
Primary central nervous system lymphoma: consolidation strategies
Introduction
Similar to diffuse large B-cell lymphomas (DLBCLs) that arise outside the central nervous system (CNS), the treatment of primary CNS lymphoma (PCNSL), mainly represented by DLBCL involves two critical steps—to reach complete remission (CR) and, then, to prevent a relapse. Most patients with systemic DLBCL or PCNSL who do not truly achieve a CR will experience progression or relapse (1). 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) is a diagnostic tool with a high sensitivity to discriminate between a CR and a partial response (PR) in patients with systemic DLBCL, which allows for PET-driven consolidation strategies aimed to reduce the risk of relapse (2). Disappointingly, discriminating sensitivity of conventional neuroimaging and 18F-FDG-PET is remarkably lower in patients with PCNSL. This resulted in a more extensive use of consolidation therapies in patients with PCNSL who achieve a CR or a PR after induction, with the aim of eliminating the residual malignant cells and preventing relapse.
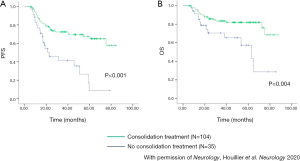
With regard to PCNSL, non-randomized studies support the beneficial role of consolidation treatment in reducing the risk of disease relapse. The long-term follow-up of patients with PCNSL treated with high-dose methotrexate (HD-MTX)-based induction chemotherapy has shown that progression-free survival (PFS) is significantly shorter in patients treated with chemotherapy alone (without consolidative radiotherapy) compared with patients treated with chemo-radiotherapy (3). However, some of these studies exhibit a selection bias related to the inclusion of patients with progressive disease during induction in the subgroup of patients treated without consolidation, whereas all patients who received consolidative radiotherapy had a lymphoma responsive to induction. Conversely, an ANOCEF retrospective study demonstrated that half of patients younger than 60 years old who did not receive consolidation radiotherapy after achieving a CR to HD-MTX-based induction chemotherapy experienced relapse, with a 3-year PFS of 28% (4). Although a control group is lacking, this is a disappointing outcome in comparison with reported series of patients treated with HD-MTX-based chemotherapy and consolidation therapy. In fact, a recent analysis of 1,002 patients registered in the French oculo-cerebral lymphoma network (LOC) database (5) has shown that the addition of consolidative whole-brain irradiation or autologous stem cell transplantation (ASCT) is associated with significantly improved PFS and overall survival (OS) in patients <60 years old who achieve CR after induction chemotherapy (Figure 1).
Whole-brain radiotherapy (WBRT) was the first and most used consolidation for decades, while more recently, consolidation strategies have evolved and new approaches are being investigated. Several studies have focused on the different available consolidation therapies used in patients with PCNSL, sometimes with contrasting results. This review aims to present the most important studies in this field, and the tolerability and efficacy of the different approaches used are analyzed in order to distinguish the best candidates for each option and provide recommendations for routine practice.
Consolidation strategies
Consolidative radiotherapy (Table 1)
Table 1
| Ref. | Type of study | N | Median age [range] (years) | Type of irradiation | CNS toxicity | Outcome |
|---|---|---|---|---|---|---|
| (6) | Prospective, multicenter phase II1 | 53 | 55 [18–60] | WBRT 40 Gy | Prospectively assessed: more than half of the patients exhibited a decline in their test score after irradiation | 2-year PFS =67%±5% |
| (7) | Prospective, multicenter phase II*2 | 53 | 57 [18–70] | WBRT 36 Gy ± boost 9 Gy | Prospectively assessed: significant impairment in some attention and executive functions after irradiation | 2-year PFS =76%±5% |
| (8) | Prospective, multicenter phase II | 53 | 55 [36–60] | WBRT 26 Gy + boost (54 Gy total dose). WBRT 40 Gy if multifocal or large-volume bifocal involvement | Not assessed | 2-year PFS =67%±5% |
| (9) | Prospective, multicenter phase II | 31 | 60 [30–79]: <60 years, n=16; ≥60 years, n=15 | WBRT 23.4 Gy in CR patients | Prospectively assessed: stable in patients <60 years; not assessed in patients ≥60 years; delayed leukoencephalopathy | <60 years: 2-year PFS =94%±5%; ≥60 years: 2-year PFS =60%±5% |
| (10) | “Real life” | 27 | 50.2 [25–60] | WBRT 23.4 Gy in patients with CR | Prospectively assessed: no cognitive impairment | 2-year PFS =65%±5% |
*, these two studies were randomized phase II studies, including an arm evaluating IC + ASCT. Only results regarding the irradiation arm are presented. 1, five patients who were nonresponders to induction chemotherapy received WBRT; 2, only patients with CR and PR received WBRT. CNS, central nervous system; WBRT, whole-brain radiotherapy; PFS, progression-free survival; CR, complete remission; IC, intensive chemotherapy; ASCT, autologous stem cell transplantation.
