
New drugs for the management of relapsed or refractory diffuse large B-cell lymphoma
Introduction
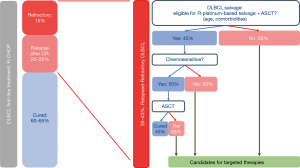
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma (NHL). Approximately 60–65% of patients can be cured with standard front-line therapy, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone). Patients who remain event-free within the first 2 years from diagnosis, have an overall survival (OS) in the range of an age and sex matched general population (1-3). The remaining 35–40% of patients will exhibit primary refractory disease or relapse following an initial response to therapy and will have a very poor outcome. Intensive salvage strategies including autologous stem cell transplantation (ASCT) provide the best chance for cure in the second-line setting. However, less than half of the patients with relapsed/refractory (rel/refr) DLBCL will be transplant-eligible based on age and co-morbidities. Of these patients, less than half will be chemotherapy-sensitive and proceed to transplant, and less than half who proceed to transplant will achieve long-term disease-free survival. All in all, 75–80% of the rel/refr population represents an unmet medical need (Figure 1). While chimeric antigen receptor T-cell (CAR-T) therapy has shown promise in this setting, many patients will be unsuitable or relapse after CAR-T therapy and require alternative options (4,5).
In the past 15 years, impressive progress on the “bench-side”, has led to improved characterization of the biologic heterogeneity of DLBCL leading to a refined classification (6). On the “bed-side”, these biological advances have led to the development of novel targeted drugs that are now being evaluated in clinical trials. In this article, we will briefly summarize the standard management of rel/refr DLBCL and explore the promise of novel therapies that are most advanced in development.
Relapsed and refractory DLBCL: standard approaches
The majority of relapses occur within 2 to 3 years from completion of initial treatment, although a low incidence of late relapse exists (7). Patients with R-CHOP-refractory disease (1,2,8) have a particularly poor prognosis with a median OS of less than a year (9-13).
Chemotherapy-based salvage regimens
Intensive strategies including ASCT (14) offer the best chance for cure for patients with rel/refr DLBCL and is the standard in the rituximab era (15). The most frequently used salvage regimens are platinum-based chemotherapy combinations, including DHAP (dexamethasone, cytarabine and cisplatin), ICE (ifosfamide, carboplatin and etoposide), and GDP (gemcitabine, dexamethasone and cisplatin) (16,17). Approximately 40–50% of these patients will exhibit chemosensitive disease and proceed to transplant (16) with a PFS at 3 years that can reach 50–60% (18-21). Patients failing to achieve a response to second-line salvage have a median OS of less than 6 months (22). For elderly and/or transplant ineligible patients, alternative chemotherapy-based regimens such as R-bendamustine, R-GEMOX (gemcitabine and oxaliplatin), R-GEM-P (gemcitabine, cisplatin and methylprednisone) or R-DHAOX (Dexamethasone, high-dose cytarabine, and oxaliplatin) can achieve an ORR ranging from 50% to 75%, with a median OS of 1 or 2 years in the majority of the studies (23-25). While CAR-T therapy may be considered for some patients who have failed 2 prior lines of therapy, cellular therapy will not be discussed within this article.
In conclusion, with a rituximab-platinum salvage regimen followed by ASCT, 20–30% of transplant-eligible patients may achieve prolonged survival. However, chemo-insensitive, as well as transplant-ineligible patients have a very poor outcome and represent a significant unmet medical need that must be addressed through alternative strategies. Recent biological insights have translated into the development on novel targeted agents that offer promise for this challenging subset of patients.
Biological heterogeneity of DLBCL
Molecular analyses have revealed DLBCL to be a complex and heterogeneous disease (26-30) which can be classified based on gene expression profiling (GEP) as germinal center B-cell (GCB) or activated B-cell (ABC), reflecting a different cell-of-origin (COO) and oncogenic pathway activation (31-33). In addition, patients with a dual rearrangement of MYC and BCL2 and/or BCL6, “double-hit” lymphoma, have been reclassified within the World Health Organization (WHO) Classification into a high-grade category with poor prognosis (6,34,35). Interestingly, a recent Nanostring-based classification has been proposed, identifying within GCB-DLBCL a subgroup of patients with a double-hit signature and a poorer outcome (36).
ABC and GCB-DLBCL subtypes are driven by different oncogenic mechanisms, and therefore may require selective therapeutic approaches. GCB-DLBCL may be preferentially sensitive to strategies targeting apoptosis, PI3K/AKT/mTOR pathway or EZH2, whereas strategies targeting the BCR, NF-κB or JAK/STAT pathways may be preferred in ABC-DLBCL. However, relying on COO classification alone may be insufficient to identify optimal treatment, as response to targeted agents may depend on the tumor’s mutational profile (30,37,38). Indeed, response to BTK inhibition has been shown to be dependent on select genetic abnormalities (such as CD79a/b, MYD88 or CARD11) (37). Based on mutational profiling, DLBCL has been further segregated into genetic subsets with distinct genotypic, epigenetic and clinical characteristics, which may become the platform upon which future targeted approaches rely (30,39,40).
While numerous studies have explored the biology of DLBCL at the time of diagnosis, fewer have focused on rel/refr disease. Importantly, a different pattern of mutations between diagnostic and relapsed samples has been shown (41). Indeed, by analyzing the exome of rel/refr DLBCL, Morin et al. (42) identified genes implicated in therapeutic resistance and reported mutations that may affect sensitivity to novel therapeutics such as MYD88 and CD79B mutations in ABC-DLBCL, and STAT6 in GCB-DLBCL. Other studies have looked at clonal evolution and suggest that oncogenic events occurring under chemotherapy selection pressure may be the main driving force at relapse (43), with a mild increase in overall mutations compared to diagnostic samples (44). In the era of precision medicine, it will become increasingly important to perform a biopsy at time of relapse in order to guide therapeutic strategies utilizing novel targeted agents.
Novel drugs and targeted strategies (Table 1)
Table 1
| Class | Target | Agent | Reference | Phase | ORR | CR |
|---|---|---|---|---|---|---|
| Antibody | CD20 | Obinutuzumab | Morschhauser (45) | 2 | 20% | – |
| Ofatumumab-DHAP | van Imhoff (18) | 2 | 38% | 15% | ||
| CD19 | MOR208 | Jurczak (46) | 2 | 26% | – | |
| MOR208 + LEN | Salles (47) | 2 | 58% | 33% | ||
| CD40 | Dacetuzumab | De Vos (48) | 2 | 9% | – | |
| Dacetuzumab/R-ICE | Fayad (49) | 2b | 36% | – | ||
| Ab drug conjugate | CD30 | Brentuximab Vedotin | Jacobsen (50) | 2 | 44% | 17% |
| CD22 | Inotuzumab ozogamicin (IO)+R | Dang (51) | 3 | 41% | – | |
| IO + R-CVP/IO + R + R-GDP | Ogura (52)/Sangha (53) | 1 | 57%/33% | – | ||
| CD79b | Polatuzumab vedotin | Palanca (54) | 1 | 56% | – | |
| Pola V + R-Benda | Sehn (55) | 1/2 | 70% | 58% | ||
| CD19 | Coltuximab ravtansine/CR + R | Trneny (56)/Coiffier (57) | 2 | 44%/31% | – | |
| Denintuzumab mafodotin | Moskowit z(58) | 1 | 33% | 22% | ||
| Loncastuximab tesirine | Radfort (59) | 1 | 40% | 22% | ||
| NF-κB and BcR | Proteasome inhibitor | Bortezomib/Bort + DA-EPOCHR | Dunleavy (60) | 2 | 4%/34%* | – |
| BTK inhibitor | Ibrutinib | Wilson (37) | 1/2 | 37% (ABC) | – | |
| SYK Inhibitor | Fostamatinib | Flinn (61) | 2 | 3% | – | |
| PI3K/AKT/mTOR | PI3K Inhibitor | Copanlisib (all/ABC) | Lenz (62) | 2 | 25%/37% | 13%/25% |
| Buparlisib | Younes (63) | 2 | 11.5% | – | ||
| mTOR | Everolimus + R | Barnes (64) | 2 | 38% | 11% | |
| Temsirolimus | Smith (65) | 2 | 28% | 12% | ||
| Pan-PI3K/mTOR | Voxtalisib | Brown (66) | 2 | 5% | – | |
| Other target | XPO1 | Selinexor | Kuruvilla (67) | 1 | 31% | – |
| BCL2 | Venetoclax | Davids (68) | 1 | 18% | – | |
| Venetoclax R-ICE | Caimi (69) | 1 | 85% | 69% | ||
| Immunomodulation | IMID | Lenalidomide | Witzig (70) | 2 | 28% | 13% |
| Len + R-Benda | Cheson (71) | 1 | 20% | – | ||
| Len + R-ESHAP + ASCT* | Martin (72) | 1b | 78.9% | 47% | ||
| Len + R-ICE + ASCT* | Feldman (73) | 1/2 | 73% | 60% | ||
| Ibru + Len + R | Ramchandren (74) | 2 | 55% | 30% | ||
| Checkpoint, PD-1 | Nivolumab | Ansell (75) | 2 | 10% | – | |
| CD47 | Hu5F9-G4 | Advani (76) | 1 | 40% | 33% | |
| BiTE | Blinatumomab | Viardot (77) | 2 | 43% | 19% | |
| RG6026 | Hutchings (78) | 1 | 33% | – | ||
| Mosunetuzumab | Budde (79) | 1 | 33% | 21% | ||
| Epigenetic | EZH2 | Tazemetostat | Morschhauser (80) | 2 | 29%** | – |
| HDAC | Panabinostat +/− R | Assouline (81) | 2 | 28% | – | |
| HDAC-PI3K | CDUC-907 | Oki (82) | 1 | 37% | 17% |
*, bortezomib single agent/bortezomib + DA-EPOCH; **, ORR among EZH2 mutated patients. DLBCL, diffuse large B-cell lymphoma.
