Radiation therapy of extranodal marginal zone lymphomas
Introduction
Extranodal marginal zone lymphoma represents 70% of all MZL. The concept of MALT lymphoma was first proposed by Isaacson and Wright in 1983 (1) and is now a well-defined clinical-pathologic entity. The diverse anatomic locations where MALT lymphoma present are often associated with certain etiologic factors such as bacterial infections, or autoimmune diseases, and some have characteristic molecular pathologic features. Even in the same site, taking the stomach as an example, etiology may vary as in H. pylori induced MALT lymphoma, versus non-H. pylori dependent disease which is often characterized by t(11; 18) translocation. The natural history of MALT lymphoma is generally indolent, with frequent relapses. The site of involvement, stage, and related symptoms drive the optimal treatment approach.
Localized MALT lymphomas tend to remain confined to one region for an extended time. Symptoms are often mild and progress slowly. Local treatment such as radiation therapy (RT) is often useful either initially, or later depending on the course of the disease. The most compelling long-term RT data for the successful control of MALT lymphomas are in orbital and gastric sites. The use of moderate dose RT with 24–30 Gy provides excellent local control, approaching 100%, with most patients remaining disease-free for 10 or more years for orbital adnexa MALT lymphoma (2), and gastric MALT lymphoma (3-5). The moderate doses of radiation used for definitive therapy are associated with a limited risk of serious long-term toxicity. However, special considerations are required for some sites including the orbit, salivary glands, lung, and stomach.
When MALT lymphoma presents as advanced (stage III/IV) disease, it is considered incurable (6). However, the progression of disease is often gradual with prolonged survival. In this setting palliative RT can have a significant role in providing local control and symptom relief. The use of RT for locoregional control of disease should be carefully considered, balancing the benefit of RT against the risk of treatment complications, which varies depending on the site being treated and the RT dose administered. We will discuss this aspect of therapeutic benefit of ultra-low dose (i.e., 2×2 Gy) or moderate dose (24–30 Gy) RT for each of the MALT sites, so that the reader can individualize the decision to use RT in a particular situation.
Principles and goals of radiation therapy in MALT lymphoma
Although preferred first line therapy for limited stage disease is often RT, no treatment consensus guidelines have been specifically developed for MZL. Consequently, the management of stage I/II MALT lymphoma often mirrors that of follicular lymphoma. In this context, RT aims to achieve durable local control with a minimal risk of acute and late effects. Taking advantage of the radiosensitivity of indolent lymphomas requiring lower doses than solid tumors, the ongoing strategy is to decrease RT intensity while maintaining high response rates (7,8).
The recommended RT dose was traditionally 30 Gy or higher. However, this has changed as a result of Lowry’s milestone phase III study which compared 40 Gy to 24 Gy for patients with early stage indolent lymphomas (mostly follicular and extranodal marginal zone lymphomas) (7). This prospective trial showed that a dose of 24 Gy in 12 fractions was as effective as 40 Gy in 20 fractions for patients with indolent lymphomas in terms of overall response (92% vs. 93%) and complete response (82% vs. 79%) rates, as well as progression-free survival (7). Moreover, there was a trend for reduced toxicities in the low-dose arms, but only erythema was significantly reduced (P=0.004). This trial established 24 Gy as the standard of care for the definitive management of MZL, although it is recognized that many single institutional series have previously used 30 Gy with a slightly higher long term local control rate (approximately 90–95%) than was reported by the study conducted by Lowry et al. (7).
Over the past two decades, the effectiveness of lower doses has been widely reported. The so-called “boom boom” radiotherapy was firstly reported by Gamen et al. (9). Low-dose radiation, 4 Gy in 2 fractions, in the palliative treatment of indolent NHL has shown satisfying results with impressive response rates [overall response rate (ORR) 88%], and mild/rare toxicity (10). This schedule was studied by Hoskin et al. (8) in a multicenter randomized trial comparing 24 Gy in 12 fractions vs. 4 Gy in 2 fractions for both curative and palliative treatment of follicular and marginal zone lymphoma patients. While the 2×2 Gy schedule was statistically inferior in terms of time to local progression, remarkable activity was demonstrated in almost 75% of patients treated to 4 Gy in 2 fractions, with roughly half of patients (49%) achieving complete remission. Moreover, several retrospective clinical series have demonstrated efficacy of ultra-low dose radiotherapy for palliation of relapsed or refractory disease or in situations when the patient is not a candidate for systemic therapy (11-14). Taken together these data provide the basis for the use of the 2×2 Gy regimen as the standard schedule in case of palliative-symptomatic lesions because of the high local response rate and the extremely rare and mild acute/late effects. Interest has emerged in utilizing this regimen in the initial management of limited stage MZL with a response adapted approach. Patients are initially treated to 4 Gy in 2 fractions with an additional RT dose of 20 Gy reserved for patients that do not achieve a complete response (ClinicalTrials.gov Identifier: NCT03680586, ClinicalTrials.gov Identifier: NCT02494700). If successful, response adapted therapeutic approaches should be evaluated in randomized trials for patients with early stage extranodal MZL.
Modern RT planning is no longer field based, rather target focused with treatment volumes delineated on volumetric radiographic (CT/MRI/PET) images. Presently the terminology is involved site RT (ISRT) and not involved field radiation therapy (IFRT) as recommended by the International Lymphoma Radiation Oncology Group (ILROG) (15). Delineation of target volumes follow the terminology of the gross tumor volume (GTV), clinical target volume (CTV) and the planning target volume (PTV). The GTV is the gross demonstrable lymphoma, while the CTV includes the GTV and/or subclinical extent of the lymphoma which must be eliminated. The PTV is defined as a RT planning volume which includes the CTV with an adequate margin to account for tumor motion (physiological such as breathing, organ filling such as it happens in the stomach), and uncertainties in geometry and day to day patient positioning and movements. The PTV is a geometric concept applied in RT planning to ensure that adequate RT dose is actually delivered to the CTV with a high level of probability. Often, RT techniques utilize immobilization devices, motion management strategies (such as deep inspiration breath-hold, DIBH), and image guided RT to minimize the CTV to PTV margin and also the exposure to surrounding organs at risk.
Generally elective nodal radiation is unnecessary for limited stage MALT lymphomas (15-19). Modern technology is utilized to achieve target coverage while minimizing doses to neighboring organs at risk (OAR) based on the ALARA (as low as reasonably achievable) principle. The definition of OAR and their uncertainties follow the same principles as defining target volumes as outline above. The choice of the set-up immobilization and radiotherapy technique will vary according to the region being targeted and the neighboring normal tissues that need to be avoided. Advanced techniques with multi-beams (IMRT) and arcs (VMAT) are standard methods to achieve a high of dose conformity with CTV/PTV coverage, while minimizing dose exposure to OARs. Given that the doses prescribed for MALT lymphoma are considered low to moderate, normal tissue constraints that are utilized for solid cancer high dose treatments are not appropriate. It is essential to reduce unnecessary radiation dose to normal tissues in order to minimize long term side effects such as secondary cancers in this patient population with highly curable disease, and prolonged survival.
Radiation therapy for specific anatomic sites
Gastric
Lymphoid tissue is scarce in normal gastric tissue. Gastric MALT lymphoma originates from the post-germinal center B-cells that reside in the marginal zone of mucosal lymphoid tissue that is often acquired in the stomach in response to infection [typically Helicobacter pylori (H. pylori)] or chronic immune stimulation (in the case of patients with auto-immune disease) (20). In 1991, Wotherspoon and colleagues reported the presence of H. pylori infection in over 90% of the 100 gastric MALT cases examined (21). In a follow up publication, the authors demonstrated gastric MALT regression after H. pylori eradication in 5 of 6 patients (22). Almost 3 decades later, anti-microbial therapy directed at H. pylori eradication remains frontline therapy for patients with localized gastric MALT lymphoma (23,24).