Since the introduction of HD-MTX in the treatment of PCNSL, the standard first-line consolidation treatment was historically represented by radiotherapy. PCNSL is a multifocal disease, with frequent involvement of the eyes, which led radiation oncologists to include the whole encephalon and orbits, or at least the posterior two-thirds of them, within the radiation volume. Following the example of systemic DLBCL, the radiation dose has been suggested according to the response to induction chemotherapy. Thus, the most common therapeutic sequence has long started with a HD-MTX-based chemotherapy followed by WBRT delivering 36–40 Gy, which provided better results than WBRT alone (11). However, such sequential treatments were retrospectively identified as a risk factor for delayed neurotoxicity leading to mild to severe cognitive impairment and gait disorder with devastating consequences on quality of life, even among patients who achieved CR (3,12-14). It also became obvious that this risk was more frequent and had a more rapid onset in patients over 60 years of age, although younger patients were not spared in the long term (12). Thus, a large randomized phase III study has been conducted in Germany with the main objective to establish the risk-benefit of avoiding consolidation radiotherapy (15). In this trial, patients received induction therapy with HD-MTX alone or associated with high-dose ifosfamide: Patients who achieved CR were randomly allocated between immediate WBRT and observation, whereas patients who did not achieve CR were randomized between complementary WBRT and high-dose cytarabine monotherapy. Neurotoxicity was only assessed by MRI. The results of that trial indicated that consolidation WBRT was associated with a significantly better PFS (median 18 vs. 12 months), but did not change OS (median 32 vs. 37 months). Unfortunately, this randomized trial failed to close the debate on the risk-benefit issue of consolidative radiotherapy because of serious protocol violations that could be source of potential bias (16,17).
Because the prognosis of PCNSL remains poor, which prevents physicians from dropping consolidation treatment, and the question on the risk-benefit of post-chemotherapy radiation therapy remains open, several groups have explored alternatives to reduce the neurotoxicity of consolidation treatment. In particular, a progressive reduction of radiation volume and doses was explored in recent studies in an effort to reduce iatrogenic neurotoxicity.
A technique of reduced and fractionated WBRT dose with a tumor-bed boost was addressed in the GELA trial “R-C5R” (8). After induction immuno-chemotherapy, patients received whole-brain irradiation with 26 Gy by two daily fractions of 1 Gy, followed by a booster dose on the initial gadolinium-enhanced tumors, up to a total dose of 54 Gy, with the same fractionation. In case of three or more lesions or large-volume bifocal involvement, which was demonstrated in half of the patients, the irradiation consisted of 40 Gy WBRT without boost. Because of the single arm design of this study lacking a prospective assessment of cognitive functions, advantages in terms of toxicity and efficacy of such an irradiation procedure cannot be demonstrated. As supportive evidence, a retrospective study performed in the pre-rituximab era reported a 2-year PFS of 80% and good cognitive safety in 60 Korean patients treated with a MPV regimen followed by reduced-dose WBRT (rdWBRT) (27 Gy) plus a 23 Gy tumor-bed boost (18).
The efficacy and toxicity of rdWBRT (23.4 Gy) with no boost in patients with radiographic CR after induction have been assessed in some prospective and retrospective studies. Planned treatment for the 52 patients (15 aged 60 or over) enrolled in a phase II study consisted of R-MVP induction chemotherapy (rituximab, HD-MTX, vincristine, and procarbazine) followed by a response-tailored WBRT dose and two cycles of high-dose cytarabine (9). Thirty-four patients entered CR after the induction chemotherapy among which 31 patients received the planned rdWBRT. In this group of patients, results were encouraging. The reduced radiation dose did not seem to impact the median PFS, which was 4.4 years in patients ≥60 years old (n=15) and not reached in patients under 60 years of age (n=16) at a median follow-up of 5.9 years. The 2-year PFS in the small series of young patients was 94%±5%. The results of neurocognitive tests in the nine tested patients under 60 years old were better than historical controls receiving a higher dose of WBRT (45 Gy). The safety of rdWBRT in elderly patients remains to be determined, since only three patients older than 60 years completed the neuropsychological evaluation. It was noteworthy that delayed leukoencephalopathy was recorded in 7 out of 12 patients.
The French network for PCNSL reported, as a meeting abstract, a series of 27 patients under 60 years of age who achieved CR after HD-MTX-based chemotherapy and received rdWBRT as consolidation, but with no post-radiation cytarabine, in a real-life setting (10). The 2-year PFS was 65%±5%, which was lower than that observed in the above-mentioned phase II study (9) but similar to that reported in previous studies using conventional-dose WBRT (6,7,15). The analysis of the results from neuropsychological tests is ongoing and survival data are maturing.
The safety and efficacy of rdWBRT was assessed in a randomized phase II trial of the Radiation Therapy Oncology Group (RTOG1114 trial). The first results were recently reported as a meeting abstract (19). A total of 87 patients with PCNSL were randomly allocated between the R-MPV regimen with or without rdWBRT (23.4 Gy) and conventional fractionation, followed by two cycles of high-dose cytarabine. As expected, the addition of rdWBRT was associated with a significantly better 2-year PFS (54% for chemo arm vs. 78% for chemo-radiation arm), without increased toxicity or clinically defined cognitive decline. These preliminary results support the role of consolidation in patients with CR. The analysis of the results from neuropsychological tests is ongoing. OS data are maturing. The effect of this strategy on OS is an important issue that could help researchers to distinguish the results of this trial from figures of the German randomized phase III study (15). In this scenario, it is noteworthy that the use of salvage radiotherapy in 44% of the chemo arm patients with relapsed lymphoma could have introduced a confounding bias.
