Recent advances in the first-line treatment of mantle cell lymphoma
Introduction
Mantle cell lymphoma (MCL) is a B-cell non-Hodgkin lymphoma with a t(11;14) translocation and cyclin D1 overexpression, that comprises 5.5% of mature B-cell neoplasms (1,2). MCL is a lymphoma of poor prognosis, with median survival of only three to four years and a continuous pattern of relapses (3-5). Several studies showed that new therapeutic approaches could improve the outcomes. In addition, MCL patients have heterogeneous clinical evolutions, with some patients not requiring treatment for months to years (6) while a small proportion of them are resistant to standard therapies and have very poor outcomes (7,8).
Prognostic factors and mantle cell international prognostic index (MIPI)
Because the therapeutic responses to treatment are heterogeneous, guidance on the therapeutic decisions would need definition of the prognostic factors. Evolving classification systems had been made to predict the outcomes of MCL. Similar to blastoid histology often associated with high number of Ki-67 positive cells, CDKN2A deletion and TP53 mutations had been identified as poor prognostic factors (9-12). The MIPI, based on four independent factors (e.g., age, performance status, leukocyte count and level of lactate dehydrogenase), had been specifically developed for MCL (13,14). This index separates high-risk MCL, which comprised 15–20% of patients who had response for a duration of less than one year after the end of treatment; the intermediate-risk group, which included patients who had an annual incidence of relapse of 10–15%; and the low-risk group, which comprised nearly 30% of patients who were in complete response (CR) for more than five years (7,15). The combined biologic index, or MIPI-c, incorporated the proliferation index Ki67 has been validated in both younger and elderly patients (16). More recently, in the prospective trials of the European MCL (EMCL) Network, TP53 expression (i.e., >50% positive cells) had been shown to confer relatively short time to treatment failure (TTF) and poor overall survival (OS) among the treated patients, independent of both MIPI score and Ki67 index (17). Moreover, the presence of TP53 mutations identified a unique subset (11%) of chemo-refractory MCL patients (median OS, 1.8 in TP53 mutated cases vs. 12.7 years in wild-type cases) (18); 50% of patients in the TP53 mutated group relapsed within one year of diagnosis. It is now accepted that MCL patients should be stratified, according to their prognostic characteristics, which may guide some newer therapeutic strategies.
Indolent mantle cell lymphoma
In selected indolent MCL cases, a watch and wait strategy should be preferred, as shown by the higher rate of survival with observation than with early treatment (6). A non-nodal or localized disease with hyperlymphocytosis and splenomegaly usually characterizes indolent MCL patients. Leukemic non-nodal MCLs showed a classical histology with a low proliferation index, had high levels of somatic mutations in the IGHV locus, a normal karyotype (19) and lacked SOX11 expression (20). However, the prognostic impact of SOX11 expression had been controversial, because some SOX11-negative MCLs can also have genomic alterations, such as TP53 mutations, which leads to poor clinical outcome (21). Moreover, in a recent study of the EMCL Network, SOX11 status, which was assessed by immunohistochemistry, did not show a strong association with OS (17). Therefore, in patients with indolent MCL, initial treatment can be deferred until the development of symptoms or disease progression. Once decided, the therapeutic strategy will depend on the age and general health of the patient.
Therapeutic goal and response assessment
In the early studies that assessed the role of ASCT, the use of the anti-CD20 monoclonal antibody rituximab combined with chemotherapy before ASCT was associated with a higher rate of overall response rate (ORR) and CR, which led to longer progression-free survival (PFS) (22). It is therefore commonly accepted that achievement of the best response before ASCT should be an important goal in the therapeutic strategy. The monitoring of minimal residual disease (MRD) is a valuable biomarker of quality of response (23,24). Indeed, the two randomized younger and elderly trials of the EMCL Network showed that molecular remission at the end of induction (before ASCT) was a strong independent prognostic factor (response duration, 87% vs. 61% patients in remission at two years, P=0.004) (24). Therefore, increasing the proportion of patients who achieve negative MRD should become the therapeutic objective of the induction therapy. The role of PET scan has not been defined, although it may have a prognostic value, both upon diagnosis and by assessing the response (15). The final analysis of the LyMa trial, which tested the efficacy of rituximab maintenance in MCL (see below), will provide answers to these questions.
