
Diffuse large B-cell lymphoma—who should we FISH?
Introduction: why is it important to diagnose high-grade B-cell lymphomas harboring rearrangements of MYC and BCL2 and/or BCL6 (HGBL-DH/TH)?
Aggressive B-cell lymphomas, including diffuse large B-cell lymphoma (DLBCL), comprise the most commonly diagnosed non-Hodgkin lymphomas in the Western world and are potentially curable with standard chemoimmunotherapy treatments in up to two-thirds of patients. However, there is increasing appreciation of underlying heterogeneity, even with similar clinical and histologic features, and there is a substantial portion of patients who will not be cured. In recent years, ongoing research efforts have identified an uncommon but clinically significant subgroup of high-risk patients with a highly aggressive clinical course and dismal long-term survival. This subgroup, commonly referred to as double hit (DHL) or triple hit lymphoma (THL), has been officially classified as “high grade B-cell lymphomas with rearrangements of MYC and BCL2 and/or BCL6” in the 2016 revision of the World Health Organization (WHO) classification of lymphoid neoplasms (1). The standard way to identify these aberrations is via fluorescence in situ hybridization (FISH) probes. MYC is located on the long arm of chromosome 8 (8q24) and is crucial for metabolism, protein synthesis, and amplification of transcription (2). MYC expression in DLBCL drives proliferation and induces genomic instability (3). BCL2, an oncogene located on the long arm of chromosome 18 (18q21), serves to promote cellular survival by preventing apoptosis (4). BCL6 normally encodes a transcriptional repressor and when overexpressed, can down-regulate several other genes, including TP53 (tumor suppressor gene), which subsequently allows DNA-damaged cells to escape from apoptosis (5). A typical translocation partner for these genes is the immunoglobulin heavy chain gene (IGH) enhancer, which is located on the long arm of chromosome 14 (14q32). The IGH enhancers activate efficient and accurate transcription of clonal IGH genes (6,7). Concurrent translocation of MYC and BCL2 and/or BCL6 molecularly generates a cellular environment of rapid growth countered by decreased apoptosis, and leads to a highly chemoresistant phenotype.
Patients with HGBL-DH/TH comprise around 10% (8-12) of newly diagnosed DLBCL and typically demonstrate poor response to standard initial therapy (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; RCHOP) and limited survival when compared to those without HGBL-DH/TH. One group retrospectively analyzed 394 patient samples and detected 19 cases of HGBL-DH (12%). After treatment with anthracycline-containing regimens, the HGBL-DH patients had a significantly shorter median OS of 8.2 months compared with 56.8 months in non- HGBL-DH patients (12). Another group retrospectively reviewed 290 patient samples and detected 14 cases of HGBL-DH (5%). After RCHOP therapy, these patients had exceedingly poor 5-year overall survival (OS) and progression-free survival (PFS) rates of 27% and 18%, respectively (11). These data, among others, led investigators to question whether more aggressive induction therapy would be more effective than RCHOP for the treatment of patients with HGBL-DH. The MD Anderson group reported their experience with 129 HGBL-DH cases. The 2-year event-free survival was much lower than historically reported outcomes of patients with DLBCL and was observed at 25%, 32%, and 67% in patients who received RCHOP, R-hyperCVAD/MA (rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, cytarabine) and REPOCH (rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin), respectively (13). A large retrospective multicenter study reviewed 311 HGBL-DH patients who received induction treatment with RCHOP, REPOCH, R-hyperCVAD/MA, or CODOX-M-IVAC (cyclophosphamide, vincristine, doxorubicin, methotrexate, ifosfamide, etoposide, and cytarabine). Patients who received REPOCH had the highest response rate. Multivariable analyses demonstrated significantly improved PFS and OS for those patients who received a more intensive induction therapy compared to R-CHOP (hazard ratio, 0.5) (14). To date, the “best” induction regimen for patients with HGBL-DH/TH remains controversial, but most clinicians prefer to use a more intensive regimen than RCHOP in patients who can tolerate the therapy based on these retrospective series.