The reduction in radiation volume may be a valid alternative to maintain the efficacy of consolidative radiotherapy while reducing neurotoxicity, especially the memory loss. For this purpose, it has been suggested to avoid the irradiation of the hippocampus, but no studies have been conducted on PCNSL, which often involves areas close to the hippocampus, because of the risk of undertreating patients. Stereotactic radiosurgery was assessed in a single study on an observational cohort of 128 patients with PCNSL treated with HD-MTX monotherapy (n=73) or followed by gamma knife radiosurgery (n=55) 11 to 16 Gy (median: 11 Gy) (20). The addition of gamma knife radiosurgery has been associated with better OS compared to chemotherapy alone (median 48 and 27 months, respectively). However, patients were not stratified according to site, size or number of lesions. The details regarding the site of relapse and results of neurocognitive tests are lacking, and confirmatory prospective studies are mandatory.
Consolidative chemo-radiation therapy
Following several other examples in oncology and, in particular, the treatment of high-grade gliomas, the addition of concomitant chemotherapy to WBRT has been proposed. Temozolomide (TMZ) is an oral alkylating agent with radiomimetic effects in CNS tumors, and it is capable of crossing the blood-brain barrier, with 38% CR in patients with relapsed PCNSL (21). The safety and efficacy of the addition of TMZ concomitant to and adjuvant after WBRT was assessed in a randomized phase III trial of the Japanese Clinical Oncology Group (JCOG1114C trial). The results of the interim analysis were recently reported as a meeting abstract (22) and suggested that this trial failed to demonstrate any benefit of this strategy. However, patients were treated with a largely demonstrated suboptimal induction (MTX 3.5 g/m2 every 3 weeks), and median follow-up was only 19 months. Study of the MGMT gene promoter methylation status is ongoing and additional follow-up is needed.
Intensive chemotherapy with autologous stem cell rescue (IC + ASCT)
IC + ASCT was first evaluated as not only a consolidation treatment in refractory or relapse (R/R) PCNSL by analogy with systemic aggressive lymphomas (23) but also as a means to overcome the blood-brain barrier, which limits the CNS penetrance of most anticancer drugs, when used at conventional doses. A couple of retrospective and prospective studies showed that IC + ASCT without radiotherapy was feasible and efficient in the specific setting of patients with R/R PCNSL (24-27). So far, no study has compared the outcomes of patients with R/R PCNSL who received ASCT consolidation vs. no or other consolidation. However, large data base studies have shown that patients who experienced the longest PFS and OS were those who were chemosensitive to the salvage chemotherapy and who received IC + ASCT (5). Despite a 60–80% overall response rate after high-dose cytarabine or ifosfamide-based salvage chemotherapies, the duration of second responses were short if not consolidated by ASCT. At relapse, most of the studies have used thiotepa-based IC (24-27). A French group (24,25) selected an IC consisting of high-dose thiotepa-busulfan-cyclophosphamide (TBC) because this combination of drugs proved feasible and effective in systemic lymphomas with poor prognosis (28) including patients with CNS involvement (29) and because thiotepa and busulfan have good CNS bioavailability (30). In a prospective phase II study for R/R PCNSL, the 2-year PFS of the 27 patients who received IC + ASCT regardless of their response to salvage treatment was 58% (24). In the retrospective French study that included 79 patients, the 5-year OS rate was 62% for patients who presented with a chemosensitive relapse (25), with a treatment-related mortality (TRM) rate of 8%. In the prospective German study (26) using rituximab-thiotepa-carmustine (BCNU) in 32 patients, the 3-year OS rate was 55%, with a TRM rate of 12%. The therapeutic results are in line with those obtained in R/R systemic aggressive lymphomas (31), although the rate of treatment-related deaths seemed higher in the patients with PCNSL receiving HD thiotepa-based IC.
The use of IC + HSCT in first-line treatment has been evaluated by several groups. To evaluate the role of ASCT in first-line treatment, only studies that did not plan WBRT after ASCT should be considered.
The first prospective study addressing this issue included 28 patients, among which 14 proceeded to ASCT (32). The IC was the BEAM regimen (carmustine 300 mg/m2; etoposide 800 mg/m2; cytarabine 1,600 mg/m2; melphalan 140 mg/m2), which is widely used for systemic lymphomas. The results were deemed poor because of a high rate of early relapses after ASCT, resulting in a median event-free survival (EFS) of only 9.3 months for the 14 patients who underwent transplantation. From then, the BEAM regimen has been less used. The combination of busulfan-etoposide-cyclophosphamide showed disappointing results in one study (33). Conversely, encouraging results were reported in retrospective or single-arm monocentric studies (34-38), either with the combination of thiotepa-BCNU or of thiotepa-busulfan +/− cyclophosphamide, which resulted in the wide use of thiotepa-based IC in first-line treatment (Table 2).
