
Targeting pathogenic mechanisms in marginal zone lymphoma: from concepts and beyond
Introduction
Marginal zone lymphomas (MZLs) are mature lymphoid malignancies that have undergone somatic hypermutation and isotypic switching and are derived from the “marginal zone” region surrounding lymphoid follicles. The disease is generally divided into three distinct categories based on clinical and molecular characteristics: extranodal MZL (EMZL) of mucosa-associated lymphoid tissue (MALT); nodal marginal zone lymphoma (NMZL); and lastly, splenic marginal zone lymphoma (SMZL) (1,2). The MZLs as a whole represents the second most common type of indolent B-cell lymphoma, comprising approximately 6% of all non-Hodgkin lymphomas (NHL) cases (3). Despite their varying clinical presentations, the three entities share common features including an association with antigen stimulation, presence of specific translocations, and importantly, the activation of the nuclear factor κB (NF-κB) pathway.
To conceptualize a new therapeutic model around how to leverage our deepening understanding of MZL pathogenesis, it is instructive to identify those unique features of MZL pathogenesis, and to consider how best to inhibit those pathways and networks in a highly disease focused fashion. In an effort to appreciate these opportunities, we have structured concepts around specific therapeutic interventions in the context of the underlying deranged biology. Genomic profiling has had a substantial impact on our understanding of the molecular pathogenesis of MZL (4-19). These experiences have identified critical aberrations in the function of the B-cell receptor (BCR), NF-κB, Janus kinase (JAK)/signal transducers and activators of transcription (STAT), and Toll-Like Receptor/Interleukin (TLR/IL) signaling, each of which has at least one therapeutic agent that could impact that biology. Herein, we underscore that biology and the agents that may have at least a theoretical application in the disease.
Pathological mechanisms driving MZL and potential therapeutic interventions
Dysregulation of NF-κB signaling
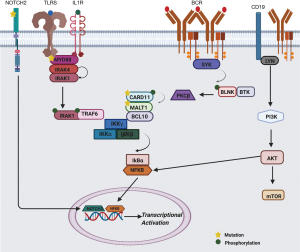
NF-κB is central to many hematological malignancies and it is a clear driver in MZL as well. The NF-κB family of transcription factors is comprised of five structurally related genes: NFKB1 (p105 and p50), NFKB2 (p100 and p52), RELA (p65), RELB (RelB), and REL (c-Rel) (20). Activation of the NF-κB family of transcription factors can occur through two distinct pathways: canonical (classical) and non-canonical (alternative). The canonical pathway is mediated through activation of BCR, Toll-like receptors (TLRs), nucleotide oligomerization domain (NOD)-like receptors, and tumor necrosis factors (TNF) family receptors, leading to the phosphorylation of I-kappa-B (IκB) by IκB kinase complex, permitting NF-κB to translocate to the nucleus. Alternatively, the non-canonical pathway is induced by B-cell activating factor belonging to the TNF family (BAFF) receptor, and CD40 (21), both of which leads to the activation of NF-κB inducible kinase (NIK) and IκB kinase α (IKKα), leading to phosphorylation and proteolysis of p100 that in turn creates the active subunit, p52. The p52-RelB heterodimer translocates into the nucleus in order to induce transcriptional activation of genes involved in cycle cell progression and proliferation (20,21).
There are several described genetic mechanisms, including recurring translocations [t(11; 18)(q21;q21); t(14;18) (q32;q21); t(1;14)(p22;q32)], that can lead to the dysregulation of the NF-κB signaling pathway in MZL (22-26). Fifteen to thirty percent of all MZL harbor inactivating mutations/deletions in TNFAIP3 (A20), which codes for a key negative regulator of NF-κB pathway, thus representing one of the most common genetic events contributing to overactivation of the NF-κB pathway in MZL (7,9,27). As discussed below, various dysregulated pathways in MZL converge to produce an overarching stimulation of NF-κB, which can be leveraged to improve therapeutic interventions. These agents may include those targeting BCR signaling through Bruton’s Tyrosine Kinase (BTK) inhibitors and phosphatidylinositol 3-kinase (PI3K) inhibitors to more novel agents in development such as MALT1 inhibitors. These pathways and their interplay are shared in Figure 1.
BCR and PI3K Pathway
BCR signaling plays a critical role in MZL pathogenesis, being one of several mechanisms that converge upon NF-κB activation. Chronic signaling through the BCR pathway, independent of antigen stimulation, is central for B-cell survival, and is mediated though the activation of the PI3K pathway (28,29). Engagement of the BCR induces phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM) in the cytoplasmic domains of CD79A and CD79B by SRC family tyrosine kinases (LYN, FYN, and BLK) (30). Spleen tyrosine kinase (SYK) is recruited to the phosphorylated ITAMs, and in turn, augments the initial signal through further phosphorylation of ITAMs as well as autophosphorylation. Moreover, upon activation of BCR, the transmembrane protein CD19 is phosphorylated by LYN leading to PI3K recruitment and subsequent production of phosphatidylinositol-3,4,5-triphophosphate (PIP3). BTK is activated by LYN and SYK kinases, and in turn, phosphorylates phospholipase Cγ2 (PLCγ2), subsequently leading to the proteolysis of PIP2 (phosphatidylinositol 4,5-bisphosphate) into DAG (diacylglycerol) and IP3 (inositol trisphosphate), triggering an influx of calcium into the cytoplasm. Consequentially, protein kinase Cβ (PKCβ) is activated by the increase in calcium and DAG, and induces the phosphorylation of CARD11, leading to the formation of the CBM complex (CARD11-BCL10-MALT1), triggering the downstream activation of NF-κB, which is critical in the development and sustainment of MZL cells (31). The CBM complex ultimately activates a cascade of events that leads to the formation of p50/RelA and p50/c-Rel heterodimers, which migrate into the nucleus and induce transcriptional activation (30,32). Activating CARD11 mutations are present in 5–10% of SMZL and NMZL, and lead to continual production or association of CBM complex components leading to IKKB activation of NF-κB pathway (12,33-35) and drives resistance in many lymphoid malignancies. Ultimately, both the BCR and PI3K pathways have the ability to promote lymphomagenesis.