Induction regimen in younger and fit patients
Although the addition of rituximab to chemotherapy improved the OS, the CR rate remained below 50% and the TTF was shorter, compared with the outcomes at two years after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) (25). The most active induction regimens included high-dose cytarabine (Ara-C). A regimen that combined rituximab-hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone, alternating with high-dose methotrexate plus cytarabine, was shown to be active and safe in a single-center experience, but was highly toxic with a non-negligible rate of stem cell collection failure in multicenter studies (26-29). In younger patients, the randomized study of the EMCL Network for Younger patients showed that Ara-C-containing induction regimen was superior to R-CHOP alone (7), confirming the previous results obtained in several phase II studies (30-33). Compared with R-CHOP alone, Ara-C treatment significantly increased the CR (39% to 55%, P=0.0005) and molecular response rates (47% to 79% in the peripheral blood), which led to a better TTF at five years (65% vs. 40%, P=0.038). The Nordic MCL2 trial, which have included six alternating courses of R-CHOP and R-Ara-C followed by an ASCT, showed impressive results of 96% ORR and 54% CR rates after induction, which led to a median OS of 12.7 years and PFS of 8.5 years (25). The LyMa study, based on four courses of rituximab, dexamethasone, cytarabine and cisplatin (R-DHAP), reported ORR and CR rates of 89% and 77% respectively (26). Of the 299 enrolled patients, 20 who were having a partial response (PR) received rescue induction therapy with four courses of R-CHOP, which improved the response in nine cases (26). Therefore, the ASCT after an induction combining rituximab and Ara-C chemotherapy has become a validated therapeutic approach in younger patients.
Autologous stem cell transplantation
In younger and fit patients, the benefit of ASCT was confirmed by a randomized study, which demonstrated better PFS with ASCT than with interferon-α (IFN) maintenance therapy (27), as suggested by several non-randomized studies on patients who received first-line treatment and those in relapse (28-30). In the only randomized study available, ASCT performed as first-line therapy, compared with IFN therapy, significantly improved the PFS but had similar three-year OS (83% vs. 77%, P=0.18) (27), a fact that can be explained by the use of ASCT as a salvage therapy in the IFN arm. Moreover, a recent large retrospective study demonstrated improved PFS (hazard ratio 0.54; 95% CI, 0.44 to 0.66; P<0.01) and a trend toward improved OS in patients who underwent consolidative ASCT (31). Certain subgroups of patients, such as those with high-risk MIPI scores, a blastoid or pleomorphic morphology or who were treated with CHOP-like induction w/o Ara-C, achieved the largest improvement in OS. Currently, the superior conditioning regimen remains unclear. The commonly used conditioning regimens included high-dose cyclophosphamide and total body irradiation (TBI) or high-dose carmustine, etoposide, cytarabine, and melphalan (BEAM). TBI only improved the PFS only in the group of patients in PR before ASCT, as shown by a retrospective comparison of the EMCL (with TBI) and the MCL Nordic group (no TBI) trials, which used similar induction with Ara-C (32). Because it is becoming clear that most of the new induction regimens intend to reach CR, TBI is not currently being used and BEAM regimen is now the new standard in Europe (15). Because the status of MRD can predict the outcome, it could be postulated that patients with negative MRD after induction may not benefit as well as that after ASCT. Therefore, the role of ASCT is currently being investigated in patients with MRD-negative MCL in the first CR (ClinicalTrials.gov identifier: NCT03267433).