Monoclonal antibodies and Antibody-drug conjugates (Figure 2)
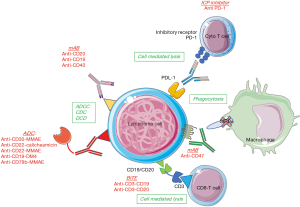
Monoclonal antibodies (mAbs)
To improve the efficiency of targeting CD20 and to overcome rituximab resistance, novel anti-CD20 mAbs have been engineered. Obinutuzumab, a type II IgG1 glycoengineered Fc-optimized mAb, has been designed to augment antibody-dependant cellular cytotoxicity compared with rituximab. The results in DLBCL have been disappointing, with an ORR of only 20% in rituximab pre-treated patients (45). Furthermore, the randomized study of obinutuzumab-CHOP vs. R-CHOP in the first-line setting (GOYA trial) did not show any difference in ORR or PFS between the 2 arms (83). Ofatumumab is a novel anti-CD20 mAb targeting a different epitope than rituximab, but in a randomized phase II trial, no difference in efficacy was found between ofatumumab-DHAP and R-DHAP in rel/refr DLBCL (18). MOR208 is an Fc-engineered, humanized, anti-CD19 antibody that demonstrated an ORR of 26% as a single agent in patients with rel/refr DLBCL in a phase 2 trial (duration of response (DoR) >12 months in 5/9 cases) (46). The favorable safety profile permits combination therapy (discussed below). Dacetuzumab (SGN-40) is a non-blocking, partial agonist, humanized IgG1 anti-CD40 mAb (48) that showed a low (9%) ORR as a single agent, and failed to show benefit when combined with R-ICE in a phase III trial that was prematurely stopped when a futility analysis failed to demonstrate higher CR rates (36% vs. 42% with placebo) (49).
Antibody-drug conjugates (ADCs)
ADCs consist of a mAb covalently linked to a small-molecule drug allowing the targeted delivery of a cytotoxic agent with aim to increase efficacy and minimize off-target effects. Brentuximab vedotin (BV) is an FDA-approved ADC, targeting CD30 and delivering the antimicrotubule agent monomethyl auristatin E (MMAE). In a phase II trial, Jacobsen et al. (50) reported an ORR of 44% (CR rate 17%) in rel/refr DLBCL, with a median DoR of 5.6 months (16.6 months in CR patients). Neutropenia and peripheral sensory neuropathy were the most frequent adverse events (AEs). The combination of BV with rituximab showed similar results (84). Inotuzumab ozogamicin (IO) is an ADC targeting CD22 linked to calicheamicin and FDA approved for the treatment of acute lymphocytic leukemia (ALL). In a phase III trial including 338 patients with rel/refr aggressive B-cell lymphoma (51), randomized between IO + rituximab versus chemotherapy + rituximab, there was no difference in ORR, PFS and OS between the 2 arms (ORR 41%, median PFS 3.7 months and median OS 9.5 months with IO + R) with more AEs leading to treatment discontinuation in the IO + R arm. Combinations of IO with R-CVP (cyclophosphamide, vincristine, and prednisone) and R-GDP have also been evaluated, with ORRs of 57% (52) and 33% (53), respectively. Polatuzumab vedotin (pola) is an ADC combining an anti-CD79b MAb with MMAE that has shown promising activity in a phase 1 trial, yielding an ORR of 56% in patients with rel/refr DLBCL (54). The most common grade 3–4 AEs were neutropenia (40%), anemia (11%), and peripheral sensory neuropathy (9%). In a phase II randomized study, the combination of pola plus bendamustine (B) and rituximab was compared to BR alone in transplant-ineligible patients with rel/refr DLBCL. With 40 patients included in each arm, the PET-CR rate was significantly higher with pola-BR vs. BR alone, 40% vs. 18%, respectively. Similarly, the median OS was significantly improved with pola-BR (12.4 vs. 4.7 months) (55). Coltuximab ravtansine, an anti-CD19 mAb conjugated to maytansinoid DM4, was evaluated in two phase II studies, demonstrating only moderate clinical benefit. As a single agent, Trneny et al. (56) reported an ORR of 44% with a modest DoR of 4.7 months. In combination with rituximab in patients with rel/refr DLBCL, Coiffier et al. (57) reported an ORR of 31% (that did not met the primary objective of the study) with a DoR of 8.6 months. SGN-CD19A or denintuzumab mafodotin is another ADC targeting CD19, conjugated with MMAF that showed similar responses in a phase 1 trial (ORR 33%, CR 22%) (58). A particular AE, microcystic keratopathy leading to visual disturbances, occurred in 84% of patients. A combination study with R-ICE is ongoing (NCT02592876). Finally, a large phase I trial including 183 patients with rel/refr DLBCL evaluated the safety and efficacy of loncastuximab tesirine, an ADC also targeting CD19 and conjugated to a pyrrolobenzodiazepine dimer toxin. The majority (73%) of patients experienced a grade 3 AE requiring a dose reduction. The ORR was 40.2% (22% CR) and median DoR was 4.2 months (59).
Overall, among the recently developed monoclonal antibodies and ADCs, polatuzumab vedotin has been the only drugs evaluated in a comparative trial, and has shown the most encouraging safety and efficacy profile.
Pathway Inhibitors (Figure 3)
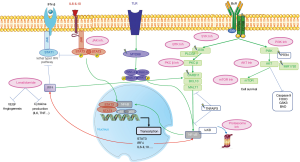
NF-κB and BCR pathway
B cells have the capacity to respond to a variety of stimuli due to the combined expression of an antigen-specific B-cell receptor (BCR) and germline-encoded receptors of the innate immune system, Toll-like receptors (TLR) (85). These stimuli activate downstream transcription factors, such as NF-κB, that controls numerous cellular processes involved in lymphoma development. In ABC-DLBCL, mutations in CARD11 (86), CD79A/B (87), and MYD88 (38), or loss of the regulating agent A20 (TNFAIP3) (88) are some of the mechanisms that can induce a constitutive activation of the BCR pathway and ultimately of NF-κB (89) with IRF-4 (interferon regulatory factor 4) upregulation. Importantly, is has been shown that cases presenting with CBM (CARD11, BCL10, MYD88) signaling mutations or MYD88 mutation without CD79A/B mutations will require inhibition downstream of this complex to kill tumor cells (such as proteasome inhibitors), compared to cases without CARD11/MYD88 mutations or with both MYD88 and CD79A/B mutations that will be sensitive to inhibition of kinases upstream of the NF-κB complex (such as BTK inhibitors) (37).
Proteasome inhibitors
Based on the biological rationale of NF-κB constitutive activation in ABC-DLBCL (89), it was hypothesized that proteasome inhibition could be beneficial in this subtype. In a phase I/II study evaluating bortezomib alone or in combination with DA-EPOCH in 49 patients with rel/refr DLBCL, bortezomib alone had no activity (ORR 4%) (60). Whereas, when combined with DA-EPOCH, a higher ORR was observed in patients with ABC-DLBCL compared with GCB-DLBCL (83% vs. 13%) leading to a higher median OS (10.8 vs. 3.4 months). Based on this promising signal of activity, bortezomib was evaluated in 2 randomized trials in combination with R-CHOP in untreated patients with non-GCB or ABC DLBCL, but no benefit was observed (90,91). The utility of bortezomib in DLBCL seems questionable, although a phase II randomized study with R-DHAP is ongoing (NCT01805557). Promising preclinical data with the novel proteasome inhibitor carfilzomib (92) in combination with a pan-HDAC inhibitor vorinostat (93,94) has led to an ongoing phase I study (NCT 01276717).