Table 2
| Ref. | N | Median age [range] (years) | Type of study | Intensive chemotherapy | Outcome |
|---|---|---|---|---|---|
| (32) | 14 | 53 [25–71] | Multicenter phase II | BEAM: carmustine 300 mg/m2; etoposide 800 mg/m2; cytarabine 1,600 mg/m2; melphalan 140 mg/m2 | 3-year EFS: 25% |
| (36) | 16 ASCT | 55 [18–70] | Multicenter phase II | Thiotepa (10 mg/kg); busulfan (16 mg/kg) | WBRT if no CR after chemo (n=3); 2-year EFS: 56%; TRM in 2 patients |
| (34) | 13 | 54 [38–67] | Pilot study | Thiotepa (20 mg/kg); carmustine (400 mg/m2) | 3-year PFS: 77%; WBRT if no CR after chemo (n=3) |
| (33) | 11 | 52 [33–65] | Retrospective, single center | Busulfan (3.2 mg/m2); cyclophosphamide (100 mg/kg); VP16 (800 mg/m2) | WBRT if no CR after chemo (n=2); 2-year EFS: 30% |
| (35) | 26 | 57 [23–67] | Monocenter phase II | Thiotepa (750 mg/m2); busulfan (8 mg/kg); cyclophosphamide (120 mg/kg) | 3-year PFS: 79%; TRM in 3 patients (11%) |
| (39) | 15 | 70 [66–75] | Retrospective multicenter | Multiple thiotepa-based regimens | 2-year PFS: 80% |
| (37) | 27 PCNSL | 57 [50–64] | Retrospective, single-center | Thiotepa (750 mg/m2); busulfan (9.6 mg/kg); cyclophosphamide (120 mg/kg) | 2-year PFS: 95% for 21 patients in CR1 at time of ASCT; TRM in 1 patient (2.1%) |
| (38) | 46 in CR1 | 59 [29–69] | Retrospective, two institutions | Thiotepa (750 mg/m2); busulfan (7.2–9.6 mg/kg); cyclophosphamide (120 mg/kg) | 2-year PFS: 92%; 2-year NRM: 2.9% |
| (7) | 54 | 58 [18–70] | Multicenter phase II*2 | Thiotepa (20 mg/kg); carmustine (400 mg/m2) | 2-year PFS from consolidation: 72%; TRM in 5 patients (9%)§ |
| (6) | 44 | 55 [18–60] | Multicenter phase II*1 | Thiotepa (750 mg/m2); busulfan (8 mg/kg); cyclophosphamide (120 mg/kg) | 2-year PFS from consolidation: 87% and 97% in responder patients before ASCT TRM in 5 patients (11%)§ |
*, these two studies were randomized phase II studies, including an arm evaluating WBRT. Only results regarding the ASCT arm are presented; 1, five patients who were non-responders to induction chemotherapy proceeded to ASCT; 2, only patients with CR and PR proceeded to WBRT; §, were considered TRM, patients who died in the first 3 months after ASCT, as well as late infection and late lymphoma-unrelated death. TRM, treatment-related mortality; NRM, non-relapse mortality; WBRT, whole-brain radiotherapy; EFS, event-free survival; ASCT, autologous stem cell transplantation; PFS, progression-free survival; CR, complete response; PCNSL, primary central nervous system lymphoma; CR1, first complete response.
More recently, two randomized phase II studies, the IELSG-32 (7) and the PRECIS trials (6), have evaluated the role of IC + ASCT as part of first-line treatment in patients with PCNSL, in parallel with a control arm with conventional WBRT consolidation. These 2 studies presented several similar features (Table 3). They were conducted during the same period. Their primary end-point was the 2-year PFS, and the WBRT modalities were very close, delivering either 36 Gy with the addition of a 9 Gy tumor-bed boost in patients with a PR in the IELSG trial or 40 Gy with no boost in the PRECIS trial. Both studies scheduled prospective neurocognitive assessments to focus on the CNS toxicities following consolidation treatments. Only patients up to 60 years of age were included in the PRECIS trial, while selected fit older patients up to 70 years of age were included in the IELSG trial, which resulted in a median age of 55 and 58 years old, respectively. The IC regimens were thiotepa-based in both studies but combined either with BCNU (thiotepa 20 mg/kg; BCNU 400 mg/m2) in the IELSG study or with busulfan-cyclophosphamide (thiotepa 750 mg/m2; busulfan 8 mg/kg; cyclophosphamide 120 mg/kg) in the PRECIS trial. An important difference between these trials is the randomization timeframe, which was at trial registration in the PRECIS trial and at response assessment after induction chemoimmunotherapy in the IELSG32 trial.
Table 3
| Features | IELSG32 | PRECIS |
|---|---|---|
| Background | Same rationale | Same rationale |
| Type of study | Randomized phase II | Randomized phase II |
| Primary endpoint | 2-year PFS | 2-year PFS |
| Randomization | After response to induction | At registration |
| Estimated sample size | 52 patients/arm | 38 patients/arm |
| Per protocol population | 113 patients | 97 patients |
| Upper age limit | ≤65 years old and PS 0–3 or (65–70 years old and PS ≤2) | 60 years old PS 0–4 |
| Induction regimen | MTX-araC ± ritux ± thiotepa | R-MBVP => R-araC |
| CRR after induction | 54% | 43% |
| Patients receiving consolidation | 54% | 73% |
| WBRT dose | 36 Gy (+ boost 9 Gy in patients with PR) | 40 Gy |
| Conditioning regimen | BCNU-thiotepa | Thiotepa-busulfan-cyclophosphamide |
| Median follow-up | 40 months | 34 months |
PFS, progression-free survival; CRR, complete remission rate; WBRT, whole brain radiotherapy; PR, partial response; PS, performance status; Gy, Gray; MTX, methotrexate; BCNU, carmustine; MTX-araC, methotrexate-cytarabine; R-MBVP, rituximab, methorexate, Carmustine, etoposide, prednisone; R-araC, rituximab, cytarabine.