Role of BTK inhibitors and PI3K inhibitors
Constitutive BCR signaling functions through a plethora of enzymes, scaffolding proteins, and protein-protein complexes, with recent advances targeting BTK and PI3K as a means to inhibit this complex pathway. The BTK inhibitor ibrutinib (36) has been FDA approved for relapsed/refractory (R/R) MZL (37) based on a phase II trial of 63 patients who progressed after receiving a prior anti-CD20 therapy (median number of prior therapies =2, range, 1–9). After a median follow-up of 19.4 months, the overall response rate (ORR), median progression free survival (PFS) and median duration of response (DOR) were 48% (95% CI, 35–62%), 14.2 months (95% CI, 8.3 to not estimable), and not reached, respectively (38). Although the numbers of each subtype are limited, there appeared to be a difference in PFS across the MZL subtypes, where the median PFS was 19.4, 13.8, and 8.3 months in SMZL (n=14), EMZL (n=32), and NMZL (n=17), respectively (38).
Among second generation BTK inhibitors, clinical data are already available for zanubrutinib (39). ORRs were 0% and 78% in two separate phase I studies enrolling two and ten R/R MZL patients, respectively, with no CRs (40-42). In a phase II study with 20 R/R MZL patients, the ORR was 80%, of which 15% attained a CR, with a median time to response of 3 months, and a 24-month PFS of 59% (43). The ORR by MZL subtype was 78% (CR, 11%) in EMZL (n.=9), 100% (CR, 40%) in NMZL (n.=5) and 67% (CR, 0%) in SMZL (n.=6) (43). Less data is available for acalabrutinib (44) which has been studied in MZL cell lines (45), and is currently being evaluated in a phase Ib/II study alone or in combination wtih rituximab (NCT02180711).
Dysregulation of the PI3K-axis has been well established as a crucial driver of malignant transformation, especially in the lymphoproliferative malignancies. Many PI3K inhibitors have emerged over the past few years, and while these drugs share class similarities, each can be differentiated from one another when one delves into the detailed pharmacology. Idelalisib was the first-in-class PI3Kδ and PI3Kd inhibitor, with essentially no activity against PI3Kα and PI3Kβ, while maintaining inhibition of PI3Kγ (albeit 40x less than PI3Kδ) (46). Idelalisib was evaluated in a phase I study which established a recommended phase 2 dose of 150 mg twice per day. This phase I study included only six patients with MZL (9% of study) (47). Across the entire population of patients in the phase I, the ORR was 47% (30/64), with a median DOR of 18.4 months and median PFS of 7.6 months (47). In the phase II study of patients with indolent lymphomas (majority with a diagnosis of FL), the ORR was 57% (71/125) with a median PFS and OS of 11 and 20.3 months, respectively (48). Only 15 patients had MZL in the phase II portion of the study. Amongst the 15 MZL patients, the median PFS was 6.6 months, ORR of 47%, CR rate of 7% and DOR of 18.4 months (49). These data led to the accelerated approval of idelalisib in patients in R/R FL and chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Despite the promising activity, idelalisib was associated with a number of immune mediated toxicities, including colitis, pneumonitis and hepatitis which have limited the general use of the drug.
Subsequently, two additional PI3K inhibitors, copanlisib and duvelisib, have been developed and approved by the U.S. FDA (50,51). These drugs have slightly different patterns of selectivity for the PI3K isoforms, with copanlisib targeting preferentially PI3Kδ and PI3Kα, while duvelisib potently inhibits PI3Kγ, along with PI3Kδ and other isoforms as well (50,51). Both drugs have been approved for R/R CLL (duvelisib) and FL (copanlisib, duvelisib). In the phase II DYNAMO trial of duvelisib in R/R indolent lymphomas, Flinn et al. demonstrated an ORR of 47% (61/129) (52). Specifically, the ORR in MZL population was 38.9% (7/18) (52). Given the fact that the chemical structures of idelalisib and duvelisib are quite similar, it is not surprising that the safety profile of the drugs are similar with regard to the immune-mediated side effects (52). In the CHRONOS-1 trial, copanlisib was evaluated in indolent B-cell lymphomas as well as peripheral T-cell lymphoma (PTCL) and demonstrated an ORR of 43.7% (14/32), median PFS of 294 days and median DOR of 390 days in the indolent cohort. Within the indolent cohort, only 3 patients had MZL (53). In an updated analysis, the ORR was 60.6%, with a median DOR, PFS, and OS of 14.1 months, 12.5 months and 42.6 months, respectively (54). Unique to the side effect profile of copanlisib was hyperglycemia and transient hypertension; however, diarrhea, colitis, pneumonitis, and neutropenia were also present. Again, to this date, neither drug has been FDA approved for MZL.
Umbralisib, an inhibitor of PI3Kδ and CK1ε (55), is the only PI3K inhibitor specifically studied in MZL. Based on the data from a phase II registration-directed trial in MZL (n=69), Zinzani et al. reported an ORR of 55%, with 4 CRs, and a 12-month PFS of 71% (56). The most common adverse events (AEs) (≥20%) of any grade included diarrhea (45%), nausea (29%), fatigue (26%) and headache (26%). Thus, in general, the toxicity profile is substantially better than for idelalisib and duvelisib, with far less immune-mediated toxicity. At this time, umbralisib has been designated ‘Breakthrough Therapy’ status with the U.S. FDA and is being submitted for accelerated FDA approval for MZL and FL and may represent the first PI3K inhibitor approved for MZL.
To build upon the potential platform of PI3K inhibition in MZL, Tarantelli et al. performed combination cell-viability screening using copanlisib as the basis in a panel of lymphomas cell lines, including MZL and mantle cell lymphoma models (57). This study revealed that amongst 15 compounds tested, venetoclax in conjunction with copanlisib was the most synergistic, and the combination was then validated using a MZL xenograft model. Based on this pre-clinical data, a phase I trial investigating this combination is underway (NCT03886649), and is again representative of a non-chemotherapeutic predicated regimen that simultaneously targets various oncogenic mechanisms.
Moreover, Arribas and colleagues have recently reported on preclinical SMZL models of secondary resistance to idelalisib. This resistance is driven by epigenetic changes leading to the upregulation of tyrosine kinase receptors (PDGFRA or ERBB4) and secretion of factors (IL6 or HBEGF), therefore, providing the basis for novel combinations in this setting that target these resistant pathways (58).
SYK inhibitors
Development of small molecule SYK inhibitors is another method in order to mitigate constitutive BCR signaling found in MZL. As stated previously, upon BCR stimulation, Syk is recruited and leads to activation of BTK, PLCγ2, and PI3K. SYK has been found to be upregulated in all three MZL subtypes, making it a rationale therapeutic target upstream from the already more established BTK inhibitors and PI3K inhibitors (4-6).