Maintenance therapy
The constant risk of MCL relapses led to propose a maintenance therapy in several trials. Responding patients included in the EMCL elderly trial underwent a second randomization to receive IFN or rituximab maintenance for two years. Compared to IFN, rituximab improved the duration of response (hazard ratio 0.55; 95% CI, 0.36 to 0.87), particularly in the subgroup of patients who received R-CHOP as induction (four-year OS of 87% in the maintenance group vs. 63%, P=0.005), a beneficial effect that has not been found with RFC (R-fludarabine and cyclophosphamide, P=0.48) (33). The phase III LyMA study confirmed the interest of rituximab maintenance compared to observation in younger MCL patients who received four courses of R-DHAP followed by ASCT as first line therapy. As a result, rituximab maintenance after ASCT is associated with higher rates of both 4-year PFS (82.2% vs. 64.6%, P=0.0005) and four-year OS (88.7% vs. 81.4%, P=0.0413) (26). In patients treated with the Nordic MCL2 protocol, rituximab maintenance was shown to significantly improve PFS, but not OS (34). However, in patients with previously untreated MCL, maintenance rituximab did not have benefits after 6 courses of rituximab and bendamustine (35). Therefore, rituximab maintenance may be a reasonable standard of care in responding younger and elderly MCL patients, at least after induction treatment including R-CHOP and/or R-DHAP. These differences among the studies are not clear but might have been due to the immunosuppression induced by purine analogs, which can reduce the antibody-dependent cellular cytotoxicity effect of rituximab. Moreover, MRD-based preemptive rituximab treatment had been shown to convert patients to MRD negativity and can probably postpone MCL relapses (36,37). Therefore, MRD monitoring may guide therapeutic interventions during the follow-up of MCL patients. These results led to a phase II trial (LyMA101) randomizing assignment for observation or MRD-based preemptive treatment after induction, ASCT and obinutuzumab maintenance (ClinicalTrials.gov identifier: NCT02896582).
Allogeneic stem cell transplantation
Around 5% to 10% of MCL were primary refractory to chemotherapy with an extremely poor prognosis and there is no consensus about salvage therapy (38). Moreover, progression within two years of diagnosis had been shown to lead to poor outcomes, with median OS of 2–12 months (versus not reached), regardless of the prognostic information obtained upon diagnosis or the induction regimen administered (39). Allogeneic hematopoietic stem cell transplantation (alloSCT), which may benefit chemosensitive MCL patients, is a salvage option in some patients. In an Italian retrospective study, alloSCT had a favorable significant impact on survival in relapsed MCL patients (39). Moreover, frontline alloSCT appeared to be feasible, with a PFS at two years of 68%, although regimen-related mortality and morbidity remained significant (40). Because alloSCT can lead to high toxicity in the first two years after administration, it may only benefit high-risk fit patients (41). These very high-risk patients may be identified, based on the presence of blastoid variants, high Ki-67expression, CDKN2A/TP53 deletions, TP53 mutations, KMT2D mutations (42) and suboptimal response after induction. However, the prognostic stratification of untreated MCL patients has to be adapted to guide an individualized targeted therapeutic strategy. Future studies should aim to develop early and risk-adapted strategies that may include frontline alloSCT, based on already identified and new biomarkers (43). However, the ability of alloSCT to overcome these putative poor prognostic parameters remains to be determined.