In addition to poor response and survival following RCHOP therapy, it is important to understand that patients with HGBL-DH/TH have an increased risk of CNS relapse. In a retrospective study of 135 patients with DLBCL, 9% were found to have a MYC translocation and the presence of this translocation held an increased risk of CNS relapse independent of all studied risk factors (15). Additional study of patients with dual expression of MYC and BCL2 proteins demonstrated a near 10% risk of CNS relapse (16). Therefore, many experts feel that HGBL-DH/TH should be offered CNS prophylaxis with initial therapy.
Thus, due to the need for more intensive induction chemotherapy than RCHOP and the potential need to implement CNS prophylaxis, it is crucial for treating physicians to clearly identify whether a patient with newly diagnosed DLBCL fits into the HGBL-DH/TH category. Despite these strong arguments, FISH studies for MYC, BCL2, and BCL6 translocations are not sent in the majority of patients newly diagnosed with DLBCL. Reasons cited for not sending these tests include: (I) lack of pathologist expertise to perform FISH analyses at the treating center; (II) the high cost of FISH analyses; (III) low prevalence of HGBL-DH/TH in the large DLBCL population; and (IV) lack of awareness that HGBL-DH/TH exists and/or the clinical consequences of HGBL-DH/TH. Since identification of this poor risk subset of DLBCL, many centers have developed provisional algorithms to limit the number of newly diagnosed DLBCL pathology cases that are sent for FISH analysis in order to reduce the cost of testing. This article will discuss the clinical phenotype, morphologic pathology features, and the potential molecular identifiers commonly found in patients with HGBL-DH/TH, and the data to inform which patient pathology samples should be evaluated for DH/TH status by FISH. These screening methods are summarized in Table 1.
Table 1
| Screening tool | Access | Cost | Sensitivity | Comments | Recommended | |
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Clinical features | ↑↑ | ↓↓ | ↓↓ | No definitive clinical phenotype | X | |
| Pathologic morphology (BCLU) | ↑↑ | ↓ | ↓↓ | No definitive morphologic phenotype; inter-reader variability | X | |
| High Ki-67 Index | ↑ | ↓ | ↓↓ | No established threshold | X | |
| COO = GCB by IHC | ↑ | ↑↑ | ↓ | Variability in methods used | X | |
| MYC ≥40% & BCL2 ≥50% by IHC | ↑ | ↑ | ↓ | X | ||
| COO = GCB & MYC ≥40% & BCL2 ≥50% by IHC | ↑ | ↑ | ↓ | X | ||
| FISH break- apart probe for MYC | ↓ | ↑ | ↑ | Misses less common MYC rearrangements: t(2;8) (IGK-MYC) and t(8;22) (IGL-MYC) | X | |
| FISH dual fusion probe for MYC | ↓ | ↑ | ↑↑ | X | ||
BCLU, B-cell lymphoma, unclassifiable; COO, cell of origin; FISH, fluorescent in situ hybridization; GCB, germinal center B-cell; IHC, immunohistochemistry.
What are the clinical characteristics of patients with HGBL-DH/TH?
Baseline clinical characteristics are easily accessible features by clinicians and would potentially be an efficient way to screen patients for whose samples should be tested for HGBL-DH/TH. Unfortunately, there are conflicting reports that describe which clinical characteristics may help to identify these patients. Petrich et al. described a cohort of 311 patients diagnosed with HGBL-DH. The authors found that lactate dehydrogenase (LDH) and white blood cell counts were elevated in 76% and 22% of the patients, respectively (14). In a series of 129 HGBL-DH patients treated at MD Anderson, 84% of patients had advanced-stage disease and 87% had an International Prognostic Index (IPI) ≥2 at diagnosis (13). A study of 252 DLBCL patients by Niitsu et al. found that patients with MYC aberrations were more likely to have bone marrow involvement, a poor performance status, and an increased LDH level at diagnosis (12). To the contrary, a comparative retrospective analysis by Savage et al. evaluated clinical characteristics of 137 patients at DLBCL diagnosis and found that there were no statistical differences in median age, sex, performance status, LDH or IPI in patients with or without MYC rearrangements (15). Another study of 53 DLBCL patients concluded that no baseline clinical characteristic, including age, serum LDH, stage, or IPI predicted which patients would be diagnosed with HGBL-DH (17).