Several SYK inhibitors, including fostamatinib (59-61), entospletinib (60,62), and cerdulatinib (inhibitor of SYK, JAK1/3 and TYK2) (63,64), have entered clinical trial development based on the rationale shared above. Much of the data available has been performed in DLBCL as well as CLL/SLL with little experience in MZL. The oral Syk inhibitor fostamatinib was evaluated in R/R DLBCL and indolent lymphomas in a phase I/II trial (59). Three patients with MZL were included in the phase II portion, with 1 patient with stable disease in a composite group of MZL and lymphoplasmacytic lymphoma. The most common AEs in the phase II portion was fatigue, diarrhea, transaminitis, and cytopenias (59). In a phase II study of entospletinib, 114 patients were enrolled, of that 17 subjects had a diagnosis of MZL (60). The ORR in the MZL subgroup was 11.8% (2/17), all of which were PRs, with 12 patients achieving stable disease (60). The most common grade ≥3 treatment emergent AEs included ALT elevation (15.8%), anemia (11.4%), fatigue (10.5%) and AST elevation (8.8%). Hamlin and colleagues evaluated cerdulatinib, a multi-kinase inhibitor, in patients with R/R B- and T-cell lymphomas, with an ORR of 61% in CLL/SLL, 50% in FL and 43% in PTCL (63); it is unclear how the MZL patients fared (n=8). Although a promising target, and perhaps due to the small numbers enrolled in the aforementioned studies, SYK inhibition has demonstrated limited activity as a single agent in MZL.
Immunomodulatory drugs
Lenalidomide is an immunomodulatory drugs (IMiD) shown to influence the ubiquitination and degradation of Ikaros, Aiolos and CK1α (65-68). The drug, possibly via pleotropic effects, including those on NF-κB and the microenvironment, has demonstrated an ORR of 61% and a CR of 33% as monotherapy in EMZL (69). To investigate a chemotherapy-free platform, the addition of lenalidomide to rituximab (R2 regimen) in untreated MZL demonstrated a remarkable ORR 93%, with CR/CRu of 70%, and a median PFS of 59.8 months and 5-year OS of 96% (70). The R2 regimen was further validated in the AUGMENT trial, which was a phase III, multicentered, randomized trial of R2vs. rituximab in conjunction with a placebo in patients with R/R follicular lymphoma (FL) or MZL (n=358) (71). At a median follow-up of 28.3 months, the PFS was significantly improved in the R2 arm (39.4 months) compared to rituximab plus placebo cohort (14.1 months) [HR, 0.46; 95% CI, 0.34–0.62, P<0.0001]. Subgroup analysis evaluating MZL patients (n=63) demonstrated that there was no statistically significant difference between the two treatment arms (HR 1.0; 95% CI, 0.47–2.13), which was attributed to a small sample size and an imbalance in baseline characteristics, with many high-risk/advance features notably in the R2 investigational arm. Nevertheless, based on the AUGMENT trial, the FDA approved R2 regimen for both R/R FL and MZL patients. Extrapolating from this platform, a single-center phase II study is currently underway evaluating the merits of the combination of ibrutinib, lenalidomide and rituximab (iR2) in previously untreated MZL and FL (NCT02532257). Results thus far have demonstrated an estimated 2-year PFS of 76% (95% CI: 60–96%), with a ORR of 80% and CR rate of 60% in MZL (n=10; 4 NMZL; 3 SMZL; 3 EMZL) (72). This data suggests that the combination of novel, targeted therapies may lead to chemotherapy-free platforms that aim to inhibit multiple oncogenic pathways in MZL.
MALT1 inhibitors
The development of MALT1 inhibitors is being intensively investigated mostly for DLBCL (73-80), though there is preclinical evidence of anti-tumor activity in MZL models as a single agent or in combination such as with the PI3K inhibitor, copanlisib (57,81). A phase I study (NCT03900598) is currently on-going with the oral MALT1 inhibitor JNJ-67856633 in R/R B-cell lymphoma and CLL patients.
SMAC mimetics
The BIRC3 gene, fused to MALT1 in the t(11;18) of EMZL, and recurrently mutated in the other MZLs, codes for the cellular inhibitor of apoptosis 2 (cIAP2) protein, an E3 ubiquitin ligase important for the activation of the CBM complex (82). Different SMAC mimetics have been developed (83-86). LCL-161 has shown anti-tumor activity in MZL primary cells (87). Some SMAC mimetics are under clinical investigation as single agents or in combination for different oncological indications (83), but not specifically for MZL patients (83,87).
NIK inhibitors
NIK inhibitors, which would target both the classical and alternative NF-κB pathways, have been developed (88-95). As noted above, NIK activation correlates with increased activity of the NF-κB pathway and has been an attractive target as a means to downregulate this oncogenic driver. Although several inhibitors have been developed and studied, including in multiple myeloma models (96), no agent to date has entered clinical evaluation.
MYD88-IRAK4 Axis
TLR/ILR signaling is mediated through the recruitment of MYD88, an adaptor molecule, which forms the core of the Myddosome complex along with IRAK4 and serves as another method to which the canonical NF-κB pathway is stimulated (97-99). Upon ligand binding with lipopolysaccharide (LPS), lipoproteins, and IL-1, TLR/ILR dimerize or oligomerize, leading to the recruitment of MYD88 which then interfaces with IRAK4 through death domains (97) (Figure 1). The formation of the Myddosome is hierarchal. After ligand stimulation of TLR/IL1R, MYD88 recruits IRAK4, and subsequently, the MYD88-IRAK4 complex recruits IRAK1 or IRAK2 to form the Myddosome (97). IRAK1/2 is phosphorylated by IRAK4 and subsequently dissociates from the Myddosome in order to interact with TRAF6 (100), in turn, leading to the activation of downstream transcription factors such as NF-κB and AP-1 (101). Gain of function MYD88 mutations are found in approximately 10–15% SMZL, NMZL and EMZL, and leads to constitutive activation of this pathway independent of ligand binding (8,102-105).
Attempts to target MYD88 has largely been unsuccessful as it is difficult to inhibit given the fact that it is an adaptor protein with no catalytic activity. ST2825 is a small molecule inhibitor that prevents dimerization of MYD88, however, it requires micromolar concentrations (>15 µM) in order to suppress the growth of lymphoma cell lines, and was found to have off target effects (106,107). T6167923, another MYD88 dimerization inhibitor, was found to protect mice from toxic-shock induced death in a dose dependent manner but has not been fully explored in malignancies addicted to MYD88 activation as a treatment option (108). On the contrary, targeting IRAK4, a key member of the Myddosome, has become a recent area of investigation. IRAK4 kinase inhibitors have been developed, including CA-4948, which is currently under investigation for patients with R/R B-cell lymphomas (NCT03328078) (109). More recently, IRAK4 degraders have been developed (110), with the thought that as IRAK4 serves as both a scaffolding and catalytic protein, the elimination of IRAK4 as a whole would lead to improved therapeutic efficacy compared to IRAK4 kinase inhibitors. To date, we await further clinical results of IRAK4 targeting, which may be a promising area of development.