Unfit MCL patients
In patients ineligible for transplantation, the benefits of chemotherapy should be weighed against its potential toxicity. Treatment with R-CHOP, R-bendamustine or targeted therapy alone can be discussed. Purine analogs had been used for the treatment of elderly MCL patients. However, a large randomized trial on the older MCL patient population showed a better outcome with R-CHOP compared to R-FC, with a four-year OS of 62% for R-CHOP and 47% for R-FC (P=0.005) (33). A recent update confirmed the better OS in the R-CHOP arm (median, 6.4 vs. 3.9 years after RFC, P=0.0054) (44).Several trials have assessed the efficacy and safety of R-bendamustine as first-line treatment. Compared with R-CHOP, R-bendamustine had a significant longer median PFS (22 vs. 35 months, P=0.004) and fewer toxic effects, in a phase 3 non-inferiority trial (45). The BRIGHT study showed that the CR rate for first-line R-bendamustine was statistically non-inferior to that for R-CHOP or R-CVP in MCL (46). The combination of R-bendamustine and lenalidomide, followed by lenalidomide maintenance, was shown to have a poor safety profile (47). The phase 3 MCL-R2 elderly trial is currently evaluating the role of cytarabine-containing induction using R-CHOP alone or alternating with the combination of rituximab, cytarabine, dexamethasone (R-HAD), followed by lenalidomide-containing maintenance in older patients (ClinicalTrials.gov identifier: NCT01865110). For frail patients who are not candidates for intensive chemotherapy, a combination of rituximab with targeted therapy should be considered. This point is discussed below in the targeted therapies section.
Targeted therapies
Some new therapeutic agents have recently emerged and are now incorporated in clinical trials. In the R-CHOP combination, vincristine has been replaced by bortezomib (i.e., VR-CAP), a regimen that significantly improved median OS in newly diagnosed MCL patients (91 versus 56 months, P=0.001) with a manageable safety profile (48). A phase II study combining lenalidomide and rituximab as first-line in unfit MCL patients was promising with a CR rate of 61% (49). The Triangle study (EMCL network) randomized the Bruton’s Tyrosine Kinase (BTK) inhibitor ibrutinib with a combination of chemotherapy (R-CHOP alone or alternating with R-DHAP) as induction, and will discuss the role of ibrutinib maintenance for younger patients (ClinicalTrials.gov identifier: NCT02858258). The use of ibrutinib and venetoclax for dual targeting of BTK and BCL2 showed promising results in relapsing patients with poor risk markers, such as a high-risk MIPI score or TP53 aberrations (50). A chemotherapy-free treatment with rituximab and ibrutinib until best response, followed by a minimum of four intensive chemotherapy courses, is currently being tested, but the preliminary ORR after induction had been excellent (ClinicalTrials.gov identifier: NCT02427620) (51). The results of the industry-sponsored phase III Shine study comparing ibrutinib and placebo in combination with bendamustine and rituximab, in elderly untreated MCL patients, are still expected. Moreover, selective inhibitors of cyclin-dependent kinases 4/6 (CDK) may allow good responses in relapsed/refractory MCL, despite the relatively modest single-agent activity (52). Therefore, these compounds could be of interest as first-line therapy, alone or in combination (53-55). These new strategies may improve the outcomes in high-risk MCL or unfit/frail patients.
Conclusions
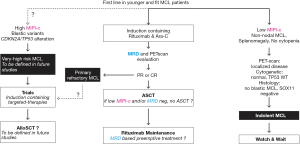
