
Risk stratification of mantle cell lymphoma (MCL)
Introduction
Mantle cell lymphoma (MCL) represents a mature B-cell lymphoma, which is characterized by the translocation t(11;14) leading to cyclin D1 overexpression and subsequently cell-cycle progression (1). MCL is typically associated with an aggressive course resulting in a dismal outcome. Historical studies report a median overall survival (OS) of only 3 to 5 years with conventional chemotherapy. The outcome has been significantly improved by intensified cytarabine-containing induction therapies followed by a consolidation treatment with high-dose chemotherapy and autologous stem cell transplantation (autoSCT) (2). Nevertheless, the clinical course of MCL patients is a very variable. There are patients who benefit from autoSCT for more than 10 years whereas others relapse within the first year after autoSCT (3). In addition, there is a subgroup of indolent MCL patients who can be followed with a watch and wait strategy without a need for treatment over years (4). Together these examples suggest a heterogeneous clinical course of MCL. Despite this heterogeneous disease course all patients with MCL are treated similar. A commonly used risk adopted strategy has been to treat patients with a poor outcome more aggressively than indolent patients. An exciting alternative strategy is to adopt the treatment to mechanistically validated genetic lesions. This approach has not yet entered the clinical routine, but holds great promise for future developments. Within this review we will focus on clinically validated prognostic factors and we will highlight individual examples of how molecular lesions could influence treatment decisions.
Clinical heterogeneity of MCL
Although most MCL patients suffer from an aggressive disease course, retrospective studies demonstrated that up to 30% of all MCL patients have a comparably indolent course even if they remain untreated (4). These patients usually present with a leukemic disease pattern and enlarged spleens, but rarely have significant nodal involvement (1,5,6). For a subset of these MCL patients a less aggressive treatment approach or even a watch and wait strategy could be justified (4). The updated WHO classification of 2016 acknowledges a subpopulation of leukemic non-nodal MCL patients, who typically present with an indolent disease course (1). However, a clear distinction of this subtype from classic MCL based on clinically validated parameters remains difficult. Some reports suggest SOX11 expression as a discriminating marker (6,7). However, two large cohort studies could not confirm its predictive and prognostic value (8,9). Thus, SOX11 should be interpreted with caution for risk stratification in MCL. The immunoglobulin heavy chain (IGHV) mutation status was evaluated as another potential marker for distinction. MCL patients with mutated IGHV gene region were more frequent in the subgroup of leukemic non-nodal MCL with a more indolent course (5), but the isolated predictive power of IGHV mutation status did not justify its use to reliably distinguish both subtypes. In a recent study, Clot et al. established a molecular assay using expression values of 16 genes to distinguish between the classic MCL and the leukemic non-nodal MCL subtype. However, further validation in larger patient cohorts are needed before this assay could be implemented in the clinical routine (10).
In the absence of an established scoring system, a combined clinical assessment of leukemic dissemination with involvement of the bone marrow, the absence of nodal involvement, an asymptomatic disease course as well as biologic and genetic features such as IGHV status, low Ki-67 index and the absence of genetic/chromosomal lesions with poor prognostic impact should be considered to identify this distinct subgroup of MCL patients with a more indolent disease course.
Current standard of risk stratification
More than two decades ago, the first prognostic score for aggressive B-cell lymphomas, the international prognostic index (IPI), was developed. A broad collection of basic clinical features was evaluated and the five most relevant prognostic makers were selected to be included in the IPI score: Ann Arbor stage, age, performance status, lactate dehydrogenase (LDH) and more than two extranodal sites. Given the success of the IPI index, additional entity specific prognostic indices such as the Follicular Lymphoma International Prognostic Index (FLIPI), were established. Hoster and colleagues analyzed pooled data from three prospective trials, GLSG1996, GLSG2000 and European MCL Trial 1, to develop an MCL specific risk score (11). As a first step they investigated if the IPI and the FLIPI are valuable prognostic scoring systems in this large cohort of 455 MCL patients. Neither the IPI nor the FLIPI could reliably distinguish between different risk groups, but the three IPI relevant risk factors: age, performance status and LDH remained independent prognostic markers for MCL in a multivariate cox regression analysis. In order to develop an MCL-specific prognostic index, numerous other parameters (patient characteristics, tumor size and location, laboratory findings) were considered but their prognostic relevance could not be confirmed in multivariate cox regression analysis. Only the leucocyte count could be confirmed as an additional prognostic marker in MCL. Thus, Hoster et al. selected these four easily available parameters (age, performance status, LDH and leucocyte count, web-based calculator: http://www.european-mcl.net/de/clinical_mipi.php) and established the MIPI score (11). Herewith, patients can be stratified into three different risk groups (low, intermediate, high). In the pooled cohort the OS was 51 and 29 months for the intermediate and high-risk group, respectively. In the low-risk group median OS was not reached. However, this index was derived from a patient cohort which was heterogeneously treated comprising only few patients who received an autoSCT. Therefore, Hoster et al. confirmed the prognostic value of the MIPI in a cohort of 958 patients who received a standardized treatment in the MCL Younger and MCL Elderly trial (12).