NOTCH signaling
NOTCH proteins are transmembrane receptors, which are comprised of four regions: an extracellular domain (NECD), a transmembrane domain, an intracellular domain (NICD), and a PEST domain (111-114). After binding with Notch ligands, the NOTCH proteins are first cleaved by an ADAM metalloproteinase, leading to the release of the NECD, and, later, by a γ-secretase, which then initiates the translocation of NCID from the cell membrane to the nucleus. In the nucleus, NCID recruits transcriptional co-factors, and acts as transcriptional factor. The PEST domain contains a ubiquitination site critical for the proteasomal degradation of the NCID. In B-cells, active NOTCH2 signaling is fundamental for the differentiation of B-cells versus the MZ phenotype (115-117), and, indeed, NOTCH signaling is activated by genetic events in MZL, especially in SMZL and NMZL (up to 40% of the cases) (8,11,12,16-18,103). Oncogenic mutations affecting the PEST domain, which induces the loss of negative regulation mediated via proteasomal degradation, are observed especially in the NOTCH2 gene (SMZL, 10–25%; NMZL, 25%; EMZL, <5%) and less frequently in NOTCH1. Notch activation is also sustained by recurrent inactivating mutations in genes coding for negative regulators such as DTX1, SPEN, and MAML2.
NOTCH inhibitors acting at different levels of the Notch signaling are available (111-114). Limited single agent anti-tumor activity has been observed using the γ-secretase inhibitor PF-03084014 (118) and the NICD inhibitor CB-103 (119) in SMZL cell lines with or without NOTCH2 mutation. More promising results are seen combining the γ-secretase inhibitor with PI3K, BTK and EZH2 inhibitors, or with the hypomethylating agent, decitabine (118). However to date, no clinical data is available with compounds targeting NOTCH in MZL patients.
Methylation and chromatin remodeling
There are genomic and preclinical data supporting the use of demethylating agents in MZL.
First, one quarter of SMZL cases are associated with a very high degree of promoter hypermethylation, leading to silencing of tumor suppressor genes and over-expression of potential therapeutic targets (NF-κB, PI3K and BCR signaling; PRC2-complex, that is EZH2, EED, and SUZ12). This phenotype is associated with inferior outcomes and a higher risk of histologic transformation (118). In fact, exposure of SMZL cell lines to the demethylating agent decitabine demonstrates a strong in vitro and in vivo anti-tumor activity in SMZL cell lines (118), and can, at least partially, revert the methylation-related phenotype observed in primary clinical specimens (118).
Another set of data supporting the importance of methylation in MZL comes from the recently observed high prevalence of somatic mutations in the TET2 gene in primary thyroid EMZLs (8,13). This gene encodes for a methylcytosine dioxygenase, which catalyzes the conversion of the modified genomic base 5-methylcytosine (5mC) into 5-hydroxymethylcytosine (5hmC), a fundamental and critical role in DNA demethylation (120,121). Importantly, the presence of the TET2 mutations in primary thyroid EMZLs is associated with increased DNA promoter methylation in genes targeted by the PRC2 complex members (EZH2, EED and SUZ12) (8). Interestingly, the transcriptome of TET2 DLBCL mutants have important overlaps with the transcriptome of CREBBP DLBCL mutants (122), and CREBBP mutants are sensitive to HDAC3 inhibitors (123). In fact, silencing of TET2 sensitizes DLBCL cells to HDAC3 inhibitors. Therefore, considering that CREBBP mutations are also common in MZL, there is a clear rationale to explore HDAC3 and other epigenetic drugs in MZL.
Conclusions
Taken together, the three MZL subtypes share common pathological mechanisms, including prominent TLR, BCR and PI3K pathway dysregulation that cumulate to induce NF-κB activation, as well as clear epigenetic deregulation. Our understanding of the deeper molecular biology of MZL, although with some overlapping themes with other indolent lymphomas, can lead to the unique targeted platforms that leverage a precision-medicine approach, which will likely require combinations of two or more novel agents to achieve the optimal therapeutic outcome of long durable responses with limited toxicity.
Acknowledgments
Funding: Work partially supported by Swiss National Science Foundation (31003A_163232/1) and Swiss Cancer Research (KFS-4727-02-2019) grants to FB.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Lymphoma for the series “Marginal Zone Lymphomas”. The article has undergone external peer review
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol-20-20). The series “Marginal Zone Lymphomas” was commissioned by the editorial office without any funding or sponsorship. JKL reports grants and research support from Kymera Therapeutics, and served on its scientific advisory board; served on Scientific Advisory Board of Astex Pharmaceuticals, Speakers’ Bureau of AstraZeneca, Scientific Advisory Board of Kura Oncology, Consulting for Daiichi Sankyo, outside the submitted work. OAO reports salary and equity stake from TG Therapeutics; consulting fees and equity stake in Kymera; consulting fees and research support from Mundipharma, Merck, Celgene/Bristol Myers Squibb, Astec, Affimed, and Trillium. FB served as the unpaid Guest Editor of the series and reports institutional research funds from Acerta, ADC Therapeutics, Bayer AG, Cellestia, CTI Life Sciences, EMD Serono, Helsinn, ImmunoGen, Menarini Ricerche, NEOMED Therapeutics 1, Nordic Nanovector ASA, Oncology Therapeutic Development, PIQUR Therapeutics AG; consultancy fee from Helsinn, Menarini; expert statements provided to HTG; travel grants from Amgen, Astra Zeneca, Jazz Pharmaceuticals, PIQUR Therapeutics AG. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Bertoni F, Rossi D, Raderer M, et al. Marginal Zone Lymphomas. Cancer J 2020;26:336-47. [Crossref] [PubMed]
- Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol 1998;16:2780-95. [Crossref] [PubMed]