In younger MCL patients, the role of rituximab combined with Ara-C as induction, followed by ASCT has been demonstrated. In both young and elderly patients, the rituximab maintenance, at least after Ara-C containing induction, is also a promising approach. Future directions should integrate a risk-adapted therapeutic approach that includes new agents alone or in combination with chemotherapy, which could overcome resistance in high-risk MCL. Obtaining a negative MRD and/or negative PET scan at the end of induction is a major goal of the treatment (Figure 1). A better understanding of the lymphoma pathogenesis is necessary to identify biomarkers, which can be specifically targeted with less toxic agents. Finally, analysis of the benefits and risks, as well as the economic burden, of such strategies will be required before proposing new standard of care.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Lymphoma for the series “Future Directions for Mantle Cell Lymphoma”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol.2019.11.04). The series “Future Directions for Mantle Cell Lymphoma” was commissioned by the editorial office without any funding or sponsorship. MD served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Laurent C, Baron M, Amara N, et al. Impact of expert pathologic review of lymphoma diagnosis: Study of patients from the French Lymphopath Network. J Clin Oncol 2017;35:2008-17. [Crossref] [PubMed]
- Andersen NS, Jensen MK, de Nully Brown P, et al. A Danish population-based analysis of 105 mantle cell lymphoma patients: incidences, clinical features, response, survival and prognostic factors. Eur J Cancer 2002;38:401-8. [Crossref] [PubMed]
- Teodorovic I, Pittaluga S, Kluin-Nelemans JC, et al. Efficacy of four different regimens in 64 mantle-cell lymphoma cases: clinicopathologic comparison with 498 other non-Hodgkin’s lymphoma subtypes. European Organization for the Research and Treatment of Cancer Lymphoma Cooperative Group. J Clin Oncol 1995;13:2819-26. [Crossref] [PubMed]
- Ganti AK, Bierman PJ, Lynch JC, et al. Hematopoietic stem cell transplantation in mantle cell lymphoma. Ann Oncol 2005;16:618-24. [Crossref] [PubMed]
- Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol 2009;27:1209-13. [Crossref] [PubMed]
- Hermine O, Hoster E, Walewski J, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet 2016;388:565-75. [Crossref] [PubMed]
- Dietrich S, Boumendil A, Finel H, et al. Outcome and prognostic factors in patients with mantle-cell lymphoma relapsing after autologous stem-cell transplantation: a retrospective study of the European Group for Blood and Marrow Transplantation (EBMT). Ann Oncol 2014;25:1053-8. [Crossref] [PubMed]
- Hernandez L, Fest T, Cazorla M, et al. p53 gene mutations and protein overexpression are associated with aggressive variants of mantle cell lymphomas. Blood 1996;87:3351-9. [Crossref] [PubMed]
- Schaffel R, Hedvat CV, Teruya-Feldstein J, et al. Prognostic impact of proliferative index determined by quantitative image analysis and the International Prognostic Index in patients with mantle cell lymphoma. Ann Oncol 2010;21:133-9. [Crossref] [PubMed]
- Determann O, Hoster E, Ott G, et al. Ki-67 predicts outcome in advanced-stage mantle cell lymphoma patients treated with anti-CD20 immunochemotherapy: results from randomized trials of the European MCL Network and the German Low Grade Lymphoma Study Group. Blood 2008;111:2385-7. [Crossref] [PubMed]
- Delfau-Larue M-H, Klapper W, Berger F, et al. High-dose cytarabine does not overcome the adverse prognostic value of CDKN2A and TP53 deletions in mantle cell lymphoma. Blood 2015;126:604-11. [Crossref] [PubMed]
- Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 2008;111:558-65. [Crossref] [PubMed]
- Hoster E, Klapper W, Hermine O, et al. Confirmation of the mantle-cell lymphoma International Prognostic Index in randomized trials of the European Mantle-Cell Lymphoma Network. J Clin Oncol 2014;32:1338-46. [Crossref] [PubMed]
- Le Gouill S, Kröger N, Dhedin N, et al. Reduced-intensity conditioning allogeneic stem cell transplantation for relapsed/refractory mantle cell lymphoma: a multicenter experience. Ann Oncol 2012;23:2695-703. [Crossref] [PubMed]