BCR pathway inhibition
The Bruton tyrosine kinase (BTK) is a member of the Tec kinase family with an early positioning within the BCR cascade. Ibrutinib is a selective and irreversible BTK inhibitor, via specific active-site occupancy (87). In vitro data showed a selective activity of ibrutinib in ABC-DLBCL cell lines with chronic active BCR signaling (87). In a phase 1/2 clinical trial including 80 patients with rel/refr DLBCL, ibrutinib resulted in an ORR of 37% in ABC-DLBCL, but only 5% in GCB-DLBCL. Furthermore, the authors showed that patients with concomitant MYD88 and CD79A/B mutations had high response to ibrutinib (37), as well as those with both wild-type (WT) BCR and MYD88, whereas those with MYD88 mutations and WT CD79A/B were resistant. The main AEs associated with ibrutinib are thrombocytopenia and bleeding risk, neutropenia, atrial fibrillation and aspergillosis infections (95). The combination of Ibrutinib, bendamustine and rituximab in a phase Ib trial led to an ORR of 37% in rel/refr DLBCL (96). Grade 3/4 toxicities included lymphopenia (77%), neutropenia (33%), thrombocytopenia (19%), and rash (25%). Recently, a randomized phase III trial evaluated the addition of ibrutinib to R-CHOP in previously untreated patients with non-GCB-DLBCL (97). The study did not meet its primary endpoint in the intention-to-treat population due to a significant interaction between treatment and age. Interestingly, in patients younger than 60 years of age, ibrutinib plus R-CHOP improved EFS, PFS and OS (HR 0.579, 0.556, 0.330 respectively). However, in patients older than 60 years of age, the increased toxicity profile of the combination compromised treatment delivery and likely reduced efficacy. The encouraging signal observed with the addition of ibrutinib in younger patients in this up-front trial justifies further exploration of BTK inhibition in patients with rel/refr disease. Acquired Ibrutinib resistance due to BTKCys481 mutations occurs in B-cell NHL, with a subclonal presentation (98), emphasizing the need for novel inhibitors. The next-generation BTK inhibitors acalabrutinib (ACP-196), tirabrutinib (ONO, GS-4059), GDC-0853 (99) and BGB-3111 (100,101) are currently being evaluated in DLBCL. Interestingly, 3 patients with BTKCys481 mutation had a response to GDC-0853 (99).
The kinase SYK, important for tonic BCR signaling (102), is another potential target in ABC-DLBCL. SYK is activated following Igα and Igβ ITAM phosphorylation, engaging additional adaptor proteins and initiating downstream signaling. Chemical SYK blockade decreases cell proliferation and induces apoptosis in DLBCL cell lines dependent on BCR signaling (103). Fostamatinib is an oral SYK kinase inhibitor recently evaluated in a phase II trial with very disappointing results (3% ORR) and many off-target effects (61). Entospletinib is an adenosine triphosphate competitive inhibitor that disrupts SYK kinase activity with a more selective action than fostamatinib. In a phase II trial including patients with CLL and NHL, the most common serious AEs were dyspnea, pneumonia, febrile neutropenia, dehydration, and pyrexia (104). ORR was reported for CLL patients only (61%). A phase Ib trial in combination with R-CHOP in first-line DLBCL is ongoing (NCT03225924).
PI3K/AKT/mTOR pathway
PI3K signals downstream of the BCR and leads to AKT activation which phosphorylates and inhibits pro-apoptotic molecules. AKT also promotes cell cycle progression and mTOR activation (mammalian target of rapamycin), a kinase that favors tumor cell survival via protein synthesis and cell proliferation. In GCB-DLBCL, deletion of the tumor suppressor PTEN and amplification of the oncogenic microRNA cluster, mir-17-92 (which inhibits PTEN translation), leads to loss of control of this pathway (33,105). Multiple forms of PI3K inhibitors exist, with differing specificities. Idelalisib (a PI3Kδ inhibitor) has been approved in rel/refr FL (106) and is currently being evaluated in a phase II trial in patients with rel/refr DLBCL (NCT03576443). Based on biological rationale (107), combination studies with the BTK inhibitor tirabrutinib are ongoing. Importantly, a phase 2 study of idelalisib with entospletinib was stopped due to serious AEs including pneumonitis in 18% of patients (with 2 fatal cases) (108). Similarly, two combination trials of idelalisib, lenalidomide and rituximab were stopped due to an excess of unexpected serious toxicities (109,110). Copanlisib (PI3K α/δ inhibitor) also has limited efficacy as a single agent in rel/refr DLBLC, with an ORR of 25%, but appeared slightly higher in ABC-DLBCL [ORR 37.5%, CR rate 25%, in a non-intention to treat analysis (ITT)] (62). Buparlisib is a pan-PI3K inhibitor that has been evaluated in a phase 2 trial (63) including 26 patients with DLBCL resulting in an ORR of only 11.5% and a short duration of response of 2.2 months, with a similar toxicity profile to other PI3K inhibitors. Umbralisib (TGR-1202) is a PI3Kδ inhibitor (111) recently evaluated in a phase I study enrolling 90 patients with rel/refr CLL and NHL. The most common grade 3–4 AEs were neutropenia (13%), anemia (9%), thrombocytopenia (7%), pneumonia (3%), and colitis (2%). A phase I combination study of umbralisib, ublituximab (UTX, a novel glycoengineered anti-CD20) and bendamustine was performed, enrolling 15 patients with rel/refr DLBCL. The only grade 3–4 AE reported in more than 10% of patients was neutropenia (22%). Among 11 patients evaluable for response, the ORR was 73%, with a CR rate of 45% (112). Everolimus (RAD001) is an orally bioavailable rapamycin analog and inhibitor of mTOR inducing inhibition of cell cycle progression in vitro by decreasing phosphorylation of mTOR targets (113). In a phase II trial including 26 patients with rel/refr DLBCL, the combination of rituxmab and everolimus (64) resulted in an ORR of 38% with 3 patients achieving a CR and a median DoR of 8 months. The most common grade 3–4 toxicities were neutropenia, anemia, and thrombocytopenia. A phase I trial in 24 patients with untreated DLBCL showed that the combination of everolimus/R-CHOP was safe and promising. Indeed, after a FU of 37 months, only one relapse with low grade follicular lymphoma has occurred and no patients have relapsed with DLBCL within the 24 months from treatment initiation (EFS24) (114). These results are even more impressive since the median time from diagnosis to treatment was rather short (14 days) in this trial, with an expected EFS24 failure rate of 44% (115). Another rapamycin analog and mTOR inhibitor, temsirolimus, administered intravenously, has been evaluated in a phase II trial including 32 patients with rel/refr DLBCL and transformed FL. The ORR was 28%, with a CR rate of 12%, but the median DoR was only 2.4 months (65). A phase II combination study of temsirolimus with R-DHAP (116) has been conducted in patients with rel/refr DLBCL who had received a maximum of 2 prior regimens, resulting in an ORR of 78% (CR rate 22%) (117). Voxtalisib, a pan-PI3K/mTOR inhibitor, has been investigated in a large series of 167 patients with rel/refr NHL exhibiting a similar safety profile but a very disappointing ORR of 5% in the 42 patients with rel/refr DLBCL (66).
JAK/STAT pathway
The signal transducer and activator of transcription (STAT) proteins is a family of transcription factors that regulate many cellular events [differentiation, proliferation, and cell survival (118)] and can be activated by cytokines and growth factors through cell surface receptor binding, leading to the activity of receptor-associated Janus kinase (JAK) family members. JAKs then phosphorylate STATs, leading to their dimerization and transit to the nucleus. Some of the transcriptional targets of STAT proteins play a role in cell-cycle progression, survival and regulation of the lethal type I IFN signaling pathway. The constitutive activation of NF-κB signaling in ABC-DLBCL leads to a positive loop regulating the production of target genes such as IL6 and IL10 leading to JAK activation, STAT3 phosphorylation (119) and intracellular signaling resulting in NF-κB nucleus transfer and synergistic activation of several genes (120). Lam et al. (121) characterized a subset of ABC-DLBCL with high STAT3, IL-6 and/or IL-10 and showed that ABC-DLBCL cell lines secreting IL-6 and/or IL-10 were selectively killed by an inhibitor of STAT3 signaling, a small JAK inhibitor, in synergy with NF-κB pathway inhibition. More recently, Lu et al. (122) showed that STAT3 also negatively regulates the lethal type I IFN signaling pathway (by inhibiting expression of IRF7, IRF9, STAT1, and STAT2) leading to an in vitro and in vivo synergistic effect of the inhibition of STAT3 by ruxolitinib with the type I IFN inducer lenalidomide on ABC-DLBCL models. Despite this biological rational, little data on the clinical utility of JAK inhibitors is available in DLBCL. Clinical trials with ruxolitinib are ongoing (NCT 01431209, in combination with bortezomib NCT 02613598) as well as with cerdulatinib, a new SYK/JAK inhibitor showing activity in an in vitro model of DLBCL (123). Younes et al. reported a phase I trial of pacritinib, an oral JAK1/2 inhibitor, in DLBCL demonstrating a favorable safety profile but modest activity (10% ORR) (124).
Others agents
XPO1 inhibitors
Exportine 1 (XPO1/CRM1) is a protein responsible for the export from the nucleus of various tumor suppressors (p53, p73, p21, p27, Rb, BRCA1/2 and IκB) leading to their inactivation (125) and is also involved in the regulation of the cytoplasmic levels of mRNA transcripts of several oncoproteins (MYC, BCL2, BCL6). Increased expression of XPO1 has been associated with disease aggressiveness (126) and mutations have been reported in different types of lymphoma, although not in DLBCL (127). Selinexor is a first-in-class oral XPO1 inhibitor. In a phase I trial, the most common grade 3/4 AEs were thrombocytopenia (47%), neutropenia (32%), anemia (27%), leukopenia (16%), fatigue (11%), and hyponatremia (10%) and the ORR was 31% (22/70) across various NHL histologies, with similar ORRs seen in ABC and GCB-DLBCL, as well as in double-hit lymphoma (3 responses/6). Interestingly, the 4 patients that achieved a CR were still alive and 3 remained on treatment with follow-up ranging from 16–35 months (67).