Gastric MALT lymphoma patients can present with various gastrointestinal symptoms including nausea, vomiting, weight loss, epigastric pain and even gastrointestinal bleeding. The gold standard for diagnosis is endoscopy with several biopsies for histological confirmation of disease as well as for the evaluation for the presence of H. pylori organisms. Patients should be advised to discontinue proton pump inhibitor (PPI) therapies as false negative H. pylori tests are common with PPI treatment within 2 weeks of endoscopy or urea breath tests (25,26).
Approximately 75–80% of patients treated with anti-microbial therapy will experience lymphoma regression. The current recommended regimen is a PPI and clarithromycin with either amoxicillin or metronidazole (27-29). In a large systematic review of over 1400 patients from 32 studies treated with up front H. pylori eradication for early stage gastric MALT lymphoma, the remission rate was 78%. For 994 patients with follow up data available, relapse occurred in 7% of patients (30). In an additional large study of 420 patients treated with initial H. pylori eradication, 77% of patients responded to therapy and long-term outcomes were excellent with 10-year overall survival rates of 95% (31). Despite the indolent nature of gastric MALT lymphoma and the excellent responses to primary antimicrobial therapy, several factors are associated with increased risk for lack of response including the presence of the API12-MALT fusion product of translocation t(11;18), regional lymph node involvement, H. pylori negativity and submucosal invasion appreciated on endoscopic ultrasonography (30-33).
For patients with localized disease that is unresponsive to H. pylori therapy, RT appears to be the most promising treatment strategy. In a pooled data analysis of 315 patients with persistent disease after H. pylori therapy, those treated with RT had a higher remission rate compared to chemotherapy (97% versus 85%, P=0.007) (34). Several series have demonstrated excellent disease outcomes after RT, regardless of H. pylori infection status and prior therapy (5,35,36). Current recommendations suggest RT for localized gastric MALT lymphoma that persists despite H. pylori eradication (23,24). While in the past RT was offered as upfront therapy to all H. pylori negative patients, increasing evidence suggests lymphoma regression can occur with anti-microbial therapy in the absence of known H. pylori infection (37-39). Therefore, even in cases where H. pylori infection is not detected, a trial of antibiotic therapy may be attempted. However, for symptomatic patients or those with risk factors that are associated with a lower chance of response to antibiotic therapy (as described above), definitive RT is contemplated (23).
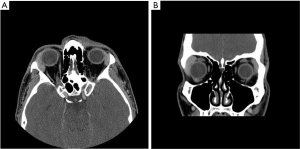
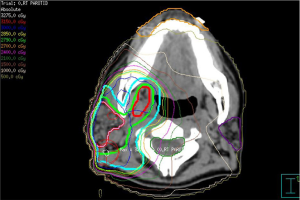
As gastric MALT lymphoma is often a multifocal disease, the radiation target volume should include the entire stomach, even in cases where the disease may appear to be confined to one region of the stomach. Lymph nodes are only included if they are suspected to be involved with the disease. In older RT series, patients were treated with extensive RT fields to the entire abdomen (5,40,41). Long term outcomes reported by Wirth et al. on behalf of the International Extranodal Lymphoma Study Group (IELSG) revealed that radiation field size and RT dose was not associated with increased treatment failure among 102 gastric MALT lymphoma patients in which 41 received RT to the whole abdomen and 61 were treated to the stomach and involved nodes (5). The German Study Group on Gastrointestinal Lymphoma performed stage adaptive RT field reductions over the course of 3 prospective trials for gastric MALT lymphoma patients without loss of local control but with lesser frequency of treatment related toxicities (42). Current recommendations from ILROG suggest that the CTV should encompass the stomach from the gastroesophageal junction to beyond the duodenal bulb as well as any pathologically involved nodes (15). Modern technology should be used to reduce doses to neighboring normal structures, including the heart, kidneys and spleen (Figure 1). As the stomach is subject to significant motion due to peristalsis, respiratory motion and variations in stomach filling, techniques and procedures directed at accounting for organ motion are essential. Patients should have NPO status at least 6 hours prior to therapy, four dimensional (4D) CT simulation or DIBH techniques should be used for treatment and PTV margins should be adjusted according to the image guidance technique utilized (Figure 1). With DIBH and daily CT image guidance, 1.0–1.5 cm margins are often used (43). With free breathing treatment and 2D imaging alignment to bony anatomy however, margins of 2.2 cm or greater have been shown to be necessary to achieve adequate PTV coverage (44). DIBH technique has been shown to be a promising strategy to reduce unintended radiation to the heart when treating gastric MALT lymphoma patients (45,46).
While older series have reported median RT doses of 40–50 Gy for the treatment of gastric MALT, many oncologists have treated to 30 Gy in 1.5 Gy fractions delivered over 4 weeks based on excellent outcomes of 17 patients treated with this approach at Memorial Sloan Kettering Cancer Center (MSKCC) in the late 1990’s (47). Since that time, the exquisitely radiosensitive nature of MALT lymphoma has been appreciated and lower RT doses have been utilized. In a prospective, multicenter study of refractory or H. pylori negative gastric MALT patients, 29 patients were randomized to definitive RT to a dose of 25.2 Gy (n=14) or 36 Gy (n=15; both in 1.8 Gy fractions). All patients experienced a complete response (CR) to RT, regardless of dose, with excellent long-term control in both arms of the study (48). In a series of 32 patients treated at MDACC with definitive RT and doses of 30–36 Gy in 1.5–2.0 Gy fractions (n=21) or 24 Gy (administered mostly in 2 Gy fractions, n=11), CR was achieved among all patients regardless of dose. Two-year treatment outcomes were not impacted by RT dose (49). Given these excellent outcomes with doses of 24–25 Gy administered in 1.8–2.0 Gy fractions, coupled with the known high response rate of other extranodal MALT lymphomas to low RT doses, an ongoing prospective study is evaluating response to 4 Gy in 2 fractions as definitive therapy for H. pylori negative or H. pylori positive refractory gastric MALT lymphoma (ClinicalTrials.gov Identifier: NCT03680586).
Orbit
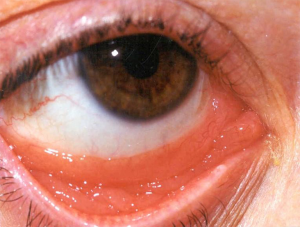
Prior to the 1990’s, much of the experience with RT for orbital adnexa MALT lymphomas are inferred from reports of patients treated for “low grade” lymphoma and “pseudolymphoma”. Many of these cases are now recognized as MALT lymphomas (50,51). The disease affects older individuals, with a median age in the sixth to seventh decade. The conjunctiva is the most commonly involved site, with characteristic “salmon-pink” infiltration (Figure 2). Another frequent orbital presentation is involvement of the lacrimal gland (Figure 3), followed by periorbital soft tissues and the retro-orbital space. Sometimes multiple lesions are found within the orbit. For conjunctival presentations, there is a tendency for bilateral involvement either at diagnosis, or later (52). Typical symptoms are irritation, pain, and epiphora. For retro-orbital presentations the patient may have proptosis and diplopia. Cervical lymph nodes are rarely involved, but distant sites of disease may be detected on detailed staging with CT, PET scan or bone marrow biopsy (53). For the patients with stage IE disease (localized to one or both orbits), treatment is directed at cure and preservation of both vision and integrity of the orbit. Therefore, extensive surgery is not indicated and should be avoided. RT is the standard treatment and achieves local control in over 95% of cases (2,3,54,55). Detailed ophthalmologic assessment prior to RT to document the vision and the presence of any ophthalmologic co-morbidity is recommended.