Complicating reliance on clinical features to predict for underlying HGBL-DH/TH, there may be subsets who have a more favorable outcome. Recently, several groups have observed that limited stage HGBL-DH/TH may overcome the adverse biology, although this is still controversial. A review of 129 patients with HGBL-DH from MD Anderson demonstrated that the 16 patients with limited stage HGBL-DH had similar complete response rates, event-free survival, and OS compared with advanced stage HGBL-DH (13). However, it is notable that all 5 of the patients with stage I HGBL-DH were in remission without events with a median follow-up of 17 months (range, 8–34 months) (13). A multicenter retrospective study pooled 201 limited stage DLBCL path samples and found that of 83 samples were FISH were available to determine double hit status, 6 patients samples (7%) were deemed HGBL-DH. After treatment with 3 cycles of RCHOP plus radiotherapy, these limited stage HGBL-DH demonstrated no statistically significant difference in 4-year PFS (85% vs. 89%, P=0.62) or 4-year OS (83% vs. 90%, P=0.70) when compared with non-HGBL-DH patients (18). Unfortunately, these sample sizes are small and until further data are available, screening by stage alone should not be performed when determining which newly diagnosed DLBCL patients should undergo FISH for MYC/BCL2/BCL6 rearrangements. In summary, although baseline clinical characteristics are a desirable way to screen patients for whose pathologic samples should be tested for HGBL-DH/TH, there is no consensus on clinical phenotype of these patients. Thus, we do not recommend using clinical characteristics to screen for HGBL-DH/TH.
Does the morphologic appearance or proliferation rate predict for underlying HGBL-DH/TH?
Since patients with HGBL-DH/TH typically have aggressive clinical behavior, one suggestion is to select lymphoma samples with an aggressive morphologic appearance for further evaluation by FISH; there is further potential that morphology may be prognostic within HGBL-DH/TH groups (19). From a histology perspective, DLBCL is classically defined as a diffuse proliferation of intermediate- to large-sized B-cells that can be described as centroblastic, immunoblastic, anaplastic or even lymphoblastic in appearance (1). The previous iteration of the WHO in 2008 included a provisional entity termed “B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma”, shortened to “B-cell lymphoma, unclassifiable” (BCLU) in many publications for simplicity (20). As the name implies, these lymphomas had shared features of both DLBCL and BL, and we now know that many of these aggressive-appearing lymphomas probably included a high proportion of HGBL-DH/TH. In the current WHO Classification, the morphology is to be noted by the hematopathologist, but if there are MYC or BCL2 and/or BCL6 rearrangements present, the diagnosis is immediately shifted to “high-grade B-cell lymphoma” with these noted rearrangements and renamed “HGBL-DHL/THL” (1).
However, can the initial morphology predict the likelihood of underlying HGBL-DH/TH? This question is unfortunately a difficult one to definitively answer, partly reflecting the heterogeneity and small size of many studies, and the lack of central pathology review in others. Nevertheless, among studies of established HGBL-DH/TH, there appears to be a concentration of variant and higher grade histologies, including BCLU from the WHO 2008 classification (1,20). One of the larger clinical series of over 300 patients with established HGBL-DH/TH found that approximately 50% of patients had DLBCL while 48% had BCLU (14). The high proportion of DLBCL in this series probably reflects that DLBCL, not otherwise specified (NOS) is the most common histology but may also be related to the lack of central pathology review whereby cases with centroblastic and immunoblastic morphology may have been lumped into one category for clinical analysis. If we approach the converse situation of just looking at patients with DLBCL, NOS, the frequency of underlying HGBL-DH/TH is much lower. A recent very large series evaluated 1,228 DLBCL biopsies from three prospective trials plus a population-based registry and found 7.9% incidence of HGBL-DH/TH; however, this series was specifically restricted to patients with DLBCL, NOS histology, and the frequency of HGBL-DH/TH may be different if higher grade morphology was included (9). Overall, the data support that patients with HGBL-DH/TH are more likely to have blastoid morphology or BCLU, but that restricting screening based on cytologic features alone would miss a high proportion of DLBCL, NOS cases that would be recategorized as HGBL-DH/TH if FISH were added. Thus, morphology alone is not a reliable predictor to screen for HGBL-DH/TH.