Apoptosis and BCL2 inhibition
The t(14;18)(q32;q21) translocation has been reported in more than 30% of GCB-DLBCL, and is associated with higher expression of the anti-apoptotic protein BCL2 compared to t(14;18) negative cases (128). Other molecular abnormalities involving the BCL2 locus, such as copy number variations (CNV), have also been reported in ABC-DLBCL (129). Venetoclax is a selective, oral small molecule inhibitor of BCL2. Davids et al. (68) recently reported the results of a phase I trial in 106 patients with R/R NHL. Venetoclax was well tolerated with only 3 cases of laboratory tumor lysis syndrome occurring. Grade 3/4 events were reported in 56% of patients, with the most common being anemia (15%), neutropenia (11%), and thrombocytopenia (9%). The ORR was 44% for the overall cohort, but was only 18% in the DLBCL population with an estimated PFS of only 1 month. Safety results of a phase I combination study of venetoclax plus R-ICE including 18 patients with R/R DLBCL were recently reported (69). The ORR of 85% was impressive, including a metabolic CR rate of 69%. Hematologic toxicities (primarily neutropenia) were common and one patient died from TLS.
In conclusion, these novel targeted therapies have shown limited efficacy as single agents and none are FDA-approved. Combination studies appear promising, but have raised concern regarding tolerability. Results from numerous ongoing combination trials are eagerly awaited.
Immunomodulation (Figure 2)
IMiDs
In ABC-DLBCL, activation of both the NF-κB and TLR pathways result in constitutive expression of IRF4, leading to the downregulation of IFN-β production and amplification of NF-κB signaling due to CARD11 transactivation (130). The immunomodulatory agent lenalidomide downregulates IRF4 and its cofactor SPIB (Spi-B Transcription Factor, a member of the ETS-family proteins) leading to IFN- production and downregulation of BCR-dependant NF-κB signaling, resulting in death of ABC-DLBCL cell lines (130,131). Blockade of BCR signaling with ibrutinib also downregulates IRF4 and consequently synergizes with lenalidomide, suggesting an attractive therapeutic combination (NCT01955499). Lenalidomide (LEN) also acts as an antiangiogenic agent, which is another rationale for its utility in DLBCL where high VEGF levels have been associated with poorer outcome (132). In a large phase II trial including 217 patients with R/R aggressive B-cell NHL, the ORR of LEN as a single agent was 35% (28% for DLBCL) with a CR rate of 13% and a DoR of 10.6 months. The most common AE was myelosuppression, with grade 4 neutropenia and thrombocytopenia observed in 17% and 6% of patients, respectively (70). As expected, ABC-DLBCL had a higher ORR and PFS than GCB-DLBCL (ORR 53% vs. 8% and median PFS 6.2 vs. 1.7 months) (133). These results led to a planned randomized phase II/III trial investigating LEN 25 mg daily versus investigators’ choice (IC: gemcitabine, rituximab, etoposide, or oxaliplatin) in R/R DLBCL (134). In the stage I part of the study, LEN-treated patients had an ORR of 27.5% versus 11.8% in the IC arm and median PFS was increased (13.6 weeks versus 7.9 weeks; in IC arm, P=0.041), with greater improvements in non-GCB patients (15.1 vs. 7.1 weeks, respectively; P=0.021) compared with GCB patients (10.1 vs. 9.0 weeks, respectively; P=0.550). However, the stage 1 results did not meet the protocol-specified threshold and therefore the study did not proceed to stage 2.
In a retrospective analysis, Ivanovo et al. reported a noteworthy ORR of 41.2%, with a CR rate of 35.3% with the combination of LEN and rituximab, with a median DoR of 26.5 months (135). However, in a phase I trial, the combination of LEN with R-Bendamustine showed limited activity in R/R DLBCL with an ORR of 20%, similar to what had been reported with LEN alone (71). In another phase Ib combination study with R-ESHAP, including transplant eligible patients, the maximum tolerated dose was 10 mg due to grade 3 angioedema at 15 mg. The ORR was 78.9% and CR rate of 47.4% (72), but different inclusion criteria preclude comparison between these studies. In a similar phase I/II trial, the combination of LEN and R-ICE showed a better tolerability profile (recommended phase 2 dose 25 mg daily), exhibiting a 73% ORR and 60% CR rate (73). Responding patients underwent ASCT followed by LEN maintenance, with neutropenia being the most frequent AE during this period, without significant infections. In rel/refr DLBCL patients responsive to salvage R-chemotherapy but not eligible for ASCT, LEN maintenance (25 mg, until lymphoma progression) was evaluated in a phase II trial: again, neutropenia was the most frequent AE leading to 4 cases of febrile neutropenia and one treatment-related death (intestinal infarction) (136). At 1 year from trial registration, 28/46 (61%) patients were progression-free, which was higher than the predetermined efficacy threshold, suggesting a possible role for LEN maintenance in this population (136). The IR2 regimen, Ibrutinib (560 mg), LEN (20 mg) and R was evaluated in a phase I/II trial with 85% of the patients experiencing a grade 3 or more AE. Among the 44 evaluable patients, the ORR was 55% and the CR rate was 30%, with median DoR of 9 months (74). The association of LEN with the anti-CD19 MOR208 has been evaluated in a phase I/II trial (81 patients, with a maximum of 3 prior therapies, and excluding patients with primary refractory disease). The main reported AEs were hematological and 42% of the patients required LEN dose reduction. CR was observed in 33% and PR in 25% of patients, with 15% of patients experiencing stable disease. The median PFS was 16.2 months and median DoR not reached (47). While these results are encouraging, the trial included better-risk patients due to strict selection criteria, making these results difficult to compare. Finally, the results of a large phase III trial comparing R-CHOP plus lenalidomide (R2-CHOP) with placebo/R-CHOP in patients with previously untreated ABC-type DLBCL were recently presented (137). The trial did not meet its PFS primary endpoint with a HR of 0.85 (2 years OS was 79% for R2-CHOP and 80% for placebo/R-CHOP). Discordant results were reported in a randomized phase II study showing an improved outcome with the R2-CHOP regimen with a 33% reduction in risk of progression or death compared to R-CHOP [HR 0.67 (95% CI: 0.44–1.03, P (one-sided 0.03)] (138). Differences in trial design, as well as population differences as highlighted by a shorter time-to-treatment from diagnosis might, in part, explain these discrepant results.
In conclusion, in the relapsed setting, LEN combined with chemotherapy and/or targeted strategies appears promising, although the tolerability profile may be an issue for elderly and/or heavily pre-treated patients. However, in previously untreated patients, a large phase III trial failed to show a better outcome with R2-CHOP compared to R-CHOP.
Checkpoint inhibitors
The program death 1 immune checkpoint (PD-1) pathway is used by lymphoma cells to avoid T cell immune surveillance. In a phase II trial, rel/refr DLBCL patients achieving at least a PR after salvage therapy prior to ASCT received the PD-1 inhibitor pidilizumab every 42 days at 1.5 mg/kg IV for 3 doses, beginning 1 to 3 months after ASCT. The most frequently reported grade 3/4 AEs were neutropenia (19%) and thrombocytopenia (8%). The PFS at 16-months was 72% and the study met its primary endpoint (139). Interestingly, the PFS of patients who were PET-positive before ASCT was comparable to those who were PET-negative. Another PD-1 inhibitor, nivolumab, has shown impressive activity in classical HL but efficacy in DLBCL as a single agent was less remarkable. In a recent phase II single arm study including 121 patients with rel/refr DLBCL, the ORR was 10% and 3% in patients who had failed ASCT and transplant-ineligible patients, respectively (median DoR, 11 and 8 months, respectively) (75). This lack of efficacy might be related to the low incidence of 9p24.1 gain/amplification (including the PD-L1 locus) in patients with rel/refr DLBCL. Indeed, only 16% of the evaluable cases had copy number gains and 3% had amplification.
Macrophage and CD47 blockade
Recently, Advani et al. (76) reported the first trial of a macrophage immune checkpoint inhibitor. Hu5F9-G4 is an Ab targeting CD47, which is expressed on lymphoma cells, inhibiting tumor-cell phagocytosis. Hu5F9-G4 may help to overcome rituximab resistance, creating a synergistic effect when both drugs are combined. In this phase I trial, 22 patients were treated (including 15 with RR DLBCL), 95% of which were rituximab-refractory. In the DLBCL population, the ORR was 40%, with 33% of patients achieving a CR and 91% exhibiting ongoing response after 6 months of follow-up. AEs were predominantly grade 1-2, the most common being anemia (an expected-on target effect) and infusion-related reactions.