Efficacy results were slightly different. An identical per-protocol 2-year PFS in the WBRT and ASCT arms {[75–76]%±5%} from the date of trial registration was reported in the IELSG study, while in the WBRT and ASCT arms, there was a per protocol 2-year PFS of 67%±5% and 86%±5%, respectively, in the PRECIS trial. PFS from the time of consolidation, which evaluates the role of each consolidation strategy more accurately, showed slightly different results between the two studies. In the IELSG trial, no difference was observed for the per-protocol population after WBRT and ASCT (2-year PFS of 70%±5% in the WBRT arm and 72%±2% in the ASCT arm). In the PRECIS trial, the exploratory analysis performed on the per-protocol population showed a significant difference of the 2-year PFS from the time of consolidation in favor of ASCT (WBRT: 2-year PFS = 69%±5%; ASCT: 2-year PFS = 87%±5%; P=0.03). In the IELSG study, only patients who achieved a response or had stable disease after induction were eligible for the second randomization, which was not the case in the PRECIS trial. In the latter study, the 2-year PFS in the subgroup of responder patients was 69.7%±5% and 97.1%±5% in the WBRT and the ASCT arm, respectively, which nevertheless did not result in different OS, mainly because of the effectiveness of the salvage strategies. Interestingly, the results of WBRT were consistent between the two studies. The apparent discrepancy in the results of ASCT might result from the type of IC used in each study, with the TBC regimen being more intensive than the thiotepa-BCNU regimen. The better PFS after the TBC regimen compared with the thiotepa-BCNU regimen was suggested in a systematic review and meta-analysis (40).
In terms of toxicity, both studies showed, at 2 years, an excess of CNS toxicity with cognitive decline identified in half of the patients (in the PRECIS trial) after WBRT but not after ASCT. However, a recent retrospective study raised concerns about long-term CNS toxicity after ASCT (41). A longer follow-up of the IELSG and PRECIS studies is needed to better consolidate the efficacy and toxicity results. ASCT resulted in early and late lymphoma-unrelated deaths in five patients in each study, representing a treatment-related death rate of 9% and 11% in the IELSG and PRECIS studies, respectively. The TRM rate of the TBC regimen seems higher than other IC regimens used for PCNSL (40), ranging from 2–11% in reported studies (Table 2). The toxicity profile of the thiotepa-based IC has never been prospectively compared to the BEAM regimen. As expected, febrile neutropenia (grade ≥3) was observed in 95–100% of patients, and grade ≥3 oral and gastrointestinal mucositis, the second more frequent toxicity, was reported in 45–81% of patients (6,7,42).
In the absence of a comparative study of the two different types of IC, a matched-pair of the IELSG and PRECIS trials could be informative to explore the differences observed in efficacy and toxicity. In the meantime, both conditioning regimens remain the main choices for patients with PCNSL eligible for consolidation with ASCT. The best PCNSL candidates for ASCT are a matter of debate. Currently, IC + ASCT is offered to patients selected by age and comorbidities. The upper age limit for ASCT indication varies both in clinical trials and routine practice, spanning between 60 and 70 years old. However, in selected elderly patients with PCNSL, consolidation with HDT-ASCT, using thiotepa-based conditioning regimens, proved feasible and effective in first-line treatment and at relapse (39,43).
“Dose-intensive” consolidation chemotherapy
Consolidation with a nonmyeloablative chemotherapy has been assessed and presented as potentially less toxic, less resource consuming and equally effective than ASCT in patients with PCNSL. The CALGB 50202 study (44) showed encouraging results with the combination of etoposide and cytarabine (EA) [etoposide 5 mg/kg by continuous IV infusion every 12 hours for eight doses (total dose, 40 mg/kg); cytarabine 2 g/m2 IV over 2 hours every 12 hours for eight doses (total dose, 16 g/m2)], in 44 patients. The 2-year PFS for the 27 patients with CR who received EA consolidation was 69%. Surprisingly, this intensive combination has been associated with a good toxicity profile, even among patients over 60 years of age, with a single case of treatment-related death (4%). The low rate of inclusion in this study, 47 patients were included from 12 US centers over a period of 5 years, and the low incidence of Eastern Cooperative Oncology Group (ECOG) performance status ≥2 (18%) suggest that patients were highly selected. As expected, the toxicity of the EA regimen was higher in a “real-life” population of 28 patients (45), particularly regarding the occurrence of neutropenic fever, documented infections and mucositis, which have been recorded in 93%, 57% and 29% of patients, respectively (16%, 18% and 8%, respectively, in the CALGB trial) (Table 4). These findings suggest that nonmyeloablative consolidation may be as resource consuming as ASCT. Two ongoing randomized phase II trials may contribute to clarify these issues on nonmyeloablative consolidation in patients with newly diagnosed PCNSL. The CALGB (Alliance) 51101 trial (46) is comparing the outcomes of nonmyeloablative consolidation with EA chemotherapy versus myeloablative consolidation with BCNU/thiotepa in patients treated with an induction consisting of HD-MTX, TMZ and rituximab (NCT01511562). Similarly, the ongoing IELSG43 study is comparing two consolidation approaches: ASCT conditioned by BCNU/thiotepa with a nonmyeloablative regimen consisting of rituximab (375 mg/m2), dexamethasone (40 mg/d; d1–3), etoposide (100 mg/m2/d; d1–3), ifosfamide (1,500 mg/m2/d; d1–3), and carboplatin (300 mg/m2) in patients who achieve CR or PR with induction of 4 courses of MATRix (NCT02531841). The accrual goals of 110 patients for the Alliance 51101 trial and 330 patients for the IELSG43 trial were recently completed and results are pending.