A correlate to morphology is proliferation rate. Given the proliferative impact of MYC rearrangements, Ki-67 has been proposed as a screening tool for underlying HGBL-DH/TH. Ki-67 is a protein that can be measured in pathologic samples by immunohistochemistry (IHC) and is used as a surrogate marker for proliferation. As HGBL-DH/TH patients typically have an aggressive clinical course and since MYC overexpression drives cellular proliferation, it has been hypothesized that HGBL-DH/TH tumor cells should express a high percentage of Ki-67 (commonly called a Ki-67 index or score). This screening approach has been shown to be ineffective in accounting for all patients with HGBL-DH/TH as demonstrated by multiple retrospective studies. In a study of 135 DLBCL patient samples, 12 were found to have a MYC translocation. Of those 12 only 7 (58%) had a Ki-67 score of >80% (15). Another study of 162 patients with high-grade B-cell lymphoma, identified 35 patients with MYC rearrangements. Of those 35, 29 patient samples had a Ki-67 score of >80%, leaving 17% of patients who would be missed by this Ki-67 score cutoff (21). A third study reviewed 53 DLBCL patient samples and found 17 cases of HGBL-DH. Interestingly, the median Ki-67 score was no different between samples with HGBL-DH/TH and non-HGBL-DH with a trend towards a higher score in the non-HGBL-DH samples (80% vs. 90%, P=0.53) (17). As such, we do not recommend screening patients by Ki-67 score to determine which patients to test for MYC/BCL2/BCL6 translocations.
Can molecular features or protein expression be used to predict for underlying HGBL-DH/TH?
As demonstrated in the preceding paragraphs, there is no clearly identifiable clinical phenotype or pathologic morphology that accurately predicts for underlying HGBL-DH/TH without missing a substantial number of cases. The molecular features of the underlying lymphoma such as cell of origin (COO) and MYC/BCL2 protein expression are considered a more objective means to screen for HGBL-DH/TH (Figure 1).
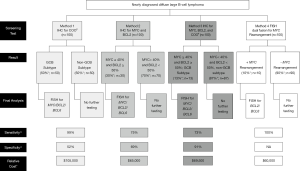
Does COO predict for underlying HGBL-DH/TH?
DLBCL can pathologically be classified by its COO, which means that molecular features of the mature cancer can be traced back to the particular stage of B-cell differentiation from which the malignant B-cells are derived. COO can be divided into Germinal Center B-cell (GCB), where differentiation of the cancer occurred in the germinal center of the lymph node, and non-GCB, where the malignant B-cell arose at a later state of B-cell differentiation (22). COO can be determined from pathologic DLBCL samples by using IHC staining and following the Hans algorithm or by gene expression profiling, such as the Lymph2Cx assay (22-24). The Hans algorithm is the most widely used method secondary to availability of IHC stains and reduced cost compared to gene expression profiling techniques. This algorithm stratifies patients based on three separate IHC stains for CD10, BCL6, and MUM1 (23). It has been reported that the concordance between the Hans algorithm and Lymph2Cx assay is around 70%, so there may be some discrepancy depending on the method used (25). Multiple retrospective studies have reported a higher prevalence of HGBL-DH in the GCB subtype of DLBCL, ranging from 64–99% (9,11,26-28). The largest study to date of 1,228 DLBCL biopsies found ~8% incidence of HGBL-DH/TH. When using the Hans algorithm or the Lymph2Cx assay, the authors reported that 99% of the HGBL-DH (MYC/BCL2) were of the GCB subtype (9). As these tests found that ~50% of the total patient samples were GCB-type, screening only the GCB samples by FISH for HGBL-DH would reduce the number of newly diagnosed DLBCL samples necessary to test by ~50% (9). However, screening only the GCB type patients by FISH would miss diagnosis of the 5% of non-GCB that are MYC-translocated and the 1.7% of non-GCB that are HGBL-DH that harbor MYC/BCL2 translocations or MYC/BCL6 translocations (9). Therefore, many HGBL-DH/TH will be missed if screening is only based upon COO. A summary of this screening method can be found in Figure 1: Method 1.