- Arribas AJ, Campos-Martín Y, Gómez-Abad C, et al. Nodal marginal zone lymphoma: gene expression and miRNA profiling identify diagnostic markers and potential therapeutic targets. Blood 2012;119:e9-21. [Crossref] [PubMed]
- Huynh MQ, Wacker HH, Wundisch T, et al. Expression profiling reveals specific gene expression signatures in gastric MALT lymphomas. Leuk Lymphoma 2008;49:974-83. [Crossref] [PubMed]
- Ruiz-Ballesteros E, Mollejo M, Rodriguez A, et al. Splenic marginal zone lymphoma: proposal of new diagnostic and prognostic markers identified after tissue and cDNA microarray analysis. Blood 2005;106:1831-8. [Crossref] [PubMed]
- Novak U, Rinaldi A, Kwee I, et al. The NF-{kappa}B negative regulator TNFAIP3 (A20) is inactivated by somatic mutations and genomic deletions in marginal zone lymphomas. Blood 2009;113:4918-21. [Crossref] [PubMed]
- Cascione L, Rinaldi A, Bruscaggin A, et al. Novel insights into the genetics and epigenetics of MALT lymphoma unveiled by next generation sequencing analyses. Haematologica 2019;104:e558-61. [Crossref] [PubMed]
- Rinaldi A, Mian M, Chigrinova E, et al. Genome-wide DNA profiling of marginal zone lymphomas identifies subtype-specific lesions with an impact on the clinical outcome. Blood 2011;117:1595-604. [Crossref] [PubMed]
- Arribas AJ, Rinaldi A, Mensah AA, et al. DNA methylation profiling identifies two splenic marginal zone lymphoma subgroups with different clinical and genetic features. Blood 2015;125:1922-31. [Crossref] [PubMed]
- Rossi D, Trifonov V, Fangazio M, et al. The coding genome of splenic marginal zone lymphoma: activation of NOTCH2 and other pathways regulating marginal zone development. J Exp Med 2012;209:1537-51. [Crossref] [PubMed]
- Spina V, Khiabanian H, Messina M, et al. The genetics of nodal marginal zone lymphoma. Blood 2016;128:1362-73. [Crossref] [PubMed]
- Moody S, Thompson JS, Chuang SS, et al. Novel GPR34 and CCR6 mutation and distinct genetic profiles in MALT lymphomas of different sites. Haematologica 2018;103:1329-36. [Crossref] [PubMed]
- Chng WJ, Remstein ED, Fonseca R, et al. Gene expression profiling of pulmonary mucosa-associated lymphoid tissue (MALT) lymphoma identifies new biological insights with potential diagnostic and therapeutic applications. Blood 2009;113:635-45. [Crossref] [PubMed]
- Veeriah S, Brennan C, Meng S, et al. The tyrosine phosphatase PTPRD is a tumor suppressor that is frequently inactivated and mutated in glioblastoma and other human cancers. Proc Natl Acad Sci U S A 2009;106:9435-40. [Crossref] [PubMed]
- Guidetti F, Bruscaggin A, Frigeni M, et al. Molecular Subtypes of Splenic Marginal Zone Lymphoma (SMZL) Are Associated with Distinct Pathogenic Mechanisms and Outcomes - Interim Analysis of the IELSG46 Study. Blood 2018;132:922. [Crossref]
- Kiel MJ, Velusamy T, Betz BL, et al. Whole-genome sequencing identifies recurrent somatic NOTCH2 mutations in splenic marginal zone lymphoma. J Exp Med 2012;209:1553-65. [Crossref] [PubMed]
- Martínez N, Almaraz C, Vaque JP, et al. Whole-exome sequencing in splenic marginal zone lymphoma reveals mutations in genes involved in marginal zone differentiation. Leukemia 2014;28:1334-40. [Crossref] [PubMed]
- Vela V, Juskevicius D, Gerlach MM, et al. High throughput sequencing reveals high specificity of TNFAIP3 mutations in ocular adnexal marginal zone B-cell lymphomas. Hematol Oncol 2020;38:284-92. [Crossref] [PubMed]
- Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 2009;1:a000034 [Crossref] [PubMed]
- Lim KH, Yang Y, Staudt LM. Pathogenetic importance and therapeutic implications of NF-kappaB in lymphoid malignancies. Immunol Rev 2012;246:359-78. [Crossref] [PubMed]
- Rosebeck S, Madden L, Jin X, et al. Cleavage of NIK by the API2-MALT1 fusion oncoprotein leads to noncanonical NF-kappaB activation. Science 2011;331:468-72. [Crossref] [PubMed]
- Streubel B, Lamprecht A, Dierlamm J, et al. T(14;18)(q32;q21) involving IGH and MALT1 is a frequent chromosomal aberration in MALT lymphoma. Blood 2003;101:2335-9. [Crossref] [PubMed]
- Murga Penas EM, Hinz K, Röser K, et al. Translocations t(11;18)(q21;q21) and t(14;18)(q32;q21) are the main chromosomal abnormalities involving MLT/MALT1 in MALT lymphomas. Leukemia 2003;17:2225-9. [Crossref] [PubMed]
- Willis TG, Jadayel DM, Du MQ, et al. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell 1999;96:35-45. [Crossref] [PubMed]
- Zhang Q, Siebert R, Yan M, et al. Inactivating mutations and overexpression of BCL10, a caspase recruitment domain-containing gene, in MALT lymphoma with t(1;14)(p22;q32). Nat Genet 1999;22:63-8. [Crossref] [PubMed]
- Honma K, Tsuzuki S, Nakagawa M, et al. TNFAIP3 is the target gene of chromosome band 6q23.3-q24.1 loss in ocular adnexal marginal zone B cell lymphoma. Genes Chromosomes Cancer 2008;47:1-7. [Crossref] [PubMed]
- Kraus M, Alimzhanov MB, Rajewsky N, et al. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell 2004;117:787-800. [Crossref] [PubMed]
- Srinivasan L, Sasaki Y, Calado DP, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell 2009;139:573-86. [Crossref] [PubMed]
- Kurosaki T, Hikida M. Tyrosine kinases and their substrates in B lymphocytes. Immunol Rev 2009;228:132-48. [Crossref] [PubMed]
- Thome M. CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat Rev Immunol 2004;4:348-59. [Crossref] [PubMed]
- Kaileh M, Sen R. NF-kappaB function in B lymphocytes. Immunol Rev 2012;246:254-71. [Crossref] [PubMed]
- Yan Q, Huang Y, Watkins AJ, et al. BCR and TLR signaling pathways are recurrently targeted by genetic changes in splenic marginal zone lymphomas. Haematologica 2012;97:595-8. [Crossref] [PubMed]
- Rossi D, Deaglio S, Dominguez-Sola D, et al. Alteration of BIRC3 and multiple other NF-kappaB pathway genes in splenic marginal zone lymphoma. Blood 2011;118:4930-4. [Crossref] [PubMed]
- Lenz G, Davis RE, Ngo VN, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 2008;319:1676-9. [Crossref] [PubMed]
- Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A 2010;107:13075-80. [Crossref] [PubMed]
- Ibrutinib Approved for Marginal Zone Lymphoma Patients. Oncology Times 2017;39:19.