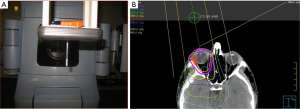
The radiation target volume need not include the whole orbit for conjunctival lesions. For other adnexal infiltrations and retro-orbital disease, the target volume includes the whole orbit. It is not necessary to cover regional lymph nodes or the contralateral orbit. For patients with conjunctival involvement and no retro-orbital extension, a single direct anterior field with either high energy electrons or photon energies ranging from 4mV to 6mV from a linear accelerator is sufficient. This technique is simple, reproducible and it also allows the option to provide shielding to the lens, anterior chamber, and the macula by suspending a 1 cm diameter cylindrical eye bar directly over the cornea (“pencil” eye shield) (Figure 4A). For electron beams, a similar eye shield of lesser thickness (1–1.5 cm, lead) can be used (51,56,57). Shielding should only be considered if there is assurance on the clinical setup that the disease will not be shielded. When photons are utilized to target the conjunctiva, bolus should be used to provide adequate surface dose. A prescribed dose of 24–25 Gy delivered in 1.5–2.0 Gy fractions specified at Dnorm will result in a dose of 20–24 Gy to most of the retro-orbital tissues (Figure 4B). For patients with retro-orbital disease, IMRT or VMAT will achieve a homogeneous dose to the CTV. In this scenario, lens shielding is omitted to avoid shielding the retro-orbital disease. Care is taken to minimize dose to the brain. For patients requiring bilateral orbital radiation, lateral opposed fields are often preferred, or a 3-field technique with addition of an anterior field with shielding of the midline structures between the orbits. For situations with bilateral involvement of the conjunctiva, bolus is used with opposed lateral photon fields to provide buildup of the prescription dose at the conjunctival surface.
The moderate doses of radiation of 24–25 Gy required to achieve a high rate of local control will result in acute side effects of skin erythema, epilation of eye lashes, and conjunctival irritation lasting a few weeks. These effects are temporary and can be managed with conservative measures. Specialized ophthalmologic care should be readily available if required. In the long term, the RT dose of 25–30 Gy without lens shielding will result in cataract formation in over 90% of patients (2,58). If the lens was shielded, cataract formation occurs in about 15% of cases (2). Apart from the cataract risk, the RT described herein is within the tolerance of the eye and deterioration of vision due to RT is rarely observed. The higher risks reported in the literature has been due to higher doses of 35–40 Gy (54) which are unnecessary for MALT lymphoma. A mild degree of permanent dryness of the eye may be observed if the lacrimal gland was treated to full dose. Therefore, ophthalmologic follow up is important.
Emerging evidence indicate that a much lower dose, e.g., 2×2 Gy regimen, can result in a high rate of local control, and can be adopted as an initial treatment strategy to minimize the orbital toxicity of higher dose treatment (12,14, ClinicalTrials.gov Identifier: NCT02494700). It is uncertain at this time if the local control is as durable compared with higher dose standard regimen, although if the patient has a local recurrence the standard 24–25 Gy regimen can still be successfully applied at that time.
Salivary gland and other head and neck locations
MALT lymphoma of the salivary gland is often seen in patients with Sjögren’s syndrome (59) which may not have been recognized at the time when lymphoma is diagnosed. Clinical features and serologic studies should be sought to confirm a diagnosis of Sjögren’s syndrome, as treatment selection with use of RT for the MALT lymphoma can be affected. Middle aged adults are often affected, with a median age of 62 years (60). The parotid gland is the most commonly involved, sometimes bilaterally. Patients usually present with a painless mass. Non-malignant changes of myoepithelial sialadenitis may be present. Cervical lymph node involvement is occasionally seen, either located in the parotid area or in the upper cervical chain (levels 2 and 3). Surgical biopsy should be pursued with caution and may involve a superficial parotidectomy, since a lesser procedure in the parotid area would not allow adequate exposure to reliably identify and preserve the facial nerve or its branches.
For stage IE disease, the radiation target volume (CTV) should include the whole parotid gland, including the deep lobe. Elective nodal coverage is not required. For those with cervical lymph node involvement (stage IIE), the ipsilateral cervical nodal chain should be covered. The RT dose is 24–30 Gy given in 1.5–2.0 Gy fractions. Conformal techniques with IMRT and VMAT are desirable, as it is important to spare the midline structures, the orbits, and the contralateral salivary glands (Figure 5). The acute side effects of parotid radiation include change of taste, xerostomia, limited mucositis and skin erythema. A pre-RT dental assessment is important, as the RT treatment may exacerbate any pre-existing dental problems. As many patients have Sjögren’s syndrome, a variable degree of permanent dryness will occur. Symptomatic management with meticulous oral and dental hygiene, consultation with dietician, avoidance of tobacco smoke and alcohol, use of salivary substitutes are all important elements of follow up care. Drugs to improve dryness such as pilocarpine can be tried but can give undesirable cholinergic side effect. Given the concern for increased risk of xerostomia in the population of patients with co-existing Sjögren’s syndrome, ultra-low dose RT is often pursued to limit treatment related morbidity (61).
MALT lymphoma can present in the other major salivary glands (sublingual and submandibular), or other minor salivary gland locations in the upper aerodigestive tract, e.g., larynx, trachea, and rarely the maxillary sinus and soft tissue. MALT lymphomas arising from Waldeyer’s ring lymphatic tissue (e.g., tonsil, nasopharynx) are exceedingly rare, accounting for only 3.6% of 329 cases of low-grade lymphomas in this site in the series from Kiel (62). RT for these head and neck locations follow the same principles as for the parotid gland, with coverage of the local organ/region. The RT dose is 24 Gy given in 1.5–2.0 Gy fractions.
Thyroid
Thyroid MALT lymphoma is typically seen in patients with Hashimoto’s thyroiditis (i.e., lymphocytic thyroiditis) (63) which may not have been clinically obvious. Thyroid function is usually normal, although a long history of Hashimoto’s thyroiditis can result in hypothyroidism. Middle-aged adults are often affected, the median age being 62 years (60). Patients commonly present with a painless mass. Regional lymph node involvement is infrequent, but if present, often affects the central neck (paratracheal, perithyroidal lymph nodes). Suspicious thyroid nodules are often investigated with a fine needle aspirate biopsy (FNAB), hence MALT lymphoma can be suspected on this basis, and additional conservative biopsy approaches can be conducted. A routine total thyroidectomy is not required as RT will generally be recommended for local control following surgery. The limited data published suggests that local therapy with surgery with or without RT results in a high likelihood of local control, and unlike MALT lymphoma presenting in other mucosal sites, a very low risk of distant dissemination (2,3,18).
For stage IE disease, the radiation target volume (CTV) should include the whole thyroid gland or in cases where biopsy was performed surgically, the entire thyroid bed. For those with cervical lymph node involvement (stage IIE), the affected cervical nodes should be included in the radiation target volume. The RT dose is 24–30 Gy given in 1.5–2.0 Gy fractions, with conformal techniques to minimize dose to neighboring salivary tissues and the spinal cord.