Can IHC for MYC/BCL2 expression predict underlying HGBL-DH/TH?
A frequently suggested method to reduce the number of DLBCL samples requiring FISH testing is to screen pathologic specimens via IHC. Arguments for this method are the lower cost (estimated at 4–5 folds less than FISH studies) and nearly universal availability of IHC staining. However, using IHC expression of MYC and BCL2 is confounded by the recent understanding that isolated dual protein expression (DPE) without underlying chromosomal rearrangements is a distinct and adverse prognostic factor in DLBCL, NOS. The definition of DPE is IHC overexpression of MYC protein (≥40%) and the BCL2 protein (≥50%). It is critical to understand that the WHO does not consider DPE as a separate entity, but as a poor prognostic factor predicting inferior survival compared to patients without DPE (1). However, the negative impact of DPE on survival is less than patients with HGBL-DH (11). The DPE subgroup is also distinguished from the HGBL-DH subgroup secondary to the higher prevalence in the DLBCL population (~30%) and the predisposition to be seen in the non-germinal GCB subtype by COO (28). Despite these differences, some experts recommend the identification of MYC and BCL2 protein overexpression by IHC to limit who should be tested for MYC/BCL2/BCL6 translocations by FISH. We caution the use of MYC and BCL2 expression to screen for HGBL-DH/TH based upon the following data. Scott et al. retrospectively evaluated 1,228 patient samples and found that 48% (n=519) expressed MYC ≥40% by IHC. If this protein expression threshold was used to screen for HGBL-DH/TH, 20% of the cases would be missed (9). This group used 905 biopsies with available data to study percentage increments of MYC positivity and demonstrated that no MYC IHC threshold (apart from 0%) that would detect all biopsies with MYC rearrangement (9). The same group found that 34% of the total cohort were considered DPE by IHC, using the standard cutoffs of MYC ≥40% and BCL2 ≥50%. Unfortunately, using these criteria as a screening study would miss 25% of samples with HGBL-DH/TH (9). Horn et al. retrospectively evaluated 442 DLBCL patient samples and found that if testing for MYC translocation was only performed on those samples that had ≥40% MYC protein expression, 30% of samples containing a MYC translocation would be missed (29). Kluk et al. reviewed 256 pathology cases of aggressive B-cell lymphoma with available MYC IHC data (30). They found that a MYC expression of >40% was used, this held a 90% sensitivity of predicting samples that also harbored a MYC rearrangement, which would miss the diagnosis in 10% of cases (30). Sakr et al. retrospectively analyzed 272 consecutive pathology samples diagnostic of aggressive B-cell lymphoma at a single center (31). Of this group, 156 cases had available data for both IHC and FISH. MYC translocations were detected in 31/35 (89%) patient samples that had MYC expression ≥40%, indicating that 11% of MYC translocations would be missed if MYC expression by IHC was used as a screening tool. In this study, 15 HGBL-DH cases were diagnosed. Three of those 15 HGBL-DH cases (20%), were negative for MYC overexpression by IHC (31). Secondary to these data indicating that many HGBL-DH/TH diagnoses will be missed by screening only for MYC and/or BCL2 protein expression, we do not recommend this approach. A summary of this screening method can be found in Figure 1: Method 2.