The MIPI is easy to implement in the clinical routine and severs as a valid prognostic score, but the complex biological heterogeneity of MCL might not be entirely reflected by this prognostic score. In order to further improve the prognostic power of the MIPI score, Hoster and colleagues analyzed the prognostic value of the MCL growth pattern (diffuse growth pattern versus mantle-zone or nodular growth), cytology (classical MCL versus blastoid variant) and the percentage Ki-67 positive cells as a surrogate for cell proliferation. The growth pattern was not found to be prognostically relevant. In contrast, blastoid cytology was associated with a more aggressive course and poor outcome, but multivariate analysis revealed a strong collinearity of blastoid cytology and high percentages of Ki-67 positive cells. Multivariate analysis revealed Ki-67 as the strongest independent biologic prognostic parameter in addition to the MIPI score. However, if blastoid histology is the only available prognostic feature, it could be considered for risk stratification as there is a high probability that multiple adverse markers (high Ki-67, TP53 mutation, complex karyotype) are associated with this morphological feature.
Hoster et al. refined the MIPI score and combined the conventional MIPI score with Ki-67 as the combined MIPI (MIPI-c) score. For the purpose of the MIPI-c score percentages of Ki-67 positive cells were dichotomized (≤ or ≥30%). It was shown that a Ki-67 ≥30% was associated with a significant worse prognosis, whereas further cutoffs below 30% did not improve the prognostic value. The MIPI-c score was finally validated in the European MCL Younger and MCL Elderly trial cohorts (13).
However, there are considerable weaknesses of the MIPI as a prognostic score. For instance, the elevated leucocyte count in the leukemic non-nodal subgroup could lead to an overestimation of the risk of progression and death in these good risk patients. The clinical diversity and the growing number of therapeutic options for MCL, justifies the need of further improvement of prognostic and potentially predictive clinical scoring systems.
The prognostic impact of recurrent chromosomal aberrations
The translocation t(11;14) is the hallmark lesion of MCL resulting in an overexpression of cyclin D1. As cyclin D1 overexpression can be found in virtually all MCL cases, it is established as the disease defining aberration. The cyclin D1 gene (CCND1) is localized on chromosome 11q13. CCND1 is juxtaposed to the IG heavy chain complex at chromosome 14q32, which causes activation of the CCND1 promoter. The resulting cyclin D1 overexpression leads to dysregulation of the cell cycle at the G1/S phase transition and thereby drives cell proliferation (14). Although the translocation t(11;14) seems to be the first step in MCL tumorigenesis, secondary lesions are proposed to increase the oncogenic potential of cyclin D1 overexpression and could provide prognostic impact.
Detailed cytogenetic analysis of structural aberrations in MCL revealed the following recurrent chromosomal lesions: del(13q), del(9p), del(9q), del(17p), del(6q), del(1p), del(10p), del(10q), del(Y), del(12p), del(1q), tri(3q) (15). Although single lesions investigated in this study did not show any prognostic impact, a complex karyotype (defined as ≥3 unrelated single aberrations) was found to be an important prognostic marker in MCL [hazard ratio (HR) 2.37] (15) independent of treatment intensity (16).
Hartmann et al. analyzed copy number alterations (CNA) in MCL by single-nucleotide polymorphism arrays and correlated them with clinical data (17). In this study several CNAs associated with an unfavorable prognosis were found: loss 9p, double loss 9p21.3, loss 1q32, double loss 1p32.3/33, amplification 12q14, double loss 2q13. However, no multivariate analysis was performed in this study to validate and rank the prognostic impact of the identified lesions (17).