- Noy A, de Vos S, Thieblemont C, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood 2017;129:2224-32. [Crossref] [PubMed]
- Guo Y, Liu Y, Hu N, et al. Discovery of Zanubrutinib (BGB-3111), a Novel, Potent, and Selective Covalent Inhibitor of Bruton's Tyrosine Kinase. J Med Chem 2019;62:7923-40. [Crossref] [PubMed]
- Zhu J, Li J, Zhou J, et al. BGB-3111, a Highly Specific BTK Inhibitor, Is Well Tolerated and Highly Active in Chinese Patients with Relapsed/Refractory B-Cell Malignancies: Initial Report of a Phase 1 Trial in China. Blood 2017;130:5347.
- Opat S, Marcus R, Portell CA, et al. Phase 2 Study of Zanubrutinib (BGB-3111) in Patients with Relapsed/Refractory Marginal Zone Lymphoma. Blood 2019;134:5256. [Crossref]
- Tam CS, Simpson D, Opat S, et al. Safety and Activity of the Highly Specific BTK Inhibitor BGB-3111 in Patients with Indolent and Aggressive Non Hodgkin's Lymphoma. Blood 2017;130:152.
- Tedeschi A, Trotman J, Tam C, et al. Phase 1/2 study of single-agent zanubrutinib in patients with relapsed/refractory marginal zone lymphoma. Available online: https://library.ehaweb.org/eha/2020/eha25th/293654/alessandra.tedeschi.phase.1.2.study.of.single-agent.zanubrutinib.in.patients.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Dzanubrutinib
- Barf T, Covey T, Izumi R, et al. Acalabrutinib (ACP-196): A Covalent Bruton Tyrosine Kinase Inhibitor with a Differentiated Selectivity and In Vivo Potency Profile. J Pharmacol Exp Ther 2017;363:240-52. [Crossref] [PubMed]
- Spriano F, Tarantelli C, Gaudio E, et al. Single and combined BTK and PI3Kdelta inhibition with acalabrutinib and ACP-319 in pre-clinical models of aggressive lymphomas. Br J Haematol 2019;187:595-601. [Crossref] [PubMed]
- Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 2011;117:591-4. [Crossref] [PubMed]
- Benson DM, Kahl BS, Furman RR, et al. Final results of a phase I study of idelalisib, a selective inhibitor of PI3Kδ, in patients with relapsed or refractory indolent non-Hodgkin lymphoma (iNHL). J Clin Oncol 2013;31:8526. [Crossref]
- Gopal AK, Kahl BS, de Vos S, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 2014;370:1008-18. [Crossref] [PubMed]
- Martin P, Armas A, Gopal AK, et al. Idelalisib Monotherapy and Durable Responses in Patients with Relapsed or Refractory Marginal Zone Lymphoma (MZL). Blood 2015;126:1543. [Crossref]
- Winkler DG, Faia KL, DiNitto JP, et al. PI3K-δ and PI3K-γ inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol 2013;20:1364-74. [Crossref] [PubMed]
- Scott WJ, Hentemann MF, Rowley RB, et al. Discovery and SAR of Novel 2,3-Dihydroimidazo[1,2-c]quinazoline PI3K Inhibitors: Identification of Copanlisib (BAY 80-6946). ChemMedChem 2016;11:1517-30. [Crossref] [PubMed]
- Flinn IW, Miller CB, Ardeshna KM, et al. DYNAMO: A Phase II Study of Duvelisib (IPI-145) in Patients With Refractory Indolent Non-Hodgkin Lymphoma. J Clin Oncol 2019;37:912-22. [Crossref] [PubMed]
- Dreyling M, Morschhauser F, Bouabdallah K, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol 2017;28:2169-78. [Crossref] [PubMed]
- Dreyling M, Santoro A, Mollica L, et al. Long-term safety and efficacy of the PI3K inhibitor copanlisib in patients with relapsed or refractory indolent lymphoma: 2-year follow-up of the CHRONOS-1 study. Am J Hematol 2019; [Epub ahead of print]. [PubMed]
- Deng C, Lipstein MR, Scotto L, et al. Silencing c-Myc translation as a therapeutic strategy through targeting PI3Kδ and CK1ε in hematological malignancies. Blood 2017;129:88-99. [Crossref] [PubMed]
- Zinzani P, Samaniego F, Jurczak W, et al. Umbralisib monotherapy demonstrates efficacy and safety in patients with relapsed/refractory marginal zone lymphoma: a multicenter, open-label, registration directed phase 2 study. Hematol Oncol 2019;37:182-3. [Crossref]
- Tarantelli C, Lange M, Gaudio E, et al. Copanlisib synergizes with conventional and targeted agents including venetoclax in B- and T-cell lymphoma models. Blood Adv 2020;4:819-29. [Crossref] [PubMed]
- Arribas AJ, Napoli S, Gaudio E, et al. Secreted Factors Determine Resistance to Idelalisib in Marginal Zone Lymphoma Models of Resistance. Blood 2019;134:2569. [Crossref]
- Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2010;115:2578-85. [Crossref] [PubMed]
- Andorsky DJ, Kolibaba KS, Assouline S, et al. An open-label phase 2 trial of entospletinib in indolent non-Hodgkin lymphoma and mantle cell lymphoma. Br J Haematol 2019;184:215-22. [Crossref] [PubMed]
- Pine PR, Chang B, Schoettler N, et al. Inflammation and bone erosion are suppressed in models of rheumatoid arthritis following treatment with a novel Syk inhibitor. Clin Immunol 2007;124:244-57. [Crossref] [PubMed]
- Currie KS, Kropf JE, Lee T, et al. Discovery of GS-9973, a selective and orally efficacious inhibitor of spleen tyrosine kinase. J Med Chem 2014;57:3856-73. [Crossref] [PubMed]
- Hamlin PA, Cheson BD, Farber CM, et al. The dual SYK/JAK inhibitor cerdulatinib demonstrates rapid tumor responses in a phase 2 study in patients with relapsed/refractory B- and T-cell non-Hodgkin lymphoma (NHL). J Clin Oncol 2018;36:7511. [Crossref]
- Coffey G, Betz A, DeGuzman F, et al. The novel kinase inhibitor PRT062070 (Cerdulatinib) demonstrates efficacy in models of autoimmunity and B-cell cancer. J Pharmacol Exp Ther 2014;351:538-48. [Crossref] [PubMed]