Some proponents of IHC as a screening tool recommend screening both for dual MYC and BCL2 expression and for GCB subtype prior to testing any samples by FISH. Scott et al. found that this would limit the population tested by FISH to only 11–14% of the total DLBCL population, however, it would result in missing up to a quarter of the cases of HGBL-DH/TH (9). As such, we also do not recommend this approach. A summary of this screening method can be found in Figure 1: Method 3.
Could sequential FISH studies for MYC followed by BCL2/BCL6 translocations be an effective screening strategy to identify patients with HGBL-DH/TH?
An alternate strategy to identify patients with HGBL-DH/TH is to screen every pathology sample of a newly diagnosed high grade B-cell lymphoma with FISH probes for dual IGH-MYC fusion. If positive, a sequential FISH study could be performed on the sample for BCL2 and BCL6 translocations. A typical FISH dual fusion probe targets the IGH-MYC fusion and can detect the classic translocation partner, t(14;18) as described in the introduction, but can also detect the less common translocations between immunoglobulin (IG) and MYC, t(2;8) (IGK-MYC) and t(8;22) (IGL-MYC). The less common mutations comprise <5% of MYC translocations, but could be missed if a FISH break-apart probe was used in lieu of the dual fusion probe (9,32). The sensitivity of this method should approach 100% in those cases where IG is the translocation partner for MYC. MYC can have other translocation partners with non-IG genes. In a study that identified 54 patients with MYC translocations, 24 were translocated with non-IG gene partners, most commonly t(8;9)(q24;p13) in 13 of 24 patient samples (33). In multivariable analysis a non-IG gene translocation partner was associated with more favorable survival compared with an IG gene partner (33). Similarly, another retrospective study identified 28/225 DLBCL patient samples that harbored a MYC translocation. They were able to identify an IG translocation partner in 12 of the 24 available samples. The authors found that MYC translocation with IG translocation partner gene was associated with worse OS compared with MYC translocation with non-IG translocation partner gene (34). Therefore, although performing FISH with a dual fusion IGH-MYC probe would not detect non-IG partner MYC translocations, but this may not be as clinically relevant. The positive predictive value of this method is not 100% as there are about 5% of all patients with DLBCL that have a single MYC translocation that is not paired with a BCL2/BCL6 translocation (9). As multiple publications have demonstrated worse prognosis and increased risk of CNS relapse with single MYC translocation in DLBCL, one could argue that it is clinically relevant to identify even patients with a single translocation (15). In summary, with the high sensitivity of screening all newly diagnosed high grade B-cell lymphoma tumor samples for MYC rearrangements with a dual fusion FISH probe, we feel that this is the most efficient way not to miss the diagnosis of HGBL-DH/TH. A summary of this screening method can be found in Figure 1: Method 4.
Should clinicians wait for final FISH results prior to initiation of chemotherapy?
As the processing times for FISH studies are highly variable between labs, a practical concern physicians often face is whether to wait for final FISH results prior to initiation of chemotherapy. This problem is of particular relevance if sequential testing of the pathology is performed, potentially leading to further delays. As high-grade B-cell lymphomas such as DLBCL are clinically very aggressive, these patients require urgent therapy. As such, we do not recommend delaying the initiation of therapy to wait for FISH results. Once FISH results are available, the treatment plan can be tailored to match the patient’s molecular risk. For example, if a patient is diagnosed with DLBCL and FISH results are unavailable, we proceed with RCHOP therapy for cycle 1. Subsequently, if the FISH studies confirm HGBCL-DH/TH, the remainder of the cycles can be switched to REPOCH and include CNS prophylaxis.
What are the approximate costs of each of these molecular screening methods?