In a PCR-based CNA study reported by Delfau-Larue and colleagues, deletions of the 13q14 locus, RB1, CDKN2A, TP53 and CDKN1B were associated with a poorer outcome (18). In multivariate analysis, only deletions of CDKN2A (HR 2.3) and TP53 (HR 2.4) were confirmed to have a prognostic impact. CDKN2A (locus 9p21) encodes for the CDK4/6 inhibitor INK4a (p16) and the TP53 activator ARF (p14). Deletion of CDKN2A is considered to foster MCL progression. Even high-dose ARA-C did not improve outcomes in these patients (18). Several studies investigated the prognostic impact of TP53/17p deletion in MCL but data are not conclusive so far (18-22). In the pooled data of the MCL2 and MCL3 trial, TP53 deletions had a prognostic impact in the univariate analysis. However, the TP53 mutations often co-occur with TP53 deletions and therefore both features were co-linear if used together in multivariate cox regression analysis. Only TP53 mutations remained significant in the final multivariate cox regression model (23).
The prognostic impact of recurrent single nucleotide variants
Several studies have identified recurrent mutations in MCL. Genes affecting cell cycle regulation (e.g., TP53, ATM, RB1, POT1 and CCND1), the epigenetic machinery (WHSC1, MLL2 and SMARCA4), cell adhesion (BIRC3, DLC1 and ROBO2) or developmental processes (NOTCH1, CCND1) were found to be frequently mutated in MCL (24,25).
Mutations in TP53 confer a poor prognosis across many tumor types, including MCL (26-28). The dismal outcome of TP53 mutated patients is illustrated by an OS of 1.8 versus 12.7 years (HR 6.2) for TP53 mutated versus unmutated MCL patients, respectively (23). This poor prognostic impact of mutated TP53 persists in patients treated with high-dose ARA-C and autoSCT. Stefancikova and colleagues demonstrated a strong association between TP53 mutation status and TP53 expression as assessed by immunohistochemistry (29). The underlying mechanism why mutated p53 accumulates in lymphoma was linked to the transformation/transcription domain-associated protein (TRRAP), which prevents the natural degradation of p53 (30). The use of p53 expression as a prognostic marker was further validated in a large cohort of MCL patients and was found to be independent of the MIPI score and Ki-67 (8). Summarized, assessment of TP53 mutation—rather than the TP53/17p deletion status should be integrated in the clinical routine. Further prognostically relevant mutations appear in the NOTCH signaling pathway. Whereas NOTCH1 mutations exclusively occur in chronic lymphatic leukemia (CLL) and NOTCH2 mutations in splenic marginal zone lymphomas, both genes have been described to be recurrently mutated in MCL (24). One first retrospective study could identify NOTCH1 mutations as an independent poor prognostic marker (OS: HR 2.82, progression-free survival (PFS): HR 2.07) (31), but further validation of the prognostic value of NOTCH1 is needed.
Although important genetic and chromosomal lesions with poor prognostic impact could be identified, comprehensive multivariate analyses, including single nucleotide alterations, chromosomal aberrations as well as clinical parameters are not yet available.
Response assessment as a prognostic parameter
Assuming that the persistence of MCL post-treatment has a prognostic relevance, the roles of post-treatment positron emission tomography (PET) and minimal residual disease (MRD) burden were studied in MCL. PET-evaluation can be a useful diagnostic tool for response assessment. However, its prognostic value for the outcome of treated MCL patients is less clear. Several studies found an association between post-treatment PET results and subsequent outcome (32-34). However, other analyses showed no prognostic value for post-treatment PET (35). Assessment of MRD is a diagnostic approach to detect remaining circulating MCL cells. Pott et al. demonstrated that MRD negativity can be a strong predictive marker for outcome in patients treated with high dose chemotherapy and autoSCT. The PFS was 92 months in the MRD-negative group and 21 months in the MRD-positive group. The median OS was 44 months in the MRD-positive group, whereas the OS has not been reached in the MRD-negative group (36). The predictive value of MRD negativity was confirmed in the MCL Younger and MCL Elderly trial of the European MCL network (37). MRD testing will be discussed separately in this series of MCL related reviews.
Prognostic parameters in relapsed patients
Most parameters for risk stratification have been validated for front line patients, but prognostic parameters for relapsed patients are scarce. The outcome of MCL patients with relapse after autoSCT is generally poor. In a large retrospective EBMT study 40% of patients relapsing after autoSCT were refractory to salvage treatment, whereas only 8% of the same cohort were refractory to first-line chemotherapy treatment (3). This observation indicates that resistant MCL clones quickly evolve during chemotherapy treatment. Therefore, we always recommend to re-biopsy relapsed MCL patients. Only few studies focused on prognostic markers for relapsed MCL patients. Dietrich et al. analyzed clinical parameters with prognostic impact for OS in relapsed patients after first autoSCT. The time to relapse after autoSCT (<12 versus >12 months) was identified as the most relevant marker for outcome after relapse (HR 0.62). This study further suggests that patients with a short remission interval after autoSCT (<12 months) might not benefit from an allogeneic stem cell transplantation (alloSCT). Further prognostic factors were primary refractory disease (HR 1.92) and prior high-dose ARA-C treatment (HR 1.43) (3).