Bi-specific antibodies
CD3-CD19 bi-specific T-cell engaging antibody (BiTE) constructs allow T-cell activation through transient ligation of CD3-positive T-cells to CD19-positive lymphoma cells leading to T-cell mediated lysis. Blinatumomab is a first-in-class BiTE approved by the FDA for the treatment of Philadelphia chromosome–negative rel/refr B-cell acute lymphoblastic leukemia (ALL). In a Phase I (140) trial including patients with rel/refr NHL, the major DLTs were neurological events and cytokine release syndrome (CRS). The MTD was 60 µg/m2/day as a continuous infusion over 4 to 8 weeks. In the subgroup of patients with DLBCL, the ORR of 55% was very promising, with 36% of patients achieving a CR and a median DoR of 404 days. A phase 2 study evaluated stepwise dosing (9/28/112 µg/d with weekly dose increases; n=23) or flat dosing (112 µg/d; n=2) by continuous infusion for up to 8 weeks, with dexamethasone prophylaxis, in heavily pretreated rel/refr DLBCL. The flat dose cohort was stopped prematurely due to neurologic AEs. Among 21 evaluable patients, the ORR after 1 cycle was 43%, including CR in 19%, and the median DoR was 11.6 months. The most common AEs were tremor (48%), pyrexia (44%), fatigue (26%), and edema (26%). Grade 3 encephalopathy and aphasia occurred in 9% and tremor, speech disorder, dizziness, somnolence, and disorientation in 4% (77). In another phase II study including patients not in CR after platinium-based salvage therapy, blinatumomab was given for a single cycle of 70 days, followed by an optional 28 days cycle. The ORR was 37% (22% CR) with grade 3 toxicities reported in 59% of the 41 patients (141). Mosunetuzumab is a full-length CD20/CD3 bi-specififc anibody being evaluated in a phase I trial with 2 different schedules: every 21 days at a fixed dose and step-up dosing during cycle 1. CRS was the most frequently reported AE (21%, all grade 1–2). Grade 3 AEs occurred in 52% of patients, including 2 deaths. ORR in the DLBCL population was 33% (13/39), with 21% (8/39) achieving a CR (79). The bi-specific antibody RG6026, with a 2:1 format (two CD20 binders in addition to a CD3 binder) and an administration schedule of every 2 weeks has demonstrated a similar ORR in RR DLBCL (33%), without CNS toxicity or significant CRS (78).
In conclusion, the response rates and DoR of novel bi-specific antibodies under evaluation are very promising with a favorable tolerability profile.
Epigenome (Figure 4)
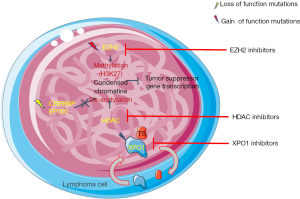
Large scale genomic studies have revealed frequent mutations in histone modifying genes in DLBCL (29,142). For instance, heterozygous mutations of the histone methyl transferase and catalytic subunit of the PRC2 chromatin remodeling complex, EZH2, have been observed in ~10% of NHL (143). These gain of function mutations are exclusively found in lymphomas of GC origin and act in concert with wild-type EZH2 to generate abnormally high levels of H3K27Me3, leading to abnormal repression of PRC2 targets, driving lymphomagenesis (144). Furthermore, EZH2 plays an essential role in GC formation in normal B-cells via a PRC2-mediated repression of target genes, allowing B-cells to undergo clonal expansion and somatic hypermutation (145). Tazemetostat is a first-in-class oral selective inhibitor of EZH2 (146). Phase I and II trials of tazemetostat as a single agent have shown a very good safety profile, with only 5% of AEs leading to dose reduction or treatment discontinuation (147). In the phase I trial, including a highly pre-treated population, the ORR was 38% among DLBCL patients (147). In the phase II trial, the ORR among EZH2 mutated DLBCL patients was 29% versus 15% among patients with WT EZH2 (median of 3 to 4 lines of prior therapy) (80). Panabinostat is a histone deacetylase inhibitor recently evaluated in a phase II study in rel/refr DLBCL (30 mg orally 3 times a week), with and without rituximab. The ORR was 28% (11/40) and the median DoR was 14.5 months, without apparent benefit of rituximab. Interestingly, early responses could be predicted by mutations in MEF2B (81). Other HDAC inhibitors have shown modest activity as single agents in phase II trials [mocetinostat (148), ORR 18.9% and belinostat (149), ORR 10.5%]. CUDC-907 is a dual PI3K/HDAC inhibitor recently evaluated in phase I trial (150). The most frequent AEs were thrombocytopenia, neutropenia and hyperglycemia. At the recommended phase 2 dose of 60 mg/day 5 days a week, the promising ORR was 37% in the expanded DLBCL cohort. The ORR in the evaluable MYC-altered DLBCL patients (defined by MYC rearrangement assessed by FISH or MYC overexpression by IHC) was 64% (7/11; 4 CR and 3 PR), while it was 29% (2/7) in MYC-unaltered, and 17% (2/12) in those with unknown MYC status (82). The median duration of response was 11.2 months in the global cohort; 13.6 months in MYC-altered patients versus 6.0 and 7.8 months in MYC-unaltered and unknown status, respectively.
The favorable safety profile of these epigenome-targeting agents suggests that combination therapy with other targeted agents may be a feasible strategy.
Conclusions
Recent progress in molecular biology has led to a better understanding of the oncogenic drivers of DLBCL, resulting in the development of a large number of targeted therapies undergoing evaluation in phase I and II trials. However, these agents have yet to earn regulatory approval, emphasizing the need for more efficient development strategies. Clinically available biomarkers to prioritize these options are also greatly needed. Importantly, as these agents emerge within the CAR-T cell era, optimal combinations and sequencing will need to be determined.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Astrid Pavlovsky) for the series “Diffuse Large B-Cell Lymphoma” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol.2019.09.01). The series “Diffuse Large B-Cell Lymphoma” was commissioned by the editorial office without any funding or sponsorship. LHS serves as an unpaid editorial board member of Annals of Lymphoma from Sep 2018 to Sep 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235-42. [Crossref] [PubMed]
- Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol 2005;23:5027-33. [Crossref] [PubMed]
- Maurer MJ, Ghesquières H, Jais JP, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol 2014;32:1066-73. [Crossref] [PubMed]
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017;377:2531-44. [Crossref] [PubMed]
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380:45-56. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Larouche JF, Berger F, Chassagne-Clément C, et al. Lymphoma recurrence 5 years or later following diffuse large B-cell lymphoma: clinical characteristics and outcome. J Clin Oncol 2010;28:2094-100. [Crossref] [PubMed]
- Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 2006;24:3121-7. [Crossref] [PubMed]
- Costa LJ, Maddocks K, Epperla N, et al. Diffuse large B-cell lymphoma with primary treatment failure: Ultra-high risk features and benchmarking for experimental therapies. Am J Hematol 2017;92:161-70. [Crossref] [PubMed]
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017;130:1800-8. [Crossref] [PubMed]
- Farooq U, Maurer MJ, Thompson CA, et al. Clinical heterogeneity of diffuse large B cell lymphoma following failure of front-line immunochemotherapy. Br J Haematol 2017;179:50-60. [Crossref] [PubMed]
- Glass B, Dohm AJ, Truemper LH, et al. Refractory or relapsed aggressive B-cell lymphoma failing (R)-CHOP: an analysis of patients treated on the RICOVER-60 trial. Ann Oncol 2017;28:3058-64. [Crossref] [PubMed]
- Hitz F, Connors JM, Gascoyne RD, et al. Outcome of patients with primary refractory diffuse large B cell lymphoma after R-CHOP treatment. Ann Hematol 2015;94:1839-43. [Crossref] [PubMed]
- Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non Hodgkin's lymphoma. N Engl J Med 1995;333:1540-5. [Crossref] [PubMed]
- Mounier N, Canals C, Gisselbrecht C, et al. High-dose therapy and autologous stem cell transplantation in first relapse for diffuse large B cell lymphoma in the rituximab era: an analysis based on data from the European Blood and Marrow Transplantation Registry. Biol Blood Marrow Transplant 2012;18:788-93. [Crossref] [PubMed]
- Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010;28:4184-90. [Crossref] [PubMed]
- Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol 2014;32:3490-6. [Crossref] [PubMed]
- van Imhoff GW, McMillan A, Matasar MJ, et al. Ofatumumab Versus Rituximab Salvage Chemoimmunotherapy in Relapsed or Refractory Diffuse Large B-Cell Lymphoma: The ORCHARRD Study. J Clin Oncol 2017;35:544-51. [Crossref] [PubMed]
- Vellenga E, van Putten WL, van 't Veer MB, et al. Rituximab improves the treatment results of DHAP-VIM-DHAP and ASCT in relapsed/progressive aggressive CD20+ NHL: a prospective randomized HOVON trial. Blood 2008;111:537-43. [Crossref] [PubMed]
- Chahoud J, Sui D, Erwin WD, et al. Updated Results of Rituximab Pre- and Post-BEAM with or without 90Yttrium-Ibritumomab Tiuxetan during Autologous Transplant for Diffuse Large B-Cell Lymphoma. Clin Cancer Res 2018;24:2304-11. [Crossref] [PubMed]
- Gisselbrecht C, Schmitz N, Mounier N, et al. Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol 2012;30:4462-9. [Crossref] [PubMed]
- Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant 2016;51:51-7. [Crossref] [PubMed]
- Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 2005;23:4117-26. [Crossref] [PubMed]
- Mounier N, El Gnaoui T, Tilly H, et al. Rituximab plus gemcitabine and oxaliplatin in patients with refractory/relapsed diffuse large B-cell lymphoma who are not candidates for high-dose therapy. A phase II lymphoma study Association trial. Haematologica 2013;98:1726-31. [Crossref] [PubMed]
- Chau I, Webb A, Cunningham D, et al. An oxaliplatin-based chemotherapy in patients with relapsed or refractory intermediate and high-grade non-Hodgkin's lymphoma. Br J Haematol 2001;115:786-92. [Crossref] [PubMed]