Sensitivity of the screening methods should certainly be taken into account when selecting an institutional standard for screening DLBCL patient samples for HGBL-DH/TH. However, another factor that should be considered is the cost of molecular analysis of these patient samples. Unfortunately, it is a bit difficult to ascertain the exact cost of these studies as the charges can be variable from one insurance company to another. In attempt to approximate the cost of each method, we roughly estimated costs based on Medicare reimbursement rates as many patients with DLBCL have Medicare insurance and many other insurance companies base reimbursement upon Medicare rates. Using current procedural terminology codes for each IHC stain and FISH study (88342, 88274, 88365, and 88291) including codes for technical procedure and for provider interpretation, we were able to estimate that each IHC stain costs approximately $100 and each FISH study is approximately $400–$500 (https://www.cms.gov/medicare-coverage-database; accessed July 8, 2018). Using these factors, we have calculated relative cost of each screening method which is pictured in Figure 1. Notably, Method 1 (screening for GCB by IHC) is the most expensive and Methods 2–4 are similar in relative cost. Therefore, we again recommend Method 4 (screening with FISH for MYC rearrangements) as the most cost-effective screening method.
Conclusions
The poor prognosis and adverse outcomes following standard chemoimmunotherapy for patients with aggressive B-cell lymphomas harboring dual rearrangements of MYC and BCL2 and/or BCL6 is now well-established. Due to the need for more intensive induction chemotherapy than RCHOP and the potential need to implement CNS prophylaxis, it is crucial for treating physicians to know whether a patient with newly diagnosed DLBCL fits into the HGBL-DH/TH category. In a cost-conscious era, routine and widespread testing for biologic determinants of outcome may not be appropriate, and a critical appraisal of predictors is warranted. As a distinct clinical phenotype or pathologic morphology cannot be identified to accurately predict for underlying HGBL-DH/TH, the molecular features of the underlying lymphoma are a more objective means to screen for HGBL-DH/TH. Herein, we have summarized the data to support various methods of screening by molecular features including COO, protein expression, and sequential FISH testing (Figure 1). We recommend screening all patient samples with newly diagnosed high-grade B-cell lymphoma with a dual fusion FISH probe for MYC-IG translocations (Figure 1: Method 4). If the FISH study is positive, the sample can then be tested for BCL2/BCL6 translocations. Based on these data, this is both the most sensitive and cost-effective method to diagnose patients with HGBL-DH/TH and to best inform treating physicians to aid in the clinical management of these patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Astrid Pavlovsky) for the series “Diffuse Large B-Cell Lymphoma” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol.2018.11.01). The series “Diffuse Large B-Cell Lymphoma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer 2008;8:976-90. [Crossref] [PubMed]
- Sesques P, Johnson NA. Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood 2017;129:280-8. [Crossref] [PubMed]
- Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988;335:440-2. [Crossref] [PubMed]
- Phan RT, Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature 2004;432:635-9. [Crossref] [PubMed]
- Weiss LM, Warnke RA, Sklar J, et al. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med 1987;317:1185-9. [Crossref] [PubMed]
- Porton B, Zaller DM, Lieberson R, et al. Immunoglobulin heavy-chain enhancer is required to maintain transfected gamma 2A gene expression in a pre-B-cell line. Mol Cell Biol 1990;10:1076-83. [Crossref] [PubMed]
- Agarwal R, Lade S, Liew D, et al. Role of immunohistochemistry in the era of genetic testing in MYC-positive aggressive B-cell lymphomas: a study of 209 cases. J Clin Pathol 2016;69:266-70. [Crossref] [PubMed]
- Scott DW, King RL, Staiger AM, et al. High-grade B-cell lymphoma with. Blood 2018;131:2060-4. [Crossref] [PubMed]
- Barrans S, Crouch S, Smith A, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol 2010;28:3360-5. [Crossref] [PubMed]
- Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012;30:3452-9. [Crossref] [PubMed]
- Niitsu N, Okamoto M, Miura I, et al. Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia 2009;23:777-83. [Crossref] [PubMed]
- Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166:891-901. [Crossref] [PubMed]
- Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood 2014;124:2354-61. [Crossref] [PubMed]
- Savage KJ, Johnson NA, Ben-Neriah S, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood 2009;114:3533-7. [Crossref] [PubMed]
- Savage KJ, Slack GW, Mottok A, et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood 2016;127:2182-8. [Crossref] [PubMed]
- Landsburg DJ, Nasta SD, Svoboda J, et al. 'Double-Hit' cytogenetic status may not be predicted by baseline clinicopathological characteristics and is highly associated with overall survival in B cell lymphoma patients. Br J Haematol 2014;166:369-74. [Crossref] [PubMed]
- Barraclough A, Alzahrani M, Schmidt Ettrup M, et al. Impact of Immunohistochemistry-Defined Cell of Origin and MYC Rearrangements on Outcomes in Patients with PET-Defined Stage I/II Diffuse Large B-Cell Lymphoma Treated with R-CHOP ± Radiotherapy: An International Multi-Center Retrospective Study. Blood 2017;130:415.