The main acute side effects of radiation to the thyroid and surrounding neck tissues are skin erythema, temporary laryngitis, tracheitis, and dysphagia to solid foods (secondary to esophagitis), all mild to moderate in degree. These effects resolve over a period of 2–3 weeks after completion of treatment. Hypothyroidism may exist prior to therapy either due to Hashimoto’s thyroiditis or as a complication of surgical biopsy (18,63). For patients with normal thyroid function prior to RT, hypothyroidism is an expected complication. Management of treatment related hypothyroidism requires periodic monitoring and thyroxine replacement. Serious long-term toxicity is not observed for the moderate RT doses used for this disease.
Breast
MALT lymphoma of the breast is a rare disease entity (64). In the largest series of histologically low-grade primary breast lymphoma, 24 patients with MALT lymphoma of the breast from multiple centers were retrospectively evaluated by the IELSG (65). The median age at diagnosis was 62 and all patients except for one had unilateral breast disease with (n=25) or without (n=71) regional nodal involvement. The treatment approaches in this study were heterogeneous and included surgery (including breast conserving resection or mastectomy), RT and chemotherapy or some combination of these. The ORR regardless of initial treatment approach was excellent at 100%, however 37% of patients relapsed, typically at distant sites. The relapses were largely salvageable, as the cause specific survival at 10 years was 80%. Of note, no patients who received RT relapsed locally, highlighting a benefit of RT to prevent local recurrence. Moreover, there was a trend towards improved PFS for breast MALT patients who received RT (HR 4.6; 95% CI: 0.9–23.3; P=0.07). The study was limited however in that no patients received immunotherapy with rituximab as a component of therapy.
When RT is used for the definitive management of MALT lymphoma of the breast the entire breast is often targeted with inclusion of regional nodal basins only in cases of disease involvement. In a MDACC series of 11 patients, those treated with RT had local control of 100%, even with low doses of 4 Gy in 2 fractions (17). Distant relapse was not uncommon however with 55% of patients eventually having recurrent disease after initial therapy. Salvage therapy with single agent rituximab was effective and no deaths occurred at a median follow up of 8 years. These limited data suggest a role for initial definitive RT in the management of MALT lymphoma of the breast.
Skin
Among cutaneous B-cell lymphomas (CBCL), primary cutaneous marginal zone B cell lymphoma (PCMZL) is a relatively rare entity, representing 2-16% of all cutaneous lymphomas (66). PCMZL is clinically characterized by multifocal small plaques or nodules mostly on arms and trunk, and histologically by small B cells, monotypic plasma cells, reactive germinal centers, and numerous T cells. RT is a preferred treatment option along with surgical excision, topical/intralesional steroids, monotherapy or combination chemotherapy regimens. The PCMZL prognosis is excellent with 5-year survival rate over 95–98%, however, up to 50% of patients manifest a cutaneous relapse (67).
Comparative data on local excision and RT are still scarce, however locoregional RT is an important option because of the high risk of local recurrence after localexcision (68). Moreover, cosmetic outcomes may be superior with RT. With an RT approach there is also a consideration of the necessity to perform multiple sites of irradiation in some, and reirradiation in case of relapses which occurred mostly outside treatment fields. For stage IE disease, the standard CTV includes the macroscopic disease with 1–2 cm lateral margins (69,70), however Servitje et al. in a retrospective series of 137 PCMZL applied 5 cm margins (19). Safety margins are needed in order to reduce the risk of local relapse. Depending on the depth of skin infiltration, radiation beam and energy selection can include electron beams (typically with energies 6–9 MeV) or low-energy orthovoltage x-rays (80–120 kV). Bolus material is required to achieve full skin dose for electron beams and higher energy x-rays from linear accelerators. Elective nodal coverage is not recommended. The standard recommended dose is 24 Gy in 1.8–2.0 Gy fractions (68,71,72).
Excellent local control and cosmetic outcomes are expected with a moderate dose of 24 Gy. Toxicity is limited and if it is observed is limited to grade 1 or 2 erythema. Late effects are rare with 24 Gy delivered with conventional fractionation and are typically characterized by skin hypo/hyperpigmentation or alopecia. For such reasons ultra-low dose RT (2×2 Gy) could be an attractive alternative that can also be applied to cutaneous disease (68,73,74), with complete response rates up to 86% and no reported late toxicity while offering excellent cosmetic outcomes. However, Oertel et al. (74) comparing standard doses to ultra-low doses showing significantly lower complete response rates (CRR 29%) for the latter group and did not recommend 2×2 Gy as standard treatment. Therefore, the standard of care is not well established because there are only retrospective and single center data with small number of patients. Clinical registry and multicenter studies may help in further explore the role of RT intensity and determine the minimal necessary dose to maintain high response rates.
Lung
Primary lung lymphomas are rare, but approximately 70–80% are MALT lymphomas (75,76). The median age at presentation is 68 years (60), and up to 30% of patients may have an associated autoimmune disease such as Sjögren’s syndrome (75,77). The etiologic factor is not known, although long-term exposure to smoking, infection and autoimmune disease may determine antigenic stimuli (76). Characteristic findings on CT imaging include the presence of a mass, consolidation, airway dilatation, air bronchograms, and surrounding ground-glass changes (78). Multifocal disease is common, occurring in 19 of 24 cases (79%) (78). Regional lymph node is frequently involved, occurring in 44% of cases according to Kurtin et al. (75).
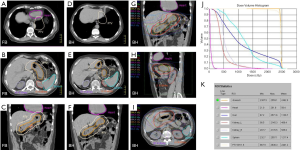
For patients presenting with stage IE or stage IIE disease, RT is recommended if the volume of lung exposed to radiation is not excessively large. Pre-RT assessment of pulmonary and cardiac function is important, and should include clinical evaluation, spirometry and diffusion capacity, and cardiac ejection fraction. The target volume encompasses the gross disease with a margin taking account of organ motion due to the respiratory cycle (Figure 6A). Established methods of motion management (e.g., DIBH) should be used whenever feasible. A RT dose of 24–30 Gy given in 1.5–2.0 Gy fractions over 2 to 3 weeks is prescribed (Figure 6B, C and D). As the lung tissue has limited tolerance to radiation (79,80), large target volumes result in a high risk of radiation pneumonitis and later pulmonary fibrosis with functional impairment. Therefore, if the tumor is bulky, or has significant pleural extension or malignant effusion, or if multiple lung nodules are present, conventional RT fractionation should not be used. Low dose RT with 2×2 Gy regimen can be very effective for local control (11). Otherwise, for more extensive disease chemotherapy is recommended. Lung tissue exposed to a dose of 30 Gy given in 2 Gy fractions or less have an approximately 40–50% chance of manifesting visible changes of pneumonitis on a CT scan (80). Factors that affect this risk include the volume of lung tissue irradiated, and the dose/fraction (81). The presence of RT changes in the lung can make response assessment difficult if residual treatment related abnormalities persist. For patients with small MALT lymphomas treated with complete surgical excision confirmed pathologically, RT may be unnecessary. If resection margins are positive, postoperative RT can be considered.
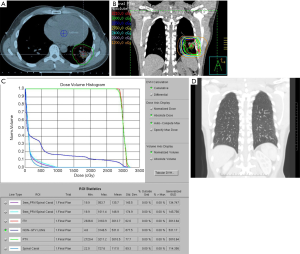
The literature describing the clinical outcome of MALT lymphoma of the lung documents an indolent disease similar to MALT lymphoma of other sites (16,82,83). Kurtin et al. reported a 10-year cause-specific survival of 72% in a series of 50 patient with lung lymphoma (52% were of MALT histology) (75). A series of 35 patients predominately treated surgically had 5- and 10-year survival of 68% and 53% respectively (76).