- Krönke J, Fink EC, Hollenbach PW, et al. Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nature 2015;523:183-8. [Crossref] [PubMed]
- Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol 2014;164:811-21. [Crossref] [PubMed]
- Lu G, Middleton RE, Sun H, et al. The Myeloma Drug Lenalidomide Promotes the Cereblon-Dependent Destruction of Ikaros Proteins. Science 2014;343:305-9. [Crossref] [PubMed]
- Krönke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014;343:301-5. [Crossref] [PubMed]
- Kiesewetter B, Troch M, Dolak W, et al. A phase II study of lenalidomide in patients with extranodal marginal zone B-cell lymphoma of the mucosa associated lymphoid tissue (MALT lymphoma). Haematologica 2013;98:353-6. [Crossref] [PubMed]
- Becnel MR, Nastoupil LJ, Samaniego F, et al. Lenalidomide plus rituximab (R(2)) in previously untreated marginal zone lymphoma: subgroup analysis and long-term follow-up of an open-label phase 2 trial. Br J Haematol 2019;185:874-82. [Crossref] [PubMed]
- Leonard JP, Trneny M, Izutsu K, et al. AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J Clin Oncol 2019;37:1188-99. [Crossref] [PubMed]
- Nastoupil LJ, Lee HJ, Hagemeister FB, et al. Safety and Efficacy of Ibrutinib in Combination with Rituximab and Lenalidomide in Previously Untreated Subjects with Follicular and Marginal Zone Lymphoma: An Open Label, Phase II Study. Blood 2018;132:447. [Crossref]
- Fontan L, Yang C, Kabaleeswaran V, et al. MALT1 small molecule inhibitors specifically suppress ABC-DLBCL in vitro and in vivo. Cancer Cell 2012;22:812-24. [Crossref] [PubMed]
- Philippar U, Lu T, Vloemans N, et al. Discovery of a novel, potential first-in-class MALT1 protease inhibitor for the treatment of B cell lymphomas. Hematol Oncol 2019;37:128. [Crossref]
- Fontan L, Hatcher J, Scott D, et al. Chemically Induced Degradation of MALT1 to Treat B-Cell Lymphomas. Blood 2019;134:2073. [Crossref]
- Hatcher JM, Du G, Fontán L, et al. Peptide-based covalent inhibitors of MALT1 paracaspase. Bioorg Med Chem Lett 2019;29:1336-9. [Crossref] [PubMed]
- Quancard J, Klein T, Fung SY, et al. An allosteric MALT1 inhibitor is a molecular corrector rescuing function in an immunodeficient patient. Nat Chem Biol 2019;15:304-13. [Crossref] [PubMed]
- Lu T, Connolly PJ, Philippar U, et al. Discovery and optimization of a series of small-molecule allosteric inhibitors of MALT1 protease. Bioorg Med Chem Lett 2019;29:126743 [Crossref] [PubMed]
- Fontán L, Qiao Q, Hatcher JM, et al. Specific covalent inhibition of MALT1 paracaspase suppresses B cell lymphoma growth. J Clin Invest 2018;128:4397-412. [Crossref] [PubMed]
- India’s first-in-class MALT1 blocker deal. Nat Biotechnol 2019;37:112. [Crossref] [PubMed]
- Robles EF, Mena-Varas M, Barrio L, et al. Homeobox NKX2-3 promotes marginal-zone lymphomagenesis by activating B-cell receptor signalling and shaping lymphocyte dynamics. Nat Commun 2016;7:11889. [Crossref] [PubMed]
- Yang Y, Kelly P, Shaffer Arthur L III, et al. Targeting Non-proteolytic Protein Ubiquitination for the Treatment of Diffuse Large B Cell Lymphoma. Cancer Cell 2016;29:494-507. [Crossref] [PubMed]
- Morrish E, Brumatti G, Silke J. Future Therapeutic Directions for Smac-Mimetics. Cells 2020;9:406. [Crossref] [PubMed]
- Benetatos CA, Mitsuuchi Y, Burns JM, et al. Birinapant (TL32711), a bivalent SMAC mimetic, targets TRAF2-associated cIAPs, abrogates TNF-induced NF-κB activation, and is active in patient-derived xenograft models. Mol Cancer Ther 2014;13:867-79. [Crossref] [PubMed]
- Flygare JA, Beresini M, Budha N, et al. Discovery of a potent small-molecule antagonist of inhibitor of apoptosis (IAP) proteins and clinical candidate for the treatment of cancer (GDC-0152). J Med Chem 2012;55:4101-13. [Crossref] [PubMed]
- Cai Q, Sun H, Peng Y, et al. A potent and orally active antagonist (SM-406/AT-406) of multiple inhibitor of apoptosis proteins (IAPs) in clinical development for cancer treatment. J Med Chem 2011;54:2714-26. [Crossref] [PubMed]
- Runckel K, Barth MJ, Mavis C, et al. The SMAC mimetic LCL-161 displays antitumor activity in preclinical models of rituximab-resistant B-cell lymphoma. Blood Adv 2018;2:3516-25. [Crossref] [PubMed]
- Li Z, Li X, Su MB, et al. Discovery of a Potent and Selective NF-κB-Inducing Kinase (NIK) Inhibitor That Has Anti-inflammatory Effects in Vitro and in Vivo. J Med Chem 2020;63:4388-407. [Crossref] [PubMed]
- Brightbill HD, Suto E, Blaquiere N, et al. NF-κB inducing kinase is a therapeutic target for systemic lupus erythematosus. Nat Commun 2018;9:179. [Crossref] [PubMed]
- Odqvist L, Sanchez-Beato M, Montes-Moreno S, et al. NIK controls classical and alternative NF-kappaB activation and is necessary for the survival of human T-cell lymphoma cells. Clin Cancer Res 2013;19:2319-30. [Crossref] [PubMed]
- Li K, McGee LR, Fisher B, et al. Inhibiting NF-kappaB-inducing kinase (NIK): discovery, structure-based design, synthesis, structure-activity relationship, and co-crystal structures. Bioorg Med Chem Lett 2013;23:1238-44. [Crossref] [PubMed]
- Pippione AC, Sainas S, Federico A, et al. N-Acetyl-3-aminopyrazoles block the non-canonical NF-κB cascade by selectively inhibiting NIK. Medchemcomm 2018;9:963-8. [Crossref] [PubMed]
- Blaquiere N, Castanedo GM, Burch JD, et al. Scaffold-Hopping Approach To Discover Potent, Selective, and Efficacious Inhibitors of NF-kappaB Inducing Kinase. J Med Chem 2018;61:6801-13. [Crossref] [PubMed]