- Swerdlow SH. Diagnosis of 'double hit' diffuse large B-cell lymphoma and B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma: when and how, FISH versus IHC. Hematology Am Soc Hematol Educ Program 2014;2014:90-9. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Harris NL, et al. World Health Organization Classification of Tumorurs of Haematopoietic and Lymphoid Tissue, 4th Edition. Lyon, France: International Agency on Research for Cancer, 2008.
- Foot NJ, Dunn RG, Geoghegan H, et al. Fluorescence in situ hybridisation analysis of formalin-fixed paraffin-embedded tissue sections in the diagnostic work-up of non-Burkitt high grade B-cell non-Hodgkin's lymphoma: a single centre's experience. J Clin Pathol 2011;64:802-8. [Crossref] [PubMed]
- Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503-11. [Crossref] [PubMed]
- Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275-82. [Crossref] [PubMed]
- Scott DW, Mottok A, Ennishi D, et al. Prognostic Significance of Diffuse Large B-Cell Lymphoma Cell of Origin Determined by Digital Gene Expression in Formalin-Fixed Paraffin-Embedded Tissue Biopsies. J Clin Oncol 2015;33:2848-56. [Crossref] [PubMed]
- Yoon N, Ahn S, Yong Yoo H, et al. Cell-of-origin of diffuse large B-cell lymphomas determined by the Lymph2Cx assay: better prognostic indicator than Hans algorithm. Oncotarget 2017;8:22014-22. [Crossref] [PubMed]
- Ye Q, Xu-Monette ZY, Tzankov A, et al. Prognostic impact of concurrent MYC and BCL6 rearrangements and expression in de novo diffuse large B-cell lymphoma. Oncotarget 2016;7:2401-16. [PubMed]
- Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012;30:3460-7. [Crossref] [PubMed]
- Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 2013;121:4021-31; quiz 250. [Crossref] [PubMed]
- Horn H, Ziepert M, Becher C, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood 2013;121:2253-63. [Crossref] [PubMed]
- Kluk MJ, Ho C, Yu H, et al. MYC Immunohistochemistry to Identify MYC-Driven B-Cell Lymphomas in Clinical Practice. Am J Clin Pathol 2016;145:166-79. [Crossref] [PubMed]
- Sakr H, Cook JR. Identification of "Double Hit" Lymphomas Using Updated WHO Criteria: Insights From Routine MYC Immunohistochemistry in 272 Consecutive Cases of Aggressive B-Cell Lymphomas. Appl Immunohistochem Mol Morphol 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Muñoz-Mármol AM, Sanz C, et al. MYC status determination in aggressive B-cell lymphoma: the impact of FISH probe selection. Histopathology 2013;63:418-24. [Crossref] [PubMed]
- Johnson NA, Savage KJ, Ludkovski O, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood 2009;114:2273-9. [Crossref] [PubMed]
- Pedersen MØ, Gang AO, Poulsen TS, et al. MYC translocation partner gene determines survival of patients with large B-cell lymphoma with MYC- or double-hit MYC/BCL2 translocations. Eur J Haematol 2014;92:42-8. [Crossref] [PubMed]
Cite this article as: Stephens DM, Smith SM. Diffuse large B-cell lymphoma—who should we FISH? Ann Lymphoma 2018;2:8.