Dura
Primary dural lymphoma is an infrequent subtype of primary CNS lymphoma that arises from the dura mater without direct involvement of the parenchyma of the brain (Figure 7). Dural lymphoma are often mistaken for meningiomas due to similarities in radiographic features and clinical presentation (84). While the majority of primary CNS lymphomas are aggressive DLBCL, dural lymphoma are typically marginal zone lymphomas (85). Dural MALT lymphoma patients can present with a range of clinical symptoms; however, headache and seizures are common (86). A female predominance has been reported. Treatment approaches are varied; however, RT has been utilized with good outcomes (86,87). In a large series of 26 marginal zone dural lymphoma patients treated at MSKCC and the University of Miami, 22 patients achieved CR, including 12 patients treated with focal RT and 7 patients that received WBRT with or without a boost (88). Most patient were treated to a dose of 30–36 Gy. At a median follow up of just over 5 years, the median PFS and OS were not reached. All patients were alive at last follow up indicating the indolent nature of the disease and success of local therapy.
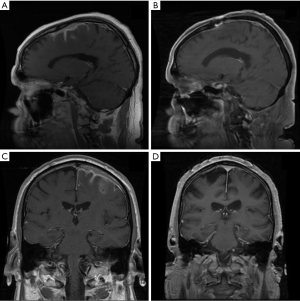
There is no standard approach for the management of MZL involving the dura, however aggressive systemic therapeutic approaches are often not warranted. In most cases the use of high dose methotrexate is discouraged, especially if RT is considered given the risk for neurotoxicity (88) and the excellent outcomes that can be achieved with single modality local therapy. Current guidelines from ILROG suggest focal therapy to the presurgical/biopsy MRI volume with margin to a dose of 30–36 Gy in cases of a single lesion (15). For multifocal disease WBRT to 24 Gy followed by a boost to involved sites with an additional 12 Gy is considered appropriate therapy. It is likely that lower RT doses such as 24 Gy would be effective, however existing published data indicates good outcomes of moderate RT doses of 30–36 Gy.
Summary/conclusions
We focused on the use of RT in the management of MALT lymphomas (Table 1). The evidence where the definitive use of RT has been successful in the management of the disease was presented. The outcome following moderate-dose RT for stage IE and IIE MALT lymphoma is that of long-term local control and possible cure. However, five to ten years of follow up is likely still insufficient to fully characterize the impact of treatment on MALT lymphomas since late recurrences can occur, and patients with recurrent disease often have prolonged survival. We are of the opinion that where local disease control can be achieved without significant toxicity, involved site RT should be offered (15).
Table 1
| Anatomic site | Author | Type of study | N. pts | Histology | Dose, median (range) | Target volume | RT technique | Local control | Follow-up, median (range) | Survival rates | Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastric | Abe 2013 (36) | Retrospective | 34, 100% failed HPE | 100% MZL | 30 Gy, 1.5–2 Gy/fr | CTV: stomach wall & perigastric lymph nodes | Opposed anterior and posterior fields or multiple field irradiation | 97.1% CR | 7.5 yr (1.2–13.0) | 97%. 5-yr RFS | No serious late events |
| 97% 5-yrOS | |||||||||||
| 100% 5-yr DSS | |||||||||||
| Wirth 2013 (4) | Retrospective | 102, 34% failed HPE | 100% MZL | 40 Gy (26–46 Gy) | Whole abdomen 50%, IFRT (stomach wall) | Antero-posterior pair and multi-field technique | 96% CR | 7.9 yr (0.3–24) | 70% 10-yr OS | 11 second malignances: 4 in field | |
| Pinnix 2019 (49) | Retrospective | 32 | 100% MZL | 21 pts: 30 to 36 Gy, 11 pts: 24 Gy, 1.5–2 Gy/fr | CTV: stomach wall | IMRT with DIBH | 100% CR; 100% 2-yr FFTF | 55.2 months (32.4–78.1) | 97% 2-yr OS | No cardiac renal late events | |
| Schmelz 2019 (48) | Prospective | 29 | 100% | 36 Gy versus 25.2 Gy | CTV: stomach and the local lymph nodes | 3D CRT | 100% CR | 79 months (36.4-143.8) | NA | 3 second malignances | |
| Orbit | Le 2002 (51) | Retrospective | 31 | 100%MZL | 30–40 Gy, 1.8–2 Gy/fr | CTV Conjunctiva; CTV Retrobulbar | 6–20 MeV electron anterior field; 4–6 MV photon multiple beam | 100% CR; 71% 10-yrs FFR | 5.9 yr (9 months – 20.3 yr) | 73% 10-yr OS | No cataract; 1 retinopathy |
| Fasola 2002 (12) | Retrospective | 20 | 40% MZL | 4 Gy 2Gy/fr | Entire conjunctiva: | 6–12 MeV electron anterior field, 4-6MV photon beam | 96% ORR; 100% 2-yrs FFLR | 26 months (7–92) | 96% 2-yr FFRR | No retinopathy or keratitis; No cataract RT-related | |
| Goda 2011 (2) | Retrospective | 89 | 100%MZL | 25 Gy 98%, 30 Gy 2%, 2.5 Gy/fr | Entire orbit | Electron anterior field, 4–6 MV photon multiple beam | 99% CR; 7-year LC rate 97%; 64% 7-yrs RFS | 5.9 yr (1–16) | 91% 7-yr OS | 25% Grade 3 cataract at 7 yrs | |
| 96% 7-yr CSS | |||||||||||
| Pinnix 2017 (14) | Retrospective | 22 | 64% MZL | 4 Gy 2Gy/fr | CTV: entire palpebral and bulbar conjunctiva or entire orbit | Electron and photon | 100% ORR | 14.1 months (range, 3.7–29.9) | NA | 1 dry eye syndrome | |
| Breast | Martinelli 2009 (65) | Retrospective | 60 | 40% MZL | Breast: median 38 Gy (range 25–50 Gy): median 36 Gy (range 30-46 Gy) | CTV: whole breast, if indicated axilla and supraclavicular node | NA | 100% ORR | 44 months (5–156 months) | 64% 5&10-yr OS | NA |
| 72% 3-yr PFS | |||||||||||
| 56% 5-yr PFS | |||||||||||
| 34% 10-yr PFS | |||||||||||
| Ludmir 2019(17) | Retrospective | 11 | 100%MZL | Median 30 Gy (range, 4–45 Gy) 1.5–2 Gy/fr | CTV: whole breast | Opposed tangent beams | 100% LC | 8 yr (4.8–10.2) | 60% 5-yr PFS | 1 pts: late grade 1 breast fibrosis | |
| Skin | Neelis 2009 (73) | Retrospective | 18 | 55% MZL | 4 Gy, 2 Gy/fr | CTV: gross tumor plus 2 cm margins | Electron beam mostly 4 MeV | 72% CRR | 13 months (2.3–42) | NA | None |
| Servitje 2013 (19) | Retrospective | 137 | 100% | Range 30–40 Gy | CTV: gross tumor plus 5 cm margins | Single electron field | 88% CRR | 54 months (12–165) | 46% 5-yr DFS | NA | |
| 93% 5-yr OS | |||||||||||
| De Felice 2018 (72) | Retrospective | 42 | 45% MZL | Median 36 Gy range 20–46 Gy | CTV: gross tumor plus 2 cm margins | Electron field | 100% CRR | 9.5 yr | 79% 5-yr RFS | ||
| 71% 10-yr RFS | |||||||||||
| 87% 10-yr OS | |||||||||||
| Gauci 2018 (71) | Retrospective | 46 | 46% MZL | Median 24 Gy (18–30 Gy) 3 Gy/fr | CTV: gross tumor plus 0.5–1 cm | Low-energy X-rays (80–120 KV) | 96% CRR | 43.5 months (0.6–100) | 55% 3-yr DFS | 78% moderate sequelae (pts reported) | |