- Lohr JG, Stojanov P, Lawrence MS, et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci U S A 2012;109:3879-84. [Crossref] [PubMed]
- Pasqualucci L, Trifonov V, Fabbri G, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet 2011;43:830-7. [Crossref] [PubMed]
- Zhang J, Grubor V, Love C, et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A 2013;110:1398-403. [Crossref] [PubMed]
- Morin RD, Mendez-Lago M, Mungall AJ, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 2011;476:298-303. [Crossref] [PubMed]
- Reddy A, Zhang J, Davis NS, et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell 2017;171:481-94.e15. [Crossref] [PubMed]
- Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000;403:503-11. [Crossref] [PubMed]
- Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002;346:1937-47. [Crossref] [PubMed]
- Lenz G, Wright GW, Emre NC, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci U S A 2008;105:13520-5. [Crossref] [PubMed]
- Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood 2014;124:2354-61. [Crossref] [PubMed]
- Sarkozy C, Traverse-Glehen A, Coiffier B. Double-hit and double-protein-expression lymphomas: aggressive and refractory lymphomas. Lancet Oncol 2015;16:e555-67. [Crossref] [PubMed]
- Ennishi D, Jiang A, Boyle M, et al. Double-Hit Gene Expression Signature Defines a Distinct Subgroup of Germinal Center B-Cell-Like Diffuse Large B-Cell Lymphoma. J Clin Oncol 2019;37:190-201. [Crossref] [PubMed]
- Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 2015;21:922-6. [Crossref] [PubMed]
- Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011;470:115-9. [Crossref] [PubMed]
- Schmitz R, Wright GW, Huang DW, et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med 2018;378:1396-407. [Crossref] [PubMed]
- Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 2018;24:679-90. [Crossref] [PubMed]
- Melchardt T, Hufnagl C, Weinstock DM, et al. Clonal evolution in relapsed and refractory diffuse large B-cell lymphoma is characterized by high dynamics of subclones. Oncotarget 2016;7:51494-502. [Crossref] [PubMed]
- Morin RD, Assouline S, Alcaide M, et al. Genetic Landscapes of Relapsed and Refractory Diffuse Large B-Cell Lymphomas. Clin Cancer Res 2016;22:2290-300. [Crossref] [PubMed]
- Rizzo D, Viailly PJ, Mareschal S, et al. Oncogenic events rather than antigen selection pressure may be the main driving forces for relapse in diffuse large B-cell lymphomas. Am J Hematol 2017;92:68-76. [Crossref] [PubMed]
- Nijland M, Seitz A, Terpstra M, et al. Mutational Evolution in Relapsed Diffuse Large B-Cell Lymphoma. Cancers (Basel) 2018; [Crossref] [PubMed]
- Morschhauser FA, Cartron G, Thieblemont C, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol 2013;31:2912-9. [Crossref] [PubMed]
- Jurczak W, Zinzani PL, Gaidano G, et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin's lymphoma. Ann Oncol 2018;29:1266-72. [Crossref] [PubMed]
- Salles G, Duell EJ, González-Barca E, et al. Single-Arm Phase II Study of MOR208 Combined with Lenalidomide in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma: L-Mind. Blood 2018;132:227. [Crossref]
- de Vos S, Forero-Torres A, Ansell SM, et al. A phase II study of dacetuzumab (SGN-40) in patients with relapsed diffuse large B-cell lymphoma (DLBCL) and correlative analyses of patient-specific factors. J Hematol Oncol 2014;7:44. [Crossref] [PubMed]
- Fayad L, Ansell SM, Advani R, et al. Dacetuzumab plus R-ICE as salvage therapy for patients with DLBCL relapsing after R-CHOP: a randomized, double-blind, placebo-controlled Phase 2b trial. Leuk Lymphoma 2015;56:2569-78. [Crossref] [PubMed]
- Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood 2015;125:1394-402. [Crossref] [PubMed]
- Dang NH, Ogura M, Castaigne S, et al. Randomized, phase 3 trial of inotuzumab ozogamicin plus rituximab versus chemotherapy plus rituximab for relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Br J Haematol 2018;182:583-586. [Crossref] [PubMed]
- Ogura M, Tobinai K, Hatake K, et al. Phase I Study of Inotuzumab Ozogamicin Combined with R-CVP for Relapsed/Refractory CD22+ B-cell Non-Hodgkin Lymphoma. Clin Cancer Res 2016;22:4807-16. [Crossref] [PubMed]
- Sangha R, Davies A, Dang NH, et al. Phase 1 study of inotuzumab ozogamicin combined with R-GDP for the treatment of patients with relapsed/refractory CD22+ B-cell non-Hodgkin lymphoma. J Drug Assess 2017;6:10-7. [Crossref] [PubMed]
- Palanca-Wessels MC, Czuczman M, Salles G, et al. Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol 2015;16:704-15. [Crossref] [PubMed]
- Sehn L, Herrera A, Matasar M, et al. Addition of Polatuzumab Vedotin to Bendamustine and Rituximab (BR) Improves Outcomes in Transplant-Ineligible Patients with Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL) Versus BR Alone: Results from a Randomized Phase 2 Study. Blood 2017;130:2821.
- Trnĕný M, Verhoef G, Dyer MJ, et al. A phase II multicenter study of the anti-CD19 antibody drug conjugate coltuximab ravtansine (SAR3419) in patients with relapsed or refractory diffuse large B-cell lymphoma previously treated with rituximab-based immunotherapy. Haematologica 2018;103:1351-8. [Crossref] [PubMed]
- Coiffier B, Thieblemont C, de Guibert S, et al. A phase II, single-arm, multicentre study of coltuximab ravtansine (SAR3419) and rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. Br J Haematol 2016;173:722-30. [Crossref] [PubMed]
- Moskowitz C, Fanale M, Shah B, et al. A Phase 1 Study of Denintuzumab Mafodotin (SGN-CD19A) in Relapsed/Refactory B-Lineage Non-Hodgkin Lymphoma. Blood 2015;S1:182. [Crossref]
- Radford J, Kahl B, Hamada H, et al. Interim Results from the First-in-Human Clinical Trial of Adct-402 (Loncastuximab Tesirine), a Novel Pyrrolobenzodiazepine-Based Antibody Drug Conjugate, in Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood 2018;S1:398. [Crossref]
- Dunleavy K, Pittaluga S, Czuczman MS, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood 2009;113:6069-76. [Crossref] [PubMed]
- Flinn IW, Bartlett NL, Blum KA, et al. A phase II trial to evaluate the efficacy of fostamatinib in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). Eur J Cancer 2016;54:11-7. [Crossref] [PubMed]
- Lenz G, Hawkes EA, Verhoef G, et al. Phase II study of single-agent copanlisib in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). J Clin Oncol 2017;35:7536. [Crossref]
- Younes A, Salles G, Martinelli G, et al. Pan-phosphatidylinositol 3-kinase inhibition with buparlisib in patients with relapsed or refractory non-Hodgkin lymphoma. Haematologica 2017;102:2104-12. [Crossref] [PubMed]
- Barnes JA, Jacobsen E, Feng Y, et al. Everolimus in combination with rituximab induces complete responses in heavily pretreated diffuse large B-cell lymphoma. Haematologica 2013;98:615-9. [Crossref] [PubMed]
- Smith SM, van Besien K, Karrison T, et al. Temsirolimus has activity in non-mantle cell non-Hodgkin's lymphoma subtypes: The University of Chicago phase II consortium. J Clin Oncol 2010;28:4740-6. [Crossref] [PubMed]
- Brown JR, Hamadani M, Hayslip J, et al. Voxtalisib (XL765) in patients with relapsed or refractory non-Hodgkin lymphoma or chronic lymphocytic leukaemia: an open-label, phase 2 trial. Lancet Haematol 2018;5:e170-80. [Crossref] [PubMed]
- Kuruvilla J, Savona M, Baz R, et al. Selective inhibition of nuclear export with selinexor in patients with non-Hodgkin lymphoma. Blood 2017;129:3175-83. [Crossref] [PubMed]
- Davids MS, Roberts AW, Seymour JF, et al. Phase I First-in-Human Study of Venetoclax in Patients With Relapsed or Refractory Non-Hodgkin Lymphoma. J Clin Oncol 2017;35:826-33. [Crossref] [PubMed]
- Caimi P, Jagadeesh D, Boughan K, et al. Safety and Efficacy of Venetoclax Combined with Rituximab, Ifosfamide, Carboplatin and Etoposide Chemoimmunotherapy (VICER) for Treatment of Relapsed Diffuse Large B Cell Lymphoma: Results from the Phase 1 Study. Blood 2018;132:397. [Crossref]
- Witzig TE, Vose JM, Zinzani PL, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin's lymphoma. Ann Oncol 2011;22:1622-7. [Crossref] [PubMed]
- Cheson BD, Crawford J. A phase I study of bendamustine, lenalidomide and rituximab in relapsed and refractory lymphomas. Br J Haematol 2015;169:528-33. [Crossref] [PubMed]
- Martín A, Redondo AM, Dlouhy I, et al. Lenalidomide in combination with R-ESHAP in patients with relapsed or refractory diffuse large B-cell lymphoma: a phase 1b study from GELTAMO group. Br J Haematol 2016;173:245-52. [Crossref] [PubMed]
- Feldman T, Mato AR, Chow KF, et al. Addition of lenalidomide to rituximab, ifosfamide, carboplatin, etoposide (RICER) in first-relapse/primary refractory diffuse large B-cell lymphoma. Br J Haematol 2014;166:77-83. [Crossref] [PubMed]
- Ramchandren R, Johnson P, Ghosh N, et al. The iR2 Regimen (Ibrutinib, Lenalidomide, and Rituximab) Is Active with a Manageable Safety Profile in Patients with Relapsed/Refractory Non-Germinal Center-like Diffuse Large B-Cell Lymphoma. Blood 2018;132:402. [Crossref]
- Ansell SM, Minnema MC, Johnson P, et al. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J Clin Oncol 2019;37:481-9. [Crossref] [PubMed]
- Advani R, Flinn I, Popplewell L, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin's Lymphoma. N Engl J Med 2018;379:1711-21. [Crossref] [PubMed]
- Viardot A, Goebeler M-E, Hess G, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 2016;127:1410-6. [Crossref] [PubMed]
- Hutchings M, Iacoboni G, Morschhauser F, et al. CD20-Tcb (RG6026), a Novel "2:1" Format T-Cell-Engaging Bispecific Antibody, Induces Complete Remissions in Relapsed/Refractory B-Cell Non-Hodgkin's Lymphoma: Preliminary Results from a Phase I First in Human Trial. Blood 2018;132:226. [Crossref]
- Budde L, Sehn L, Assouline S, et al. Mosunetuzumab, a Full-Length Bispecific CD20/CD3 Antibody, Displays Clinical Activity in Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma (NHL): Interim Safety and Efficacy Results from a Phase 1 Study. Blood 2018;132.