- Castanedo GM, Blaquiere N, Beresini M, et al. Structure-Based Design of Tricyclic NF-kappaB Inducing Kinase (NIK) Inhibitors That Have High Selectivity over Phosphoinositide-3-kinase (PI3K). J Med Chem 2017;60:627-40. [Crossref] [PubMed]
- Wang B, Parobchak N, Martin A, et al. Screening a small molecule library to identify inhibitors of NF-κB inducing kinase and pro-labor genes in human placenta. Sci Rep 2018;8:1657. [Crossref] [PubMed]
- Demchenko YN, Brents LA, Li Z, et al. Novel inhibitors are cytotoxic for myeloma cells with NFκB inducing kinase-dependent activation of NFκB. Oncotarget 2014;5:4554-66. [Crossref] [PubMed]
- Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 2010;465:885-90. [Crossref] [PubMed]
- Motshwene PG, Moncrieffe MC, Grossmann JG, et al. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J Biol Chem 2009;284:25404-11. [Crossref] [PubMed]
- de Groen RAL, Schrader AMR, Kersten MJ, et al. MYD88 in the driver's seat of B-cell lymphomagenesis: from molecular mechanisms to clinical implications. Haematologica 2019;104:2337-48. [Crossref] [PubMed]
- Cao Z, Xiong J, Takeuchi M, et al. TRAF6 is a signal transducer for interleukin-1. Nature 1996;383:443-6. [Crossref] [PubMed]
- Martin MU, Wesche H. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim Biophys Acta 2002;1592:265-80. [Crossref] [PubMed]
- Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011;470:115-9. [Crossref] [PubMed]
- Parry M, Rose-Zerilli MJ, Ljungstrom V, et al. Genetics and Prognostication in Splenic Marginal Zone Lymphoma: Revelations from Deep Sequencing. Clin Cancer Res 2015;21:4174-83. [Crossref] [PubMed]
- Trøen G, Warsame A, Delabie J. CD79B and MYD88 Mutations in Splenic Marginal Zone Lymphoma. ISRN Oncol 2013;2013:252318 [Crossref] [PubMed]
- Li ZM, Rinaldi A, Cavalli A, et al. MYD88 somatic mutations in MALT lymphomas. Br J Haematol 2012;158:662-4. [Crossref] [PubMed]
- Shiratori E, Itoh M, Ohtaka M, et al. Mechanisms of Suppressive Effects of MYD88 Inhibitors on the Growth of Lymphoma and Leukemia Cells. Blood 2016;128:2773. [Crossref]
- Shiratori E, Itoh M, Tohda S. MYD88 Inhibitor ST2825 Suppresses the Growth of Lymphoma and Leukaemia Cells. Anticancer Res 2017;37:6203-9. [PubMed]
- Olson MA, Lee MS, Kissner TL, et al. Discovery of small molecule inhibitors of MyD88-dependent signaling pathways using a computational screen. Sci Rep 2015;5:14246. [Crossref] [PubMed]
- Booher RN, Nowakowski GS, Patel K, et al. Preclinical Activity of IRAK4 Kinase Inhibitor CA-4948 Alone or in Combination with Targeted Therapies and Preliminary Phase 1 Clinical Results in Non-Hodgkin Lymphoma. Blood 2018;132:4168. [Crossref]
- Kelleher J, Audoly L, Campbell V, et al. Targeted Degradation of IRAK4 Protein Via Heterobifunctional Small Molecules for Treatment of MYD88 Mutant Lymphoma. Blood 2018;132:2953. [Crossref]
- Moorthy S. NOTCH Pathway. In: Karp DD, Falchook GS, editors. Handbook of Targeted Cancer Therapy and Immunotherapy. 2nd ed. Philadelphia: Wolters Kluwer, 2019:244-5.
- Tamagnone L, Zacchigna S, Rehman M. Taming the Notch Transcriptional Regulator for Cancer Therapy. Molecules 2018;23:431. [Crossref] [PubMed]
- Sorrentino C, Cuneo A, Roti G. Therapeutic Targeting of Notch Signaling Pathway in Hematological Malignancies. Mediterr J Hematol Infect Dis 2019;11:e2019037 [Crossref] [PubMed]
- Chiang MY, Radojcic V, Maillard I. Oncogenic Notch signaling in T-cell and B-cell lymphoproliferative disorders. Curr Opin Hematol 2016;23:362-70. [Crossref] [PubMed]
- Descatoire M, Weller S, Irtan S, et al. Identification of a human splenic marginal zone B cell precursor with NOTCH2-dependent differentiation properties. J Exp Med 2014;211:987-1000. [Crossref] [PubMed]
- Moran ST, Cariappa A, Liu H, et al. Synergism between NF-kappa B1/p50 and Notch2 during the development of marginal zone B lymphocytes. J Immunol 2007;179:195-200. [Crossref] [PubMed]
- Witt CM, Won WJ, Hurez V, et al. Notch2 haploinsufficiency results in diminished B1 B cells and a severe reduction in marginal zone B cells. J Immunol 2003;171:2783-8. [Crossref] [PubMed]
- Arribas A, Gaudio E, Arcaini L, et al. Targeting the Epigenome and the Cell Signaling As Novel Therapeutic Approaches for Splenic Marginal Zone Lymphoma. Blood 2016;128:4186. [Crossref]
- Spriano F, Tarantelli C, Arribas A, et al. Abstract B061: Targeting lymphomas with the novel first-in-class pan-NOTCH transcription inhibitor CB-103. Mol Cancer Ther 2018;17:B061.
- Feng Y, Li X, Cassady K, et al. TET2 Function in Hematopoietic Malignancies, Immune Regulation, and DNA Repair. Front Oncol 2019;9:210. [Crossref] [PubMed]
- Lio CJ, Yuita H, Rao A. Dysregulation of the TET family of epigenetic regulators in lymphoid and myeloid malignancies. Blood 2019;134:1487-97. [Crossref] [PubMed]
- Dominguez PM, Ghamlouch H, Rosikiewicz W, et al. TET2 Deficiency Causes Germinal Center Hyperplasia, Impairs Plasma Cell Differentiation, and Promotes B-cell Lymphomagenesis. Cancer Discov 2018;8:1632-53. [PubMed]
- Mondello P, Tadros S, Teater M, et al. Selective Inhibition of HDAC3 Targets Synthetic Vulnerabilities and Activates Immune Surveillance in Lymphoma. Cancer Discov 2020;10:440-59. [Crossref] [PubMed]
Cite this article as: Lue JK, O’Connor OA, Bertoni F. Targeting pathogenic mechanisms in marginal zone lymphoma: from concepts and beyond. Ann Lymphoma 2020;4:7.