| Oertel 2020 (74) | Retrospective | 26 | 38.5% MZL | Median 40 Gy (4–50 Gy) | NA | Electron and photon | ORR: 92% vs. 86% | NA | 55% 10-yr PFS | 54% grade 1 late toxicity (conventional RT); None in low dose RT | |
| CRR: 84% vs. 29% conventional RT vs. low dose RT | |||||||||||
| Lung | Girinsky 2012 (11) | Retrospective | 10 | 100% MZL | 4 Gy 2 Gy/fr | PTV: gross tumor plus1 cm isotropic margin | 3D CRT | 80% CR | 56 months (2–103 months) | 86% 5-yr PFS | None |
| 100% 5-yr OS | |||||||||||
| Central Nervous system Dura | de la Fuente 2017 (88) | Retrospective | 26 | 100% MZL | 16 to 36 Gy in 9 to 20 fr | 13 pts focal RT | IMRT | 85% CR | 64 months (2–209 months) | 89% 3-yr PFS | NA |
| 36 to 39 Gy in 20 to 26 fr | 6 whole brain RT | ||||||||||
| Sunderland 2020 (87) | Retrospective | 26, 27% Sugery+ RT | 100% primary or secondary MZL | NA | NA | NA | 77% CR in primary MZL | 1.9 yr (0.1–11.4) | 59% 2-yr PFS | NA | |
| 2% CR in secondary MZL | 80% 2-yr OS | ||||||||||
| Mixed anatomic site | Tsang 2001 (9) | Retrospective | 75 | 100% MZL | 25 Gy for orbital lymphoma, 30–35 Gy fractions for other sites 1–2.5 Gy/fr | CTV: involved organ/lymph node, with or without the adjacent first echelon lymph node region | IFRT | 96% CRR | 4.2 yr (0.3–11.4) | 76%5-yr DFS | No serious toxicity observed |
| 96%5-yr OS | |||||||||||
| Goda 2010 (2) | Retrospective | 167, Salivary glands 28; Thyroid 21; Other head and neck sites 6 | 100% MZL | Median 30 Gy (range, 17.5–35 Gy) | CTV: whole organ plus locoregional lymph nodes for thyroid | Electron field/photon beam 2D, 3DCRT & IMRT | 99% CRR | 7.4 yr (0.67–16.20) | 68% 10-yr DFS | NA | |
| 76% 10-yr RFR | 87% 10-yr OS | ||||||||||
| Salivary gland: 68% 10-yr RFR | 98% 10-yr CSS | ||||||||||
| Thyroid: 95% 10-yr RFR | |||||||||||
| Teckie 2015 (3) | Retrospective | 244 | 100% MZL | Median 30 Gy | NA | Electron fields 3-D CRT or IMRT | 88% CR | 5.2 yr (0.2–21.3) | 92% 5-yr OS | NA | |
| 74% 5-yr RFS |
N, number; Pts, patients; HPE,
The technical aspects of delivering effective and safe RT were illustrated for both common and rare presentations of the disease and expected side effects of therapy were discussed. The concern about late effects of radiation, mainly induction of second cancers should lead to the use of more restricted involved site RT volumes, lower RT doses, and techniques that optimize normal tissue protection (15). Given the unique biologic behavior of MALT lymphoma with a tendency to relapse in extranodal MALT sites and an indolent course, it is not surprising that RT is often the treatment of choice again for patients with localized relapses. We suggest clinicians must remain vigilant in weighing the relative benefit of radiotherapy and chemotherapy, and the toxicities of both modalities to optimize the management of patients with MALT lymphomas.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francesco Bertoni, Davide Rossi, Thomas Habermann, Emanuele Zucca) for the series “Marginal Zone Lymphomas” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-23). The series “Marginal Zone Lymphomas” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer 1983;52:1410-6. [Crossref] [PubMed]
- Goda JS, Le LW, Lapperriere NJ, et al. Localized orbital mucosa-associated lymphoma tissue lymphoma managed with primary radiation therapy: efficacy and toxicity. Int J Radiat Oncol Biol Phys 2011;81:e659-666. [Crossref] [PubMed]
- Teckie S, Qi S, Lovie S, et al. Long-term outcomes and patterns of relapse of early-stage extranodal marginal zone lymphoma treated with radiation therapy with curative intent. Int J Radiat Oncol Biol Phys 2015;92:130-7. [Crossref] [PubMed]
- Goda JS, Gospodarowicz M, Pintilie M, et al. Long-term outcome in localized extranodal mucosa-associated lymphoid tissue lymphomas treated with radiotherapy. Cancer 2010;116:3815-24. [Crossref] [PubMed]
- Wirth A, Gospodarowicz M, Aleman BM, et al. Long-term outcome for gastric marginal zone lymphoma treated with radiotherapy: a retrospective, multi-centre, International Extranodal Lymphoma Study Group study. Ann Oncol 2013;24:1344-51. [Crossref] [PubMed]
- Fisher RI, Dahlberg S, Nathwani BN, et al. A clinical analysis of two indolent lymphoma entities: mantle cell lymphoma and marginal zone lymphoma (including the mucosa-associated lymphoid tissue and monocytoid B-cell subcategories): a Southwest Oncology Group study. Blood 1995;85:1075-82. [Crossref] [PubMed]
- Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol 2011;100:86-92. [Crossref] [PubMed]
- Hoskin PJ, Kirkwood AA, Popova B, et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): a randomised phase 3 non-inferiority trial. Lancet Oncol 2014;15:457-463. [Crossref] [PubMed]
- Ganem G, Lambin P, Socié G, et al. Potential role for low dose limited-fi eld radiation therapy (2 x 2 grays) in advanced low-grade non-Hodgkin’s lymphomas. Hematol Oncol 1994;12:1-8. [Crossref] [PubMed]
- Chan EK, Fung S, Gospodarowicz M, et al. Palliation by low-dose local radiation therapy for indolent non-Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2011;81:e781-6. [Crossref] [PubMed]
- Girinsky T, Paumier A, Ferme C, et al. Low-dose radiation treatment in pulmonary mucosa-associated lymphoid tissue lymphoma: a plausible approach? A single-institution experience in 10 patients. Int J Radiat Oncol Biol Phys 2012;83:e385-9. [Crossref] [PubMed]
- Fasola CE, Jones JC, Huang DD, et al. Low-dose radiation therapy (2 Gy × 2) in the treatment of orbital lymphoma. Int J Radiat Oncol Biol Phys 2013;86:930-5. [Crossref] [PubMed]
- König L, Hörner-Rieber J, Bernhardt D, et al. Response rates and recurrence patterns after low-dose radiotherapy with 4 Gy in patients with low-grade lymphomas. Strahlenther Onkol 2018;194:454-61. [Crossref] [PubMed]
- Pinnix CC, Dabaja BS, Milgrom SA, et al. Ultra-low-dose radiotherapy for definitive management of ocular adnexal B-cell lymphoma. Head Neck 2017;39:1095-100. [Crossref] [PubMed]
- Yahalom J, Illidge T, Specht L, et al. Modern radiation therapy for extranodal lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 2015;92:11-31. [Crossref] [PubMed]
- Tsang RW, Gospodarowicz MK, Pintilie M, et al. Stage I and II MALT lymphoma: results of treatment with radiotherapy. Int J Radiat Oncol Biol Phys 2001;50:1258-64. [Crossref] [PubMed]
- Ludmir EB, Milgrom SA, Pinnix CC, et al. Treatment Strategies for Primary Breast Extranodal Marginal Zone Lymphoma of Mucosa-associated Lymphoid Tissue. Clin Lymphoma Myeloma Leuk 2019;19:244-250. [Crossref] [PubMed]