- Morschauser F, Salles G, McKay P, et al. Interim report from a phase 2 multicenter study of Tazemetostat, an EZH2 inhibitor, in patients with relapseed or refractory B-cell non-hodgkin lymphomas. Hematol Oncol 2017;35:24-5. [Crossref]
- Assouline SE, Nielsen TH, Yu S, et al. Phase 2 study of panobinostat with or without rituximab in relapsed diffuse large B-cell lymphoma. Blood 2016;128:185-94. [Crossref] [PubMed]
- Oki Y, Kelly KR, Flinn I, et al. CUDC-907 in relapsed/refractory diffuse large B-cell lymphoma, including patients with MYC-alterations: results from an expanded phase I trial. Haematologica 2017;102:1923-30. [Crossref] [PubMed]
- Vitolo U, Trneny M, Belada D, et al. Obinutuzumab or Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Previously Untreated Diffuse Large B-Cell Lymphoma. J Clin Oncol 2017;35:3529-37. [Crossref] [PubMed]
- Bartlett NL, Smith MR, Siddiqi T, et al. Brentuximab vedotin activity in diffuse large B-cell lymphoma with CD30 undetectable by visual assessment of conventional immunohistochemistry. Leuk Lymphoma 2017;58:1607-16. [Crossref] [PubMed]
- Rawlings DJ, Schwartz MA, Jackson SW, et al. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat Rev Immunol 2012;12:282-94. [Crossref] [PubMed]
- Rawlings DJ, Sommer K, Moreno-Garcia ME. The CARMA1 signalosome links the signalling machinery of adaptive and innate immunity in lymphocytes. Nat Rev Immunol 2006;6:799-812. [Crossref] [PubMed]
- Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010;463:88-92. [Crossref] [PubMed]
- Compagno M, Lim WK, Grunn A, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature 2009;459:717-21. [Crossref] [PubMed]
- Davis RE, Brown KD, Siebenlist U, et al. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med 2001;194:1861-74. [Crossref] [PubMed]
- Leonard JP, Kolibaba KS, Reeves JA, et al. Randomized Phase II Study of R-CHOP With or Without Bortezomib in Previously Untreated Patients With Non-Germinal Center B-Cell-Like Diffuse Large B-Cell Lymphoma. J Clin Oncol 2017;35:3538-46. [Crossref] [PubMed]
- Davies A, Barrans S, Maishman T. Differential Efficacy of Bortezomib In Subtypes of Diffuse Large B‐Cell Lymphoma (DLBL): A Prospective Randomised Study Stratified By Transcriptome Profiling: Remodl‐B. Hematol Oncol 2017;35:130-1. [Crossref]
- Gu JJ, Hernandez-Ilizaliturri FJ, Kaufman GP, et al. The novel proteasome inhibitor carfilzomib induces cell cycle arrest, apoptosis and potentiates the anti-tumour activity of chemotherapy in rituximab-resistant lymphoma. Br J Haematol 2013;162:657-69. [Crossref] [PubMed]
- Dasmahapatra G, Lembersky D, Kramer L, et al. The pan-HDAC inhibitor vorinostat potentiates the activity of the proteasome inhibitor carfilzomib in human DLBCL cells In vitro and In vivo. Blood 2010;115:4478-87. [Crossref] [PubMed]
- Dasmahapatra G, Lembersky D, Son MP, et al. Obatoclax interacts synergistically with the irreversible proteasome inhibitor carfilzomib in GC- and ABC-DLBCL cells In vitro and In vivo. Mol Cancer Ther 2012;11:1122-32. [Crossref] [PubMed]
- Brown JR, Moslehi J, O'Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica 2017;102:1796-805. [Crossref] [PubMed]
- Maddocks K, Christian B, Jaglowski S, et al. A phase 1/1b study of rituximab, bendamustine, and ibrutinib in patients with untreated and relapsed/refractory non-Hodgkin lymphoma. Blood 2015;125:242-8. [Crossref] [PubMed]
- Younes A, Sehn L, Johnson P, et al. Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2019;37:1285-95. [Crossref] [PubMed]
- Chen JG, Liu X, Munshi M, et al. BTKCys481Ser drives ibrutinib resistance via ERK1/2, and protects BTKWild-Type MYD88 mutated cells by a paracrine mechanism. Blood 2018;131:2047-59. [Crossref] [PubMed]
- Byrd JC, Smith S, Wagner-Johnston N, et al. First-in-human phase 1 study of the BTK inhibitor GDC-0853 in relapsed or refractory B-cell NHL and CLL. Oncotarget 2018;9:13023-35. [Crossref] [PubMed]
- Walter HS, Rule SA, Dyer MJS, et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood 2016;127:411-9. [Crossref] [PubMed]
- Byrd JC, Harrington B, O'Brien S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med 2016;374:323-32. [Crossref] [PubMed]
- Chen L, Juszczynski P, Takeyama K, et al. Protein tyrosine phosphatase receptor-type O truncated (PTPROt) regulates SYK phosphorylation, proximal B-cell-receptor signaling, and cellular proliferation. Blood 2006;108:3428-33. [Crossref] [PubMed]
- Chen L, Monti S, Juszczynski P, et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood 2008;111:2230-7. [Crossref] [PubMed]
- Sharman J, Hawkins M, Kolibaba K, et al. An open-label phase 2 trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in chronic lymphocytic leukemia. Blood 2015;125:2336-43. [Crossref] [PubMed]
- Pfeifer M, Grau M, Lenze D, et al. PTEN loss defines a PI3K/AKT pathway-dependent germinal center subtype of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A 2013;110:12420-5. [Crossref] [PubMed]
- Miller BW, Przepiorka D, de Claro RA, et al. FDA approval: idelalisib monotherapy for the treatment of patients with follicular lymphoma and small lymphocytic lymphoma. Clin Cancer Res 2015;21:1525-9. [Crossref] [PubMed]
- Yahiaoui A, Meadows SA, Sorensen RA, et al. PI3Kdelta inhibitor idelalisib in combination with BTK inhibitor ONO/GS-4059 in diffuse large B cell lymphoma with acquired resistance to PI3Kdelta and BTK inhibitors. PloS one 2017;12:e0171221 [Crossref] [PubMed]
- Barr PM, Saylors GB, Spurgeon SE, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood 2016;127:2411-5. [Crossref] [PubMed]
- Smith SM, Pitcher BN, Jung SH, et al. Safety and tolerability of idelalisib, lenalidomide, and rituximab in relapsed and refractory lymphoma: the Alliance for Clinical Trials in Oncology A051201 and A051202 phase 1 trials. Lancet Haematol 2017;4:e176-82. [Crossref] [PubMed]
- Cheah CY, Nastoupil LJ, Neelapu SS, et al. Lenalidomide, idelalisib, and rituximab are unacceptably toxic in patients with relapsed/refractory indolent lymphoma. Blood 2015;125:3357-9. [Crossref] [PubMed]
- Burris HA 3rd, Flinn IW, Patel MR, et al. Umbralisib, a novel PI3Kdelta and casein kinase-1epsilon inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol 2018;19:486-96. [Crossref] [PubMed]
- Lunning MA, Vose J, Bierman P, et al. Combination study of TGT-1202, Ublituximab, and bendamustine is safe and highly active in patients with advanced DLBCL and follicular lymphoma. Hematol Oncol 2017;35:266-7. [Crossref]
- Wanner K, Hipp S, Oelsner M, et al. Mammalian target of rapamycin inhibition induces cell cycle arrest in diffuse large B cell lymphoma (DLBCL) cells and sensitises DLBCL cells to rituximab. Br J Haematol 2006;134:475-84. [Crossref] [PubMed]
- Witzig TE, LaPlant B, Habermann TM, et al. High rate of event-free survival at 24 months with everolimus/RCHOP for untreated diffuse large B-cell lymphoma: updated results from NCCTG N1085 (Alliance). Blood Cancer J 2017;7:e576 [Crossref] [PubMed]
- Maurer MJ, Ghesquieres H, Link BK, et al. Diagnosis-to-Treatment Interval Is an Important Clinical Factor in Newly Diagnosed Diffuse Large B-Cell Lymphoma and Has Implication for Bias in Clinical Trials. J Clin Oncol 2018;36:1603-10. [Crossref] [PubMed]