Table 4
| Ref. | Type of study | N | Median age [range] (years) | Consolidation chemotherapy* | Toxicity | Outcome |
|---|---|---|---|---|---|---|
| (43) | Prospective multicenter phase II | 27 | 61 [12–76]† | AraC: 16 g/m2;VP16: 40 mg/kg | Myelotoxicity (50% grade 4 neutropenia and thrombocytopenia, one TRD) | 2-year TTP**: 69% |
| (44) | Retrospective monocentric | 14 | 60 [39–77] | AraC: 16 g/m2; VP16: 40 mg/kg | Neutropenic fever in 93%; median duration of grade 4 neutropenia: 11 days (range, 9–14 days) | 2-year PFS: 83% |
*, the consolidation chemotherapy was administered only in patients with CR; **, from start of consolidation chemotherapy; †, age of the 44 patients included in the study, age of the 27 patients who completed consolidation chemotherapy not available. TTP, time to progression; TRD, treatment-related death; CR, complete response.
In addition to the role of the consolidation strategies, these prospective studies highlight the need to improve the activity of induction treatments, which is suggested by the fact that a high proportion of patients still do not proceed to consolidation because of failure of induction chemotherapy. The proportion of patients receiving consolidation treatment was 64% in the CALGB 50202 trial (43), 49% in the phase III CALGB 51101 trial (45), 55% in the IELSG32 study (7) and 72% in the PRECIS trial (6).
Perspectives
With current induction chemotherapies, consolidation remains an essential part of the first-line treatment in patients with PCNSL to reduce the risk of relapse. Indeed, relapses and salvage treatments, in addition to the psychological stress, put patients at increased risk of neurotoxicity. Conventional WBRT tends to be abandoned because of the radiation-induced CNS toxicity. Several other efficient consolidation options are currently available for patients in CR after induction chemotherapy and present a lower risk of neurotoxicity. However, the optimal consolidative therapy has not yet been defined. In clinical practice, rdWBRT, nonmyeloablative chemotherapy and myeloablative chemotherapy followed by ASCT can be offered to younger patients and to a subgroup of selected fit patients over 60 years of age.
Ongoing randomized studies focused on consolidation strategies (Table 5) will provide useful additional information on the consolidation strategies in the first-line treatment of PCNSL by comparing nonmyeloablative with myeloablative chemotherapy. If any of these strategies proves better, then it should be compared also with rdWBRT. Long-term follow-up is mandatory in this population. The international efforts to identify biomarkers and imaging criteria will hopefully better identify prognostic factors at diagnosis and at the end of induction treatment that will allow for risk-driven consolidation strategies. Nevertheless, with a median age of 68 years, a significant proportion of patients with PCNSL are not eligible for consolidation treatment. For this population, maintenance treatments are being evaluated in several studies that are developed in a dedicated article on this issue. Regardless of the best consolidation and maintenance treatments, some effort will be needed to increase the CR rate after induction chemotherapy. Future perspectives might also include immunotherapies with chimeric antigen receptor T (CAR-T) cells, which are starting to be evaluated in PCNSL.