- Hoster E, Rosenwald A, Berger F, et al. Prognostic Value of Ki-67 Index, Cytology, and Growth Pattern in Mantle-Cell Lymphoma: Results From Randomized Trials of the European Mantle Cell Lymphoma Network. J Clin Oncol 2016;34:1386-94. [Crossref] [PubMed]
- Aukema SM, Hoster E, Rosenwald A, et al. Expression of TP53 is associated with the outcome of MCL independent of MIPI and Ki-67 in trials of the European MCL Network. Blood 2018;131:417-20. [Crossref] [PubMed]
- Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood 2017;130:1903-10. [Crossref] [PubMed]
- Sarkozy C, Terré C, Jardin F, et al. Complex karyotype in mantle cell lymphoma is a strong prognostic factor for the time to treatment and overall survival, independent of the MCL international prognostic index. Genes Chromosomes Cancer 2014;53:106-16. [Crossref] [PubMed]
- Fernàndez V, Salamero O, Espinet B, et al. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer Res 2010;70:1408-18. [Crossref] [PubMed]
- Nordström L, Sernbo S, Eden P, et al. SOX11 and TP53 add prognostic information to MIPI in a homogenously treated cohort of mantle cell lymphoma--a Nordic Lymphoma Group study. Br J Haematol 2014;166:98-108. [Crossref] [PubMed]
- Schulz H, Bohlius JF, Trelle S, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. J Natl Cancer Inst 2007;99:706-14. [Crossref] [PubMed]
- Pott C, Schrader C, Gesk S, et al. Quantitative assessment of molecular remission after high-dose therapy with autologous stem cell transplantation predicts long-term remission in mantle cell lymphoma. Blood 2006;107:2271-8. [Crossref] [PubMed]
- Pott C, Hoster E, Delfau-Larue M-H, et al. Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: a European MCL intergroup study. Blood 2010;115:3215-23. [Crossref] [PubMed]
- Eskelund CW, Kolstad A, Jerkeman M, et al. 15-year follow-up of the Second Nordic Mantle Cell Lymphoma trial (MCL2): prolonged remissions without survival plateau. Br J Haematol 2016;175:410-8. [Crossref] [PubMed]
- Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after Autologous Stem-Cell Transplantation in Mantle-Cell Lymphoma. N Engl J Med 2017;377:1250-60. [Crossref] [PubMed]
- Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood 2005;105:2677-84. [Crossref] [PubMed]
- Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood 2008;112:2687-93. [Crossref] [PubMed]
- Gianni AM, Magni M, Martelli M, et al. Long-term remission in mantle cell lymphoma following high-dose sequential chemotherapy and in vivo rituximab-purged stem cell autografting (R-HDS regimen). Blood 2003;102:749-55. [Crossref] [PubMed]
- Touzeau C, Leux C, Bouabdallah R, et al. Autologous stem cell transplantation in mantle cell lymphoma: a report from the SFGM-TC. Ann Hematol 2014;93:233-42. [Crossref]
- Gerson JN, Handorf E, Villa D, et al. Survival Outcomes of Younger Patients With Mantle Cell Lymphoma Treated in the Rituximab Era. J Clin Oncol 2019;37:471-80. [Crossref] [PubMed]
- Rubio-Moscardo F, Climent J, Siebert R, et al. Mantle-cell lymphoma genotypes identified with CGH to BAC microarrays define a leukemic subgroup of disease and predict patient outcome. Blood 2005;105:4445-54. [Crossref] [PubMed]
- Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of Older Patients with Mantle-Cell Lymphoma. N Engl J Med 2012;367:520-31. [Crossref] [PubMed]
- Klener P, Salek D, Pytlik R, et al. Rituximab maintenance significantly prolongs progression-free survival of patients with newly diagnosed mantle cell lymphoma treated with the Nordic MCL2 protocol and autologous stem cell transplantation. Am J Hematol 2019;94:E50-3. [PubMed]
- Rummel MJ, Knauf W, Goerner M, et al. Two years rituximab maintenance vs. observation after first-line treatment with bendamustine plus rituximab (B-R) in patients with mantle cell lymphoma: First results of a prospective, randomized, multicenter phase II study (a subgroup study of the StiL NHL7-2008 MAINTAIN trial). J Clin Oncol 2016;34:7503. [Crossref]