- Witzens-Harig M, Memmer ML, Dreyling M, et al. A phase I/II trial to evaluate the safety, feasibility and activity of salvage therapy consisting of the mTOR inhibitor Temsirolimus added to standard therapy of Rituximab and DHAP for the treatment of patients with relapsed or refractory diffuse large cell B-Cell lymphoma - the STORM trial. BMC Cancer 2013;13:308. [Crossref] [PubMed]
- Witzens M, Viardot A, Keller U, et al. Safety and clinical activity of Temsirolimus in combination with rituximab and DHAP in patients with relapsed or refractory diffuse large B-cell lymphoma-Report of the prospective, multicenter phase II STORM trial. Hematol Oncol 2016;35:191. [Crossref]
- Calò V, Migliavacca M, Bazan V, et al. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol 2003;197:157-68. [Crossref] [PubMed]
- Kishimoto T. Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol 2005;23:1-21. [Crossref] [PubMed]
- Yang J, Liao X, Agarwal MK, et al. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev 2007;21:1396-408. [Crossref] [PubMed]
- Lam LT, Wright G, Davis RE, et al. Cooperative signaling through the signal transducer and activator of transcription 3 and nuclear factor-{kappa}B pathways in subtypes of diffuse large B-cell lymphoma. Blood 2008;111:3701-13. [Crossref] [PubMed]
- Lu L, Zhu F, Zhang M, et al. Gene regulation and suppression of type I interferon signaling by STAT3 in diffuse large B cell lymphoma. Proc Natl Acad Sci U S A 2018;115:E498-505. [Crossref] [PubMed]
- Ma J, Xing W, Coffey G, et al. Cerdulatinib, a novel dual SYK/JAK kinase inhibitor, has broad anti-tumor activity in both ABC and GCB types of diffuse large B cell lymphoma. Oncotarget 2015;6:43881-96. [Crossref] [PubMed]
- Younes A, Romaguera J, Fanale M, et al. Phase I study of a novel oral Janus kinase 2 inhibitor, SB1518, in patients with relapsed lymphoma: evidence of clinical and biologic activity in multiple lymphoma subtypes. J Clin Oncol 2012;30:4161-7. [Crossref] [PubMed]
- Gravina GL, Senapedis W, McCauley D, et al. Nucleo-cytoplasmic transport as a therapeutic target of cancer. J Hematol Oncol 2014;7:85. [Crossref] [PubMed]
- Tan DSP, Bedard PL, Kuruvilla J, et al. Promising SINEs for embargoing nuclear-cytoplasmic export as an anticancer strategy. Cancer discovery 2014;4:527-37. [Crossref] [PubMed]
- Jardin F, Pujals A, Pelletier L, et al. Recurrent mutations of the exportin 1 gene (XPO1) and their impact on selective inhibitor of nuclear export compounds sensitivity in primary mediastinal B-cell lymphoma. Am J Hematol 2016;91:923-30. [Crossref] [PubMed]
- Iqbal J, Sanger WG, Horsman DE, et al. BCL2 Translocation Defines a Unique Tumor Subset within the Germinal Center B-Cell-Like Diffuse Large B-Cell Lymphoma. Am J Pathol 2004;165:159-66. [Crossref] [PubMed]
- Knittel G, Liedgens P, Korovkina D, et al. B-cell-specific conditional expression of Myd88p.L252P leads to the development of diffuse large B-cell lymphoma in mice. Blood 2016;127:2732-41. [Crossref] [PubMed]
- Yang Y, Shaffer AL 3rd, Emre NC, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell 2012;21:723-37. [Crossref] [PubMed]
- Zhang LH, Kosek J, Wang M, et al. Lenalidomide efficacy in activated B-cell-like subtype diffuse large B-cell lymphoma is dependent upon IRF4 and cereblon expression. Br J Haematol 2013;160:487-502. [Crossref] [PubMed]
- Cardesa-Salzmann TM, Colomo L, Gutierrez G, et al. High microvessel density determines a poor outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus chemotherapy. Haematologica 2011;96:996-1001. [Crossref] [PubMed]
- Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, et al. Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer 2011;117:5058-66. [Crossref] [PubMed]
- Czuczman MS, Trneny M, Davies A, et al. A Phase 2/3 Multicenter, Randomized, Open-Label Study to Compare the Efficacy and Safety of Lenalidomide Versus Investigator's Choice in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Clin Cancer Res 2017;23:4127-37. [Crossref] [PubMed]
- Ivanov V, Coso D, Chetaille B, et al. Efficacy and safety of lenalinomide combined with rituximab in patients with relapsed/refractory diffuse large B-cell lymphoma. Leuk Lymphoma 2014;55:2508-13. [Crossref] [PubMed]
- Ferreri AJM, Sassone M, Zaja F, et al. Lenalidomide maintenance in patients with relapsed diffuse large B-cell lymphoma who are not eligible for autologous stem cell transplantation: an open label, single-arm, multicentre phase 2 trial. Lancet Haematol 2017;4:e137-46. [Crossref] [PubMed]
- Vitolo U, Witzig TE, Gascoyne RD, et al. ROBUST: First report of phase III randomized study of lenalidomide/R‐CHOP (R2‐CHOP) vs placebo/R‐CHOP in previously untreated ABC‐type diffuse large B‐cell lymphoma. Hematol Oncol 2019. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/hon.5_2629
- Nowakowski GS, Hong F, Scott DW, et al. Addition of lenalidomideto R-CHOP (R2CHOP) improves outcomes in newly diagnosed diffuse large B-cell lymphoma (DLBCL): first report of ECOG-ACRIN1412 a randomized phase 2 US intergroup study of R2CHOP vs R-CHOP. Hematol Oncol 2019. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/hon.6_2629
- Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol 2013;31:4199-206. [Crossref] [PubMed]
- Goebeler ME, Knop S, Viardot A, et al. Bispecific T-Cell Engager (BiTE) Antibody Construct Blinatumomab for the Treatment of Patients With Relapsed/Refractory Non-Hodgkin Lymphoma: Final Results From a Phase I Study. J Clin Oncol 2016;34:1104-11. [Crossref] [PubMed]
- Coyle L, Morley N, Rambaldi A, et al. Open-Label, Phase 2 Study of Blinatumomab As Second Salvage Therapy in Adults with Relapsed/Refractory Aggressive B-Cell Non-Hodgkin Lymphoma. Blood 2018;400. [Crossref]
- Pasqualucci L, Dominguez-Sola D, Chiarenza A, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature 2011;471:189-95. [Crossref] [PubMed]
- Bödör C, Grossmann V, Popov N, et al. EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood 2013;122:3165-8. [Crossref] [PubMed]
- Velichutina I, Shaknovich R, Geng H, et al. EZH2-mediated epigenetic silencing in germinal center B cells contributes to proliferation and lymphomagenesis. Blood 2010;116:5247-55. [Crossref] [PubMed]
- Béguelin W, Popovic R, Teater M, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 2013;23:677-92. [Crossref] [PubMed]
- Brach D, Johnston-Blackwell D, Drew A, et al. EZH2 Inhibition by Tazemetostat Results in Altered Dependency on B-cell Activation Signaling in DLBCL. Mol Cancer Ther 2017;16:2586-97. [Crossref] [PubMed]
- Italiano A, Soria JC, Toulmonde M, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol 2018;19:649-59. [Crossref] [PubMed]
- Batlevi CL, Crump M, Andreadis C, et al. A phase 2 study of mocetinostat, a histone deacetylase inhibitor, in relapsed or refractory lymphoma. Br J Haematol 2017;178:434-41. [Crossref] [PubMed]
- Puvvada SD, Li H, Rimsza LM, et al. A phase II study of belinostat (PXD101) in relapsed and refractory aggressive B-cell lymphomas: SWOG S0520. Leuk Lymphoma 2016;57:2359-69. [Crossref] [PubMed]
- Younes A, Berdeja JG, Patel MR, et al. Safety, tolerability, and preliminary activity of CUDC-907, a first-in-class, oral, dual inhibitor of HDAC and PI3K, in patients with relapsed or refractory lymphoma or multiple myeloma: an open-label, dose-escalation, phase 1 trial. Lancet Oncol 2016;17:622-31. [Crossref] [PubMed]
Cite this article as: Sarkozy C, Sehn LH. New drugs for the management of relapsed or refractory diffuse large B-cell lymphoma. Ann Lymphoma 2019;3:10.