- Thieblemont C, Mayer A, Dumontet C, et al. Primary thyroid lymphoma is a heterogeneous disease. J Clin Endocrinol Metab 2002;87:105-111. [Crossref] [PubMed]
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol 2013;69:357-65. [Crossref] [PubMed]
- Du MQ, Isaccson PG. Gastric MALT lymphoma: from aetiology to treatment. Lancet Oncol 2002;3:97-104. [Crossref] [PubMed]
- Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, et al. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991;338:1175-6. [Crossref] [PubMed]
- Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993;342:575-7. [Crossref] [PubMed]
- Zucca E, Copie-Bergman C, Ricardi U, et al. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi144-148. [Crossref] [PubMed]
- Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, et al. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut 2011;60:747-58. [Crossref] [PubMed]
- Graham DY, Opekun AR, Hammoud F, et al. Studies regarding the mechanism of false negative urea breath tests with proton pump inhibitors. Am J Gastroenterol 2003;98:1005-9. [Crossref] [PubMed]
- Dickey W, Kenny BD, McConnell JB. Effect of proton pump inhibitors on the detection of Helicobacter pylori in gastric biopsies. Aliment Pharmacol Ther 1996;10:289-93. [Crossref] [PubMed]
- Chey WD, Leontiadis GI, Howden CW, et al. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol 2017;112:212-39. [Crossref] [PubMed]
- Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017;66:6-30. [Crossref] [PubMed]
- Crowe SE. Helicobacter pylori Infection. N Engl J Med 2019;380:1158-1165. [Crossref] [PubMed]
- Zullo A, Hassan C, Cristofari F, et al. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol 2010;8:105-10. [Crossref] [PubMed]
- Nakamura S, Sugiyama T, Matsumoto T, et al. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut 2012;61:507-13. [Crossref] [PubMed]
- Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet 2001;357:39-40. [Crossref] [PubMed]
- Ruskoné-Fourmestraux A, Lavergne A, Aegerter PH, et al. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut 2001;48:297-303. [Crossref] [PubMed]
- Zullo A, Hassan C, Andriani A, et al. Treatment of low-grade gastric MALT-lymphoma unresponsive to Helicobacter pylori therapy: a pooled-data analysis. Med Oncol 2010;27:291-5. [Crossref] [PubMed]
- Ryu KD, Kim GH, Park SO, et al. Treatment outcome for gastric mucosa-associated lymphoid tissue lymphoma according to Helicobacter pylori infection status: a single-center experience. Gut Liver 2014;8:408-14. [Crossref] [PubMed]
- Abe S, Oda I, Inaba K, et al. A retrospective study of 5-year outcomes of radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma refractory to Helicobacter pylori eradication therapy. Jpn J Clin Oncol 2013;43:917-22. [Crossref] [PubMed]
- Kim JS, Kang SH, Moon HS, et al. Clinical Outcome of Eradication Therapy for Gastric Mucosa-Associated Lymphoid Tissue Lymphoma according to H. pylori Infection Status. Gastroenterol Res Pract 2016;2016:6794848 [Crossref] [PubMed]
- Asano N, Iijima K, Terai S, et al. Eradication therapy is effective for Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma. Tohoku J Exp Med 2012;228:223-7. [Crossref] [PubMed]
- Strati P, Lee ST, Teegavarupu P, et al. Frontline antibiotic therapy for early-stage Helicobacter pylori-negative gastric MALT lymphoma. Am J Hematol 2019;94:E150-3. [Crossref] [PubMed]
- Vrieling C, de Jong D, Boot H, et al. Long-term results of stomach-conserving therapy in gastric MALT lymphoma. Radiother Oncol 2008;87:405-11. [Crossref] [PubMed]
- Koch P, Probst A, Berdel WE, et al. Treatment results in localized primary gastric lymphoma: data of patients registered within the German multicenter study (GIT NHL 02/96). J Clin Oncol 2005;23:7050-9. [Crossref] [PubMed]
- Reinartz G, Pyra RP, Lenz G, et al. Favorable radiation field decrease in gastric marginal zone lymphoma: Experience of the German Study Group on Gastrointestinal Lymphoma (DSGL). Strahlenther Onkol 2019;195:544-57. [Crossref] [PubMed]
- Wang H, Milgrom SA, Dabaja BS, et al. Daily CT guidance improves target coverage during definitive radiation therapy for gastric MALT lymphoma. Pract Radiat Oncol 2017;7:e471-8. [Crossref] [PubMed]
- Johnson ME, Pereira GC, El Naqa IM, et al. Determination of planning target volume for whole stomach irradiation using daily megavoltage computed tomographic images. Pract Radiat Oncol 2012;2:e85-8. [Crossref] [PubMed]
- Christopherson KG, Milgrom S, Wong P, et al. Primary Gastric Diffuse Large B-Cell Lymphoma treated with Abbreviated Chemoimmunotherapy and Contemporary Radiation Therapy has Excellent Outcomes with Minimal Toxicity. In American Society for Radiation Oncology (ASTRO) annual Meeting. San Antonio, Texas: 2018.
- Choi SH, Park SH, Lee JJB, et al. Combining deep-inspiration breath hold and intensity-modulated radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma: Dosimetric evaluation using comprehensive plan quality indices. Radiat Oncol 2019;14:59. [Crossref] [PubMed]
- Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol 1998;16:1916-21. [Crossref] [PubMed]
- Schmelz R, Miehlke S, Thiede C, et al. Sequential H. pylori eradication and radiation therapy with reduced dose compared to standard dose for gastric MALT lymphoma stages Ie & II1e: A prospective randomized trial. J Gastroenterol 2019;54:388-95. [Crossref] [PubMed]
- Pinnix CC, Gunther JR, Milgrom SA, et al. Outcomes After Reduced-Dose Intensity Modulated Radiation Therapy for Gastric Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma. Int J Radiat Oncol Biol Phys 2019;104:447-55. [Crossref] [PubMed]
- Cahill M, Barnes C, Moriarty P, et al. Ocular adnexal lymphoma-comparison of MALT lymphoma with other histological types. Br J Ophthalmol 1999;83:742-7. [Crossref] [PubMed]
- Le QT, Eulau SM, George TI, et al. Primary radiotherapy for localized orbital MALT lymphoma. Int J Radiat Oncol Biol Phys 2002;52:657-63. [Crossref] [PubMed]
- Hardman-Lea S, Kerr-Muir M, Wotherspoon AC, et al. Mucosal-associated lymphoid tissue lymphoma of the conjunctiva. Arch Ophthalmol 1994;112:1207-12. [Crossref] [PubMed]
- Thuro BA, Ning J, Peng SA, et al. Rates of Positive Findings on Positron Emission Tomography and Bone Marrow Biopsy in Patients With Ocular Adnexal Lymphoma. Ophthalmic Plast Reconstr Surg 2017;33:355-60. [Crossref] [PubMed]
- Stafford SL, Kozelsky TF, Garrity JA, et al. Orbital lymphoma: radiotherapy outcome and complications. Radiother Oncol 2001;59:139-44. [Crossref] [PubMed]