Table 5
| Clinicaltrials.gov identification | Type of study | Purpose | Objective | Status |
|---|---|---|---|---|
| NCT02531841 | Randomized phase III | Nonmyeloablative consolidation (R-DeVIC) vs. ASCT (thiotepa-carmustine) | 2-year PFS | Accrual completed (330 patients) |
| NCT01511562 | Randomized phase II | Nonmyeloablative consolidation (EA) vs. ASCT (thiotepa-carmustine) | 2-year PFS | Accrual completed (110 patients) |
EA, etoposide-cytarabine; R-DeVIC, rituximab, dexamethasone, etoposide, ifosfamide, and carboplatin; ASCT, autologous stem cell transplantation; PFS, progression-free survival.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Lymphoma for the series “Central Nervous System Lymphomas”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-29). The series “Central Nervous System Lymphomas” was commissioned by the editorial office without any funding or sponsorship. AJMF served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Lymphoma from Mar 2020 to Feb 2022. AJMF discloses speaker fee from Adienne; research grants from BMS, Beigene, Pharmacyclics, Hutchison Medipharma, Amgen, Genmab, ADC Therapeutics, Gilead, Novartis, and Pfizer; advisory boards from Gilead, Novartis, Juno, and PletixaPharm; inventor of patents on NGR-hTNF/RCHOP in relapsed or refractory PCNSL and SNGR-hTNF in brain tumours. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059-68. [Crossref] [PubMed]
- Le Gouill S, Casasnovas RO. Interim PET-driven strategy in de novo diffuse large B-cell lymphoma: do we trust the driver? Blood 2017;129:3059-70. [Crossref] [PubMed]
- Gavrilovic IT, Hormigo A, Yahalom J, et al. Long-term follow-up of high dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol 2006;24:4570-4. [Crossref] [PubMed]
- Omuro A, Taillandier L, Chinot O, et al. Primary CNS lymphoma in patients younger than 60: can whole-brain radiotherapy be deferred? J Neurooncol 2011;104:323-30. [Crossref] [PubMed]
- Houillier C, Soussain C, Ghesquières H, et al. Management and outcome of primary CNS lymphoma in the modern era: An LOC network study. Neurology 2020;94:e1027-39. [Crossref] [PubMed]
- Houillier C, Taillandier L, Dureau S, et al. Radiotherapy or Autologous Stem-Cell Transplantation for Primary CNS Lymphoma in Patients 60 Years of Age and Younger: Results of the Intergroup ANOCEF-GOELAMS Randomized Phase II PRECIS Study. J Clin Oncol 2019;37:823-33. [Crossref] [PubMed]
- Ferreri AJM, Cwynarski K, Pulczynski E, et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol 2017;4:e510-23. [Crossref] [PubMed]
- Ghesquieres H, Tilly H, Sonet A, et al. A Multicentric Prospective Phase 2 Study of Intravenous Rituximab and Intrathecal Liposomal Cytarabine in Combination with C5R Protocol Followed by Brain Radiotherapy for Immunocompetent Patients with Primary CNS Lymphoma: A Lymphoma Study Association (LYSA) Trial. Blood 2012;120:796. </jrn>. [Crossref]
- Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol 2013;31:3971-9. [Crossref] [PubMed]
- Lesueur P, Damaj G, Hoang-Xuan K, et al. Toxicity and outcomes of reduced-dose whole brain radiotherapy as consolidation treatment for patients with cns lymphoma in real life setting. 14th Meeting of the European Association of Neuro-Oncology; September 19–22; Lyon, France. Neuro Oncol, Volume 21, Issue Supplement_3, August 2019.
- Ferreri AJ, Reni M, Pasini F, et al. A multicenter study of treatment of primary CNS lymphoma. Neurology 2002;58:1513-20. [Crossref] [PubMed]
- Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol 1998;16:859-63. [Crossref] [PubMed]
- Omuro AM, Ben-Porat LS, Panageas KS, et al. Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol 2005;62:1595-600. [Crossref] [PubMed]
- Prica A, Chan K, Cheung MC. Combined modality therapy versus chemotherapy alone as an induction regimen for primary central nervous system lymphoma: a decision analysis. Br J Haematol 2012;158:600-7. [Crossref] [PubMed]
- Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol 2010;11:1036-47. [Crossref] [PubMed]
- Cabanillas F. How important is whole brain radiotherapy for treatment of primary CNS lymphoma? Lancet Oncol 2010;11:1011-2. [Crossref] [PubMed]
- Ferreri AJ, DeAngelis L, Illerhaus G, et al. Whole-brain radiotherapy in primary CNS lymphoma. Lancet Oncol 2011;12:118-9. [Crossref] [PubMed]
- Kim BH, Kim IH, Park SH, et al. Low-dose whole brain radiotherapy with tumor bed boost after methotrexate-based chemotherapy for primary central nervous system lymphoma. Cancer Res Treat 2014;46:261-9. [Crossref] [PubMed]
- Omuro A, DeAngelis L, Karrison T, et al. NRG;Randomized phase II study of rituximab, methotrexate (MTX), procarbazine, vincristine, and cytarabine (R-MPV-A) with and without low-dose whole-brain radiotherapy (LD-WBRT) for newly diagnosed primary CNS lymphoma (PCNSL). J Clin Oncol 2020;38:abstr 2501.
- Alvarez-Pinzon AM, Wolf AL, Swedberg H, et al. Primary Central Nervous System Lymphoma (PCNSL): Analysis of Treatment by Gamma Knife Radiosurgery and Chemotherapy in a Prospective, Observational Study. Cureus 2016;8:e697 [Crossref] [PubMed]
- Reni M, Zaja F, Mason W, et al. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer 2007;96:864-7. [Crossref] [PubMed]
- Mishima K, Nishikawa R, Narita Y, et al: Randomized phase III study of high-dose methotrexate and whole brain radiotherapy with or without concomitant and adjuvant temozolomide in patients with newly diagnosed primary central nervous system lymphoma: JCOG1114C. J Clin Oncol 2020;38:abstr 2500.
- Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med 1995;333:1540-5. [Crossref] [PubMed]
- Soussain C, Hoang-Xuan K, Taillandier L, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol 2008;26:2512-8. [Crossref] [PubMed]
- Soussain C, Choquet S, Fourme E, et al. Intensive chemotherapy with thiotepa, busulfan and cyclophosphamide and hematopoietic stem cell rescue in relapsed or refractory primary central nervous system lymphoma and intraocular lymphoma: a retrospective study of 79 cases. Haematologica 2012;97:1751-6. [Crossref] [PubMed]
- Kasenda B, Ihorst G, Schroers R, et al. High-dose chemotherapy with autologous haematopoietic stem cell support for relapsed or refractory primary CNS lymphoma: a prospective multicentre trial by the German Cooperative PCNSL study group. Leukemia 2017;31:2623-9. [Crossref] [PubMed]
- Cheng T, Forsyth P, Chaudhry A, et al. High-dose thiotepa, busulfan, cyclophosphamide and ASCT without whole-brain radiotherapy for poor prognosis primary CNS lymphoma. Bone Marrow Transplant 2003;31:679-85. [Crossref] [PubMed]
- Przepiorka D, Nath R, Ippoliti C, et al. A phase I-II study of high-dose thiotepa, busulfan and cyclophosphamide as a preparative regimen for autologous transplantation for malignant lymphoma. Leuk Lymphoma 1995;17:427-33. [Crossref] [PubMed]
- van Besien K, Przepiorka D, Mehra R, et al. Impact of preexisting CNS involvement on the outcome of bone marrow transplantation in adult hematologic malignancies. J Clin Oncol 1996;14:3036-42. [Crossref] [PubMed]
- Muldoon LL, Soussain C, Jahnke K, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol 2007;25:2295-305. [Crossref] [PubMed]
- Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010;28:4184-90. Erratum in: J Clin Oncol 2012 May 20;30(15):1896. [Crossref] [PubMed]
- Abrey LE, Moskowitz CH, Mason WP, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol 2003;21:4151-6. [Crossref] [PubMed]
- Yoon DH, Lee DH, Choi DR, et al. Feasibility of BU, CY and etoposide (BUCYE), and auto-SCT in patients with newly diagnosed primary CNS lymphoma: a single-center experience. Bone Marrow Transplant 2011;46:105-9. [Crossref] [PubMed]
- Illerhaus G, Müller F, Feuerhake F, et al. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica 2008;93:147-8. [Crossref] [PubMed]
- Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood 2015;125:1403-10. [Crossref] [PubMed]
- Montemurro M, Kiefer T, Schüler F, et al. Primary central nervous system lymphoma treated with high-dose methotrexate, high-dose busulfan/thiotepa, autologous stem-cell transplantation and response-adapted whole-brain radiotherapy: results of the multicenter Ostdeutsche Studiengruppe Hamato-Onkologie OSHO-53 phase II study. Ann Oncol 2007;18:665-71. [Crossref] [PubMed]
- Young PA, Gaut D, Kimaiyo DK, et al. Durable Survival Outcomes in Primary and Secondary Central Nervous System Lymphoma After High-dose Chemotherapy and Autologous Stem Cell Transplantation Using a Thiotepa, Busulfan, and Cyclophosphamide Conditioning Regimen. Clin Lymphoma Myeloma Leuk 2020;20:468-79. [Crossref] [PubMed]
- DeFilipp Z, Li S, El-Jawahri A, et al. High-dose chemotherapy with thiotepa, busulfan, and cyclophosphamide and autologous stem cell transplantation for patients with primary central nervous system lymphoma in first complete remission. Cancer 2017;123:3073-9. [Crossref] [PubMed]
- Schorb E, Fox CP, Fritsch K, et al. High-dose thiotepa-based chemotherapy with autologous stem cell support in elderly patients with primary central nervous system lymphoma: a European retrospective study. Bone Marrow Transplant 2017;52:1113-9. [Crossref] [PubMed]
- Alnahhas I, Jawish M, Alsawas M, et al. Autologous Stem-Cell Transplantation for Primary Central Nervous System Lymphoma: Systematic Review and Meta-analysis. Clin Lymphoma Myeloma Leuk 2019;19:e129-41. [Crossref] [PubMed]
- Correa DD, Braun E, Kryza-Lacombe M, et al. Longitudinal cognitive assessment in patients with primary CNS lymphoma treated with induction chemotherapy followed by reduced-dose whole-brain radiotherapy or autologous stem cell transplantation. J Neurooncol 2019;144:553-62. [Crossref] [PubMed]
- Scordo M, Bhatt V, Hsu M, et al. A Comprehensive Assessment of Toxicities in Patients with Central Nervous System Lymphoma Undergoing Autologous Stem Cell Transplantation Using Thiotepa, Busulfan, and Cyclophosphamide Conditioning. Biol Blood Marrow Transplant 2017;23:38-43. [Crossref] [PubMed]
- Schorb E, Kasenda B, Ihorst G, et al. High-dose chemotherapy and autologous stem cell transplant in elderly patients with primary CNS lymphoma: a pilot study. Blood Adv 2020;4:3378-81. [Crossref] [PubMed]
- Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol 2013;31:3061-8. [Crossref] [PubMed]
- Birsen R, Willems L, Pallud J, et al. Efficacy and safety of high-dose etoposide cytarabine as consolidation following rituximab methotrexate temozolomide induction in newly diagnosed primary central nervous system lymphoma in immunocompetent patients. Haematologica 2018;103:e296-9. [Crossref] [PubMed]
- Batchelor T, Giri S, Amy S et al. Myeloablative versus non-myeloablative consolidative chemotherapy for newly diagnosed primary central nervous system lymphoma: Results of induction therapy in Alliance 51101. J Clin Oncol 2020;38:abstr 8042.
Cite this article as: Soussain C, Ferreri AJM. Primary central nervous system lymphoma: consolidation strategies. Ann Lymphoma 2020;4:14.