- Andersen NS, Pedersen LB, Laurell A, et al. Pre-emptive treatment with rituximab of molecular relapse after autologous stem cell transplantation in mantle cell lymphoma. J Clin Oncol 2009;27:4365-70. [Crossref] [PubMed]
- Cheminant M, Derrieux C, Touzart A, et al. Minimal residual disease monitoring by 8-color flow cytometry in mantle cell lymphoma: an EU-MCL and LYSA study. Haematologica 2016;101:336-45. [Crossref] [PubMed]
- Robinson SP, Goldstone AH, Mackinnon S, et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood 2002;100:4310-6. [Crossref] [PubMed]
- Visco C, Tisi MC, Evangelista A, et al. Time to progression of mantle cell lymphoma after high-dose cytarabine-based regimens defines patients risk for death. Br J Haematol 2019;185:940-4. [Crossref] [PubMed]
- Rule S, Cook G, Russell NH, et al. Allogeneic stem cell transplantation as part of front line therapy for Mantle cell lymphoma. Br J Haematol 2019;184:999-1005. [Crossref] [PubMed]
- Fenske TS, Zhang MJ, Carreras J, et al. Autologous or reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chemotherapy-sensitive mantle-cell lymphoma: analysis of transplantation timing and modality. J Clin Oncol 2014;32:273-81. [Crossref] [PubMed]
- Ferrero S, Rossi D, Rinaldi A, et al. KMT2D mutations and TP53 disruptions are poor prognostic biomarkers in mantle cell lymphoma receiving high-dose therapy: a FIL study. Haematologica 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Robinson S, Dreger P, Caballero D, et al. The EBMT/EMCL consensus project on the role of autologous and allogeneic stem cell transplantation in mantle cell lymphoma. Leukemia 2015;29:464-73. [Crossref] [PubMed]
- Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle cell lymphoma (MCL): Long-term follow-up of the randomized European MCL elderly trial. J Clin Oncol 2020;38:248-56. [Crossref] [PubMed]
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203-10. [Crossref] [PubMed]
- Flinn IW, Jagt R, van der , Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 2014;123:2944-52. [Crossref] [PubMed]
- Albertsson-Lindblad A, Kolstad A, Laurell A, et al. Lenalidomide-bendamustine-rituximab in patients older than 65 years with untreated mantle cell lymphoma. Blood 2016;128:1814-20. [Crossref] [PubMed]
- Robak T, Jin J, Pylypenko H, et al. Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: final overall survival results of a randomised, open-label, phase 3 study. Lancet Oncol 2018;19:1449-58. [Crossref] [PubMed]
- Ruan J, Martin P, Shah B, et al. Lenalidomide plus Rituximab as Initial Treatment for Mantle-Cell Lymphoma. N Engl J Med 2015;373:1835-44. [Crossref] [PubMed]
- Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus Venetoclax for the Treatment of Mantle-Cell Lymphoma. N Engl J Med 2018;378:1211-23. [Crossref] [PubMed]
- Wang X, Werneck MBF, Wilson BG, et al. TCR-dependent transformation of mature memory phenotype T cells in mice. J Clin Invest 2011;121:3834-45. [Crossref] [PubMed]
- Martin P, Bartlett NL, Blum KA, et al. A phase I trial of ibrutinib plus palbociclib in previously treated mantle cell lymphoma. Blood 2019;133:1201-4. [Crossref] [PubMed]
- Leonard JP, LaCasce AS, Smith MR, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood 2012;119:4597-607. [Crossref] [PubMed]
- Kouroukis CT, Belch A, Crump M, et al. Flavopiridol in untreated or relapsed mantle-cell lymphoma: results of a phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2003;21:1740-5. [Crossref] [PubMed]
- Seftel MD, Kuruvilla J, Kouroukis T, et al. The CDK inhibitor AT7519M in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) and mantle cell lymphoma. A Phase II study of the Canadian Cancer Trials Group. Leuk Lymphoma 2017;58:1358-65. [Crossref] [PubMed]
Cite this article as: Cheminant M, Dreyling M, Hermine O. Recent advances in the first-line treatment of mantle cell lymphoma. Ann Lymphoma 2020;4:2.