Despite the generally dismal outcome of relapsed MCL patients, individual relapsed patients exhibit a long-term outcome. A retrospective study of 118 MCL patients, who underwent autoSCT, could identify a small subset of four patients who lived for more than 5 years after relapse although they did not receive intensive salvage treatment. All four patients had a long recurrence-free period after autoSCT (38) and the percentage of Ki-67 positive cells was as low as 5% in one of these patients. This underlines the biological heterogeneity of MCL not only at diagnosis but also in the relapsed situation. However, there is a lack of markers which could identify these patients.
The response duration after frontline treatment and eventually the percentage of Ki-67 positive cells in relapse MCL biopsies could be considered as prognostic markers for relapsed MCL patients. To our knowledge, there are no studies focusing on prognostic markers in relapsed patients who did not receive intensive frontline therapy with autoSCT. Thus, further studies in larger cohorts are necessary, to extend our knowledge about prognostic markers in relapsed MCL patients.
Predictive markers for targeted therapies
In the context of targeted treatments, drug specific resistance mechanisms emerge and play an important role for targeted drug resistance. MCL patients with such mutations would not benefit from a specific treatment. Gene mutations can pre-exist and lead to an apriori resistance or can be acquired during treatment and foster clonal evolution and drug resistance. For some novel agents, potential resistance markers were identified in preclinical studies, but not all were clinically validated yet.
Ibrutinib is the best-studied small molecule in MCL. In CLL, acquired resistance mutations for ibrutinib were described for the first time. A mutation of the Bruton’s tyrosine kinase (BTK) gene (C481S mutation) was identified to induce resistance by altering the binding of ibrutinib. Furthermore, gain-of-function mutations in the PLCγ2 (R665W and L845F) directly downstream of BTK lead to an autonomous B-cell receptor activity in CLL (39). Similar mutations could be found in ibrutinib resistant MCL patients (40). Another mechanism for ibrutinib resistance represents the bypass of classical nuclear factor κB (NF-κB) signaling through activating mutations of the alternative NF-κB pathway. Activating mutations of the alternative NF-κB were found in TRAF2, BIRC3 and MAP3K14 (NIK) (41). These mutations preexist and could cause apriori drug resistance. In-vitro studies in MCL revealed CARD11 mutations as a further potential mechanism of primary ibrutinib resistance. CARD11 mutations occur in approximately 5% of MCL patients independent of newly diagnosed or relapsed disease state (42). An activating mutation of CARD11 can lead to a chronic activation of the B-cell receptor (BCR) and drive classical NF-κB activation. Due to the fact, that ibrutinib targets BTK, which lies upstream of CARD11, lymphoma cells with a CARD11 mutation are expected to be insensitive to ibrutinib.
Another important small molecule for treatment of MCL is the Bcl-2 inhibitor venetoclax. Recently, a study described genetic aberrations (BTG1 mutation, homozygous deletion CDKN2A/B, BRAF mutation, amplification of CD274) in venetoclax resistant CLL patients (43). The predictive impact of these alterations and the relevance for MCL patients is not clear yet.
Apart from genetic mutations, one study described an association between the response to the combination treatment with ibrutinib and rituximab and the percentage of Ki-67 positive MCL cells in relapsed or refractory MCL patients. Whereas all patients with a Ki-67 below 50% achieved objective responses, patients with a Ki-67 over 50% showed only stable disease or even progressive disease (44).
With the increasing use of targeted therapies, a better understanding of the mutational landscape conferring treatment resistance is needed. Clinical studies are necessary to validate such alterations as prognostic treatment specific markers.
Risk adopted therapy
The heterogeneity of disease courses justifies the development of risk adopted treatment approaches in MCL (45). Based on the above discussed considerations we propose a risk-stratified treatment algorithm for newly diagnosed, transplant-eligible MCL patients (Figure 1). A combined assessment of clinical and biological parameter could be used to identify the small proportion of MCL patients with an indolent course. Leukemic and non-nodal presentation or a low-risk MIPI score might justify a watch and wait strategy and surveillance every 3 months. Of note, the MIPI does not always reliably identify this subgroup of MCL patients as it can be disproportionately influenced by single parameters like white blood cell count and age. If treatment has to be initiated for these low-risk patients, we propose an immunochemotherapy treatment