- Tran KH, Campbell BA, Fua T, et al. Efficacy of low dose radiotherapy for primary orbital marginal zone lymphoma. Leuk Lymphoma 2013;54:491-6. [Crossref] [PubMed]
- Donaldson SS, Findley DO. Treatment of orbital lymphoid tumors with electron beams. Front Radiat Ther Oncol 1991;25:187-200. [Crossref] [PubMed]
- Dunbar SF, Linggood RM, Doppke KP, et al. Conjunctival lymphoma: results and treatment with a single anterior electron field. A lens sparing approach. Int J Radiat Oncol Biol Phys 1990;19:249-57. [Crossref] [PubMed]
- Esik O, Ikeda H, Mukai K, et al. A retrospective analysis of different modalities for treatment of primary orbital non-Hodgkin's lymphomas. Radiother Oncol 1996;38:13-8. [Crossref] [PubMed]
- Tzioufas AG. B-cell lymphoproliferation in primary Sjögren's syndrome. Clin Exp Rheumatol 1996;14:S65-70. [PubMed]
- Khalil MO, Morton LM, Devesa SS, et al. Incidence of Marginal Zone Lymphoma in the United States, 2001-2009 With a Focus on Primary Anatomic Site. Br J Haematol 2014;165:67-77. [Crossref] [PubMed]
- Gunther JR, Park C, Dabaja BS, et al. Radiation Therapy for Salivary Gland MALT Lymphoma: Ultra-Low Dose Treatment Achieves Encouraging Early Outcomes and Spares Salivary Function. Leuk Lymphoma 2020;61:171-5. [Crossref] [PubMed]
- Paulsen J, Lennert K. Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type in Waldeyer's ring. Histopathology 1994;24:1-11. [Crossref] [PubMed]
- Aozasa K. Hashimoto's thyroiditis as a risk factor of thyroid lymphoma. Acta Pathol Jpn 1990;40:459-68. [Crossref] [PubMed]
- Cheah CY, Campbell BA, Seymour JF. Primary breast lymphoma. Cancer Treat Rev 2014;40:900-8. [Crossref] [PubMed]
- Martinelli G, Ryan G, Seymour JF, et al. Primary follicular and marginal-zone lymphoma of the breast: clinical features, prognostic factors and outcome: a study by the International Extranodal Lymphoma Study Group. Ann Oncol 2009;20:1993-9. [Crossref] [PubMed]
- Hoefnagel JJ, Vermeer MH, Jansen PM, et al. Primary cutaneous marginal zone B-cell lymphoma: clinical and therapeutic features in 50 cases. Arch Dermatol 2005;141:1139-45. [Crossref] [PubMed]
- Kempf W, Zimmermann AK, Mitteldorf C. Cutaneous lymphomas—An update 2019 Hematol Oncol 2019;37:43-7. [Crossref] [PubMed]
- Goyal A, Carter JB, Pashtan I, et al. Very low-dose versus standard dose radiation therapy for indolent primary cutaneous B-cell lymphomas: A retrospective study. J Am Acad Dermatol 2018;78:408-10. [Crossref] [PubMed]
- Senff NJ, Hoefnagel JJ, Neelis K, et al. Results of Radiotherapy in 153 Primary Cutaneous B-Cell Lymphomas Classified According to the WHO-EORTC Classification. Arch Dermatol 2007;143:1520-6. [Crossref] [PubMed]
- Pashtan I, Mauch PM, Chen YH, et al. Radiotherapy in the management of localized primary cutaneous B-cell lymphoma. Leuk Lymphoma 2013;54:726-30. [Crossref] [PubMed]
- Gauci ML, Quero L, Ram-Wolff C, et al. Outcomes of radiation therapy of indolent cutaneous B-cell lymphomas and literature review. J Eur Acad Dermatol Venereol 2018;32:1668-73. [Crossref] [PubMed]
- De Felice F, Grapulin L, Pieroni A, et al. Radiation therapy in indolent primary cutaneous B cell lymphoma: a single institute experience. Ann Hematol 2018;97:2411-6. [Crossref] [PubMed]
- Neelis KJ, Schimmel EC, Vermeer MH, et al. Low-dose palliative radiotherapy for cutaneous B- and T-cell lymphomas. Int J Radiat Oncol Biol Phys 2009;74:154-8. [Crossref] [PubMed]
- Oertel M, Elsayad K, Weishaupt C, et al. De-escalated radiotherapy for indolent primary cutaneous B-cell lymphoma. Strahlenther Onkol 2020;196:126-31. [Crossref] [PubMed]
- Kurtin PJ, Myers JL, Adlakha H, et al. Pathologic and clinical features of primary pulmonary extranodal marginal zone B-cell lymphoma of MALT type. Am J Surg Pathol 2001;25:997-1008. [Crossref] [PubMed]
- Ferraro P, Trastek VF, Adlakha H, et al. Primary non-Hodgkin's lymphoma of the lung. Ann Thorac Surg 2000;69:993-7. [Crossref] [PubMed]
- Royer B, Cazals-Hatem D, Sibilia J, et al. Lymphomas in patients with Sjogren's syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood 1997;90:766-75. [Crossref] [PubMed]
- King LJ, Padley SP, Wotherspoon AC, et al. Pulmonary MALT lymphoma: imaging findings in 24 cases. Eur Radiol 2000;10:1932-8. [Crossref] [PubMed]
- McDonald S, Rubin P, Phillips TL, et al. Injury to the lung from cancer therapy: clinical syndromes, measurable endpoints, and potential scoring systems. Int J Radiat Oncol Biol Phys 1995;31:1187-203. [Crossref] [PubMed]
- Mah K, Van Dyk J, Keane T, et al. Acute radiation-induced pulmonary damage: a clinical study on the response to fractionated radiation therapy. Int J Radiat Oncol Biol Phys 1987;13:179-88. [Crossref] [PubMed]
- Mah K, Keane TJ, Van Dyk J, et al. Quantitative effect of combined chemotherapy and fractionated radiotherapy on the incidence of radiation-induced lung damage: a prospective clinical study. Int J Radiat Oncol Biol Phys 1994;28:563-74. [Crossref] [PubMed]
- Zinzani PL, Magagnoli M, Galieni P. Nongastrointestinal low-grade mucosa-associated lymphoid tissue lymphoma: analysis of 75 patients. J Clin Oncol 1999;17:1254. [Crossref] [PubMed]
- Thieblemont C, Bastion Y, Berger F, et al. Mucosa-associated lymphoid tissue gastrointestinal and nongastrointestinal lymphoma behavior: analysis of 108 patients. J Clin Oncol 1997;15:1624-30. [Crossref] [PubMed]
- Tu PH, Giannini C, Judkins AR, et al. Clinicopathologic and genetic profile of intracranial marginal zone lymphoma: a primary low-grade CNS lymphoma that mimics meningioma. J Clin Oncol 2005;23:5718-27. [Crossref] [PubMed]
- Swerdlow SH CE, Harris NL, Jaffe ES, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer. & World Health Organization 2017.
- Ayanambakkam A, Ibrahimi S, Bilal K, et al. Extranodal Marginal Zone Lymphoma of the Central Nervous System. Clin Lymphoma Myeloma Leuk 2018;18:34-37.e8. [Crossref] [PubMed]
- Sunderland AJ, Steiner RE, Al Zahrani M, et al. An international multicenter retrospective analysis of patients with extranodal marginal zone lymphoma and histologically confirmed central nervous system and dural involvement. Cancer Med 2020;9:663-70. [Crossref] [PubMed]
- de la Fuente MI, Haggiagi A, Moul A, et al. Marginal zone dural lymphoma: the Memorial Sloan Kettering Cancer Center and University of Miami experiences. Leuk Lymphoma 2017;58:882-8. [Crossref] [PubMed]
Cite this article as: Deantonio L, Pinnix CC, Tsang R. Radiation therapy of extranodal marginal zone lymphomas. Ann Lymphoma 2020;4:16.