases, is comprised of two dominant subtypes: “GATA-3” and “T-bet” PTCL (11).
The utility of the “cell-of-origin” as a predictive biomarker in the setting of relapsed/refractory disease, while poorly understood, is best appreciated for AITL/PTCL-TFH. In the PROPEL study, 32% of PTCL, NOS patients achieved a response with pralatrexate, whereas only a single AITL patient achieved a response with pralatrexate, and a partial response at that (18). In contrast to the 8% overall response rate (ORR) observed with pralatrexate, the ORR observed in AITL with romidepsin, belinostat, or brentuximab vedotin range from 30–54% (19-21). Among the≈15% of PTCL, NOS/AITL patients achieving a CR with romidepsin, a majority achieved a durable CR, lasting at least 1 year in 60%, and >2 years in 40% (22). As such, romidepsin was elected, but he progressed after receiving three cycles of therapy with PET imaging demonstrating disease progression, with nodal disease above and below the diaphragm (summarized in Figure 2). In a phase II study, an ORR of 54%, most of which were complete, was observed in AITL patients treated with brentuximab vedotin, irrespective of CD30 expression (21). He subsequently received brentuximab vedotin, but nodal progression was observed on PET.

What is the optimal management of multiply relapsed or refractory PTCL?
Survival outcomes are dismal for most patients with refractory PTCL with currently available therapies. For example, a recent retrospective, single institution study examined outcomes following salvage therapy in the setting of primary refractory disease (n=93). As anticipated, outcomes were dismal, with median event-free and overall survival of <4 and 9 months, respectively, observed (23). A significant difference in survival was not observed when comparing patients who received “traditional” salvage regimens (e.g., ICE) with those who received single agents. While the benefits of clinical trial participation have not been systematically examined in PTCL, one study performed in Hodgkin lymphoma and selected B-cell non-Hodgkin lymphomas (NHL) examined outcomes between clinical trial participants and matched patients who met eligibility criteria but did not participate in a clinical trial (24). Significant improvements in event-free survival were observed for clinical trial participants among all the lymphomas examined. Among the 47 phase I–III trials included in this study, 17 trials included novel agents that subsequently received FDA approval. As clinical trial participation should be highly encouraged in patients with relapsed/refractory PTCL, our patient was enrolled in a phase II trial with the oral proteasome inhibitor ixazomib (25). Unfortunately, nodal disease progression was observed (by PET).
The genetic landscape in AITL and its therapeutic implications.
Epigenetic dysregulation is a hallmark of TFH-derived malignancies. Ten-Eleven-Translocation (TET) 2 promotes DNA demethylation and recurrent loss-of-function mutations affecting its catalytic domain are observed in the majority of AITL. In addition, isocitrate dehydrogenase (IDH) 2 catalyzes the decarboxylation of isocitrate to α-ketoglutarate (α-KG) and indirectly regulates α-KG dependent dioxygenases, including TET2. IDH2 is recurrently mutated at arginine-172 in approximately one-third of AITL, conferring neomorphic activity such that α-KG is converted to the R-enantiomer of 2-hydroxyglutarate, an oncometabolite that antagonizes TET2 and histone demethylases. Loss-of-function mutations in the DNA methyltransferase DNMT3A are also observed, and usually occur with TET2 mutations, in approximately one-third of AITL cases. Interestingly, TET2 and DNMT3A mutations are observed in hematopoietic stem and progenitor cells in patients with clonal hematopoiesis (26-28), and are not restricted to malignant T cells in AITL, but are also present in progenitor cells and constituents of the tumor microenvironment (29,30). In contrast, other recurrent mutations (e.g., RhoA, IDH2) are restricted to malignant T cells (30), suggesting that TET2 and/or DNMT3A mutations are early events in disease pathogenesis that promote the malignant transformation of T cells following the acquisition of additional genetic hits. Conventional T cells are dependent upon the integration of both antigen-dependent and antigen-independent (e.g., costimulation and cytokine-dependent) signals. These pathways are prime candidates for the “second hits” that promote T-cell transformation (31) and ripe for therapeutic exploitation. The constellation of mutational profiling and next-generation sequencing studies completed to date generally support this contention (32-37).
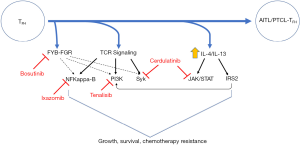
Lymphoma patients are increasingly subjected to next-generation sequencing (http://mctp.med.umich.edu/physicians/mi-oncoseq-study) at our institution (38,39), and this testing was performed for our patient. In contrast to the highly recurrent mutations commonly observed in AITL, which were not observed in our patient, a novel fusion between the SRC family kinase FGR, including its kinase domain, and a binding protein (i.e., FYB) for a related SRC family kinase (i.e., Fyn) was observed (Figure 3). While the function of this novel FYB-FGR fusion is unknown, activating mutations in the SRC family kinase Fyn are observed in ≤4% of AITL and PTCL, NOS (33,35). Treatment with bosutinib, a potent SRC family kinase inhibitor with IC50’s <10 nM (40), was initiated, but he failed to respond with PET scan showing disease progression, including new nodal sites of disease. Mechanisms of resistance to most novel agents are poorly understood, and this is a fertile area for future study. Nonetheless, it is noteworthy that FGR was identified in 2016 as a mediator of resistance to vorinostat in B-cell NHL (41).
At this point, having relapsed or progressed after six prior lines of therapy, our patient had developed bulky and symptomatic retroperitoneal disease, prompting the selection of therapy that may rapidly debulk his disease. As gemcitabine, either alone or in combination, is associated with ORR exceeding 50% in PTCL (42-46), GemOx was initiated. Unfortunately, continued disease progression and clinical deterioration was observed, despite treatment. As durable remissions may be achieved with corticosteroids alone (47), and in the setting of continued clinical deterioration, high-dose dexamethasone (40 mg daily for 4 days) was initiated. Rapid and significant debulking of his disease and symptomatic improvement was observed. After experiencing disease relapse or progression with seven prior lines of therapy, a partial response was achieved with corticosteroids. He appreciated use of an oral agent, and lenalidomide is associated with an ORR of 31% (15% CR) in AITL. Therefore, lenalidomide was initiated, as previously described (48). While stable disease was achieved for 7 months, and treatment well tolerated, he ultimately progressed, developing biopsy-confirmed bone marrow involvement.
Many of the recurrent mutations and other genetic alterations observed in AITL converge on the phosphoinositide 3-kinase (PI3K) pathway (Figure 3). The costimulatory receptor ICOS plays a fundamental role in TFH cell biology (49), is highly expressed in AITL (31), and also culminates in PI3K activation. Among PTCL patients treated with the PI3Kγ/δ inhibitor duvelisib in a phase I study, 50% achieved a response, including an AITL patient (out of three treated) that achieved a durable and ongoing complete response. Therefore, our patient was enrolled in a clinical trial investigating a novel PI3Kγ/δ inhibitor (Tenalisib), but did not achieve a response. Similarly, many recurrent abnormalities observed in AITL implicate the T-cell receptor in disease pathogenesis (33), and suggest that downstream kinases, including Syk (50), among others (15), are suitable therapeutic targets. In addition, multiple cytokines (e.g., IL-4/IL-13) have been implicated in AITL pathogenesis (31), suggesting that inhibition of cytokine receptor-dependent activation of Janus kinases (JAK) is attractive. Therefore, our patient was enrolled in a phase I/II trial investigating the dual JAK/Syk inhibitor cerdulatinib, achieving a complete response. Whether the “cell of origin” and TFH-cell derivation are predictive biomarkers for response to cerdulatinib is unknown, but will be investigated in a multinational phase IIb study (CELTIC-1, ClinicalTrials.gov Identifier: NCT04021082).
What’s the optimal twelfth-line therapy?
We don’t know what the future holds for our patient, but are convinced that clinical trial participation will continue to be an important consideration in his management. For example, PD-L1, while rarely expressed by malignant T cells in AITL, is commonly expressed by constituents of the tumor microenvironment (51). Furthermore, a significant mutational burden was observed in our patient (119 somatic mutations), which may portend a favorable response to checkpoint blockade (52). Therefore, checkpoint blockade, ideally in combination with additional immunomodulatory strategies (52), is attractive.
The case presented (summarized in Figure 2) illustrates both the importance of expert hematopathology review and the significant benefits associated with clinical trial participation. In fact, clinical trial participation may become increasingly important, as we now face a challenge that was almost unimaginable 10 years ago. That is, the smorgasbord of therapeutic targets and novel agents continues to rapidly expand, necessitating the development of predictive biomarkers that will optimize the selection of patients for conventional and novel therapies, as a “good physician treats the disease”, but “the great physician treats the patient who has the disease” (Sir William Osler).
Acknowledgments
Funding: Leukemia & Lymphoma Society (6503-16), the American Cancer Society (129084-RSG-16–045-01-LIB), and the NIH-NCI (K08CA172215).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol.2019.10.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this article.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Laurent C, Baron M, Amara N, et al. Impact of Expert Pathologic Review of Lymphoma Diagnosis: Study of Patients From the French Lymphopath Network. J Clin Oncol 2017;35:2008-17. [Crossref] [PubMed]
- Eladl AE, Satou A, Elsayed AA, et al. Clinicopathological Study of 30 Cases of Peripheral T-cell Lymphoma with Hodgkin and Reed-Sternberg-like B-cells from Japan. Am J Surg Pathol 2017;41:506-16. [Crossref] [PubMed]
- Nicolae A, Pittaluga S, Venkataraman G, et al. Peripheral T-cell lymphomas of follicular T-helper cell derivation with Hodgkin/Reed-Sternberg cells of B-cell lineage: both EBV-positive and EBV-negative variants exist. Am J Surg Pathol 2013;37:816-26. [Crossref] [PubMed]
- Hsi ED, Horwitz SM, Carson KR, et al. Analysis of Peripheral T-cell Lymphoma Diagnostic Workup in the United States. Clin Lymphoma Myeloma Leuk 2017;17:193-200. [Crossref] [PubMed]
- Casulo C, Horwitz S. Should eligible patients with T-cell lymphoma receive high-dose therapy and autologous stem cell transplant in the upfront setting? Curr Oncol Rep 2010;12:374-82. [Crossref] [PubMed]
- d'Amore F, Jantunen E, Relander T. Hemopoietic stem cell transplantation in T-cell malignancies: who, when, and how? Curr Hematol Malig Rep 2009;4:236-44. [Crossref] [PubMed]
- d'Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol 2012;30:3093-9. [Crossref] [PubMed]
- Kharfan-Dabaja MA, Kumar A, Ayala E, et al. Clinical Practice Recommendations on Indication and Timing of Hematopoietic Cell Transplantation in Mature T Cell and NK/T Cell Lymphomas: An International Collaborative Effort on Behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2017;23:1826-38. [Crossref] [PubMed]
- Carson KR, Horwitz SM, Pinter-Brown LC, et al. A prospective cohort study of patients with peripheral T-cell lymphoma in the United States. Cancer 2017;123:1174-83. [Crossref] [PubMed]
- Kyriakou C, Canals C, Goldstone A, et al. High-dose therapy and autologous stem-cell transplantation in angioimmunoblastic lymphoma: complete remission at transplantation is the major determinant of Outcome-Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 2008;26:218-24. [PubMed]
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Wang T, Feldman AL, Wada DA, et al. GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features. Blood 2014;123:3007-15. [Crossref] [PubMed]
- Iqbal J, Wright G, Wang C, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood 2014;123:2915-23. [Crossref] [PubMed]
- Tindemans I, Serafini N, Di Santo JP, et al. GATA-3 function in innate and adaptive immunity. Immunity 2014;41:191-206. [Crossref] [PubMed]
- Wang T, Lu Y, Polk A, et al. T-cell Receptor Signaling Activates an ITK/NF-kappaB/GATA-3 axis in T-cell Lymphomas Facilitating Resistance to Chemotherapy. Clin Cancer Res 2017;23:2506-15. [Crossref] [PubMed]
- Zhang W, Wang Z, Luo Y, et al. GATA3 expression correlates with poor prognosis and tumor-associated macrophage infiltration in peripheral T cell lymphoma. Oncotarget 2016;7:65284-94. [PubMed]
- Manso R, Bellas C, Martin-Acosta P, et al. C-MYC is related to GATA3 expression and associated with poor prognosis in nodal peripheral T-cell lymphomas. Haematologica 2016;101:e336-8. [Crossref] [PubMed]
- O'Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol 2011;29:1182-9. [Crossref] [PubMed]
- Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol 2012;30:631-6. [Crossref] [PubMed]
- O'Connor OA, Horwitz S, Masszi T, et al. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol 2015;33:2492-9. [Crossref] [PubMed]
- Horwitz SM, Advani RH, Bartlett NL, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood 2014;123:3095-100. [Crossref] [PubMed]
- Coiffier B, Pro B, Prince HM, et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses. J Hematol Oncol 2014;7:11. [Crossref] [PubMed]
- Zhang JY, Briski R, Devata S, et al. Survival following salvage therapy for primary refractory peripheral T-cell lymphomas (PTCL). Am J Hematol 2018;93:394-400. [Crossref] [PubMed]
- Nowakowski GS, Ansell SM, Witzig TE, et al. Participation in clinical trials to improve outcomes of patients with relapsed lymphoma. J Clin Oncol 2016;34:7523. [Crossref]
- Boonstra PS, Polk A, Brown N, et al. A single center phase II study of ixazomib in patients with relapsed or refractory cutaneous or peripheral T-cell lymphomas. Am J Hematol 2017;92:1287-94. [Crossref] [PubMed]
- Quivoron C, Couronne L, Della Valle V, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell 2011;20:25-38. [Crossref] [PubMed]
- Shlush LI, Zandi S, Mitchell A, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 2014;506:328-33. [Crossref] [PubMed]
- Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 2014;371:2477-87. [Crossref] [PubMed]
- Schwartz FH, Cai Q, Fellmann E, et al. TET2 mutations in B cells of patients affected by angioimmunoblastic T-cell lymphoma. J Pathol 2017;242:129-33. [Crossref] [PubMed]
- Nguyen TB, Sakata-Yanagimoto M, Asabe Y, et al. Identification of cell-type-specific mutations in nodal T-cell lymphomas. Blood Cancer J 2017;7:e516 [Crossref] [PubMed]
- Wilcox RA. A three-signal model of T-cell lymphoma pathogenesis. Am J Hematol 2016;91:113-22. [Crossref] [PubMed]
- Boddicker RL, Razidlo GL, Dasari S, et al. Integrated mate-pair and RNA sequencing identifies novel, targetable gene fusions in peripheral T-cell lymphoma. Blood 2016;128:1234-45. [Crossref] [PubMed]
- Vallois D, Dobay MP, Morin RD, et al. Activating mutations in genes related to TCR signaling in angioimmunoblastic and other follicular helper T-cell-derived lymphomas. Blood 2016;128:1490-502. [Crossref] [PubMed]
- Sakata-Yanagimoto M, Enami T, Yoshida K, et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat Genet 2014;46:171-5. [Crossref] [PubMed]
- Palomero T, Couronne L, Khiabanian H, et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat Genet 2014;46:166-70. [Crossref] [PubMed]
- Yoo HY, Sung MK, Lee SH, et al. A recurrent inactivating mutation in RHOA GTPase in angioimmunoblastic T cell lymphoma. Nat Genet 2014;46:371-5. [Crossref] [PubMed]
- Fujisawa M, Sakata-Yanagimoto M, Nishizawa S, et al. Activation of RHOA-VAV1 signaling in angioimmunoblastic T-cell lymphoma. Leukemia 2018;32:694-702. [Crossref] [PubMed]
- Robinson DR, Wu YM, Lonigro RJ, et al. Integrative clinical genomics of metastatic cancer. Nature 2017;548:297-303. [Crossref] [PubMed]
- Roychowdhury S, Iyer MK, Robinson DR, et al. Personalized oncology through integrative high-throughput sequencing: a pilot study. Sci Transl Med 2011;3:111ra121 [Crossref] [PubMed]
- Remsing Rix LL, Rix U, Colinge J, et al. Global target profile of the kinase inhibitor bosutinib in primary chronic myeloid leukemia cells. Leukemia 2009;23:477-85. [Crossref] [PubMed]
- Joosten M, Ginzel S, Blex C, et al. A novel approach to detect resistance mechanisms reveals FGR as a factor mediating HDAC inhibitor SAHA resistance in B-cell lymphoma. Mol Oncol 2016;10:1232-44. [Crossref] [PubMed]
- Zinzani PL, Magagnoli M, Bendandi M, et al. Therapy with gemcitabine in pretreated peripheral T-cell lymphoma patients. Ann Oncol 1998;9:1351-3. [Crossref] [PubMed]
- Mahadevan D, Unger JM, Spier CM, et al. Phase 2 trial of combined cisplatin, etoposide, gemcitabine, and methylprednisolone (PEGS) in peripheral T-cell non-Hodgkin lymphoma: Southwest Oncology Group Study S0350. Cancer 2013;119:371-9. [Crossref] [PubMed]
- Pellegrini C, Dodero A, Chiappella A, et al. A phase II study on the role of gemcitabine plus romidepsin (GEMRO regimen) in the treatment of relapsed/refractory peripheral T-cell lymphoma patients. J Hematol Oncol 2016;9:38. [Crossref] [PubMed]
- Qi F, Dong M, He X, et al. Gemcitabine, dexamethasone, and cisplatin (GDP) as salvage chemotherapy for patients with relapsed or refractory peripheral T cell lymphoma-not otherwise specified. Ann Hematol 2017;96:245-51. [Crossref] [PubMed]
- Yao YY, Tang Y, Zhu Q, et al. Gemcitabine, oxaliplatin and dexamethasone as salvage treatment for elderly patients with refractory and relapsed peripheral T-cell lymphoma. Leuk Lymphoma 2013;54:1194-200. [Crossref] [PubMed]
- Siegert W, Agthe A, Griesser H, et al. Treatment of angioimmunoblastic lymphadenopathy (AILD)-type T-cell lymphoma using prednisone with or without the COPBLAM/IMVP-16 regimen. A multicenter study. Kiel Lymphoma Study Group. Ann Intern Med 1992;117:364-70. [Crossref] [PubMed]
- Morschhauser F, Fitoussi O, Haioun C, et al. A phase 2, multicentre, single-arm, open-label study to evaluate the safety and efficacy of single-agent lenalidomide (Revlimid) in subjects with relapsed or refractory peripheral T-cell non-Hodgkin lymphoma: the EXPECT trial. Eur J Cancer 2013;49:2869-76. [Crossref] [PubMed]
- Wilcox RA, Ansell SM, Lim MS, et al. The B7 homologues and their receptors in hematologic malignancies. Eur J Haematol 2012;88:465-75. [Crossref] [PubMed]
- Wilcox RA, Sun DX, Novak A, et al. Inhibition of Syk protein tyrosine kinase induces apoptosis and blocks proliferation in T-cell non-Hodgkin's lymphoma cell lines. Leukemia 2010;24:229-32. [Crossref] [PubMed]
- Wilcox RA, Feldman AL, Wada DA, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood 2009;114:2149-58. [Crossref] [PubMed]
- Phillips T, Devata S, Wilcox RA. Challenges and opportunities for checkpoint blockade in T-cell lymphoproliferative disorders. J Immunother Cancer 2016;4:95. [Crossref] [PubMed]
Cite this article as: Scott AJ, Ross CW, Gabali AM, Wilcox RA. A survey of the therapeutic landscape in peripheral T-cell lymphomas: the importance of expert hematopathology review in the era of targeted therapies and precision medicine. Ann Lymphoma 2019;3:9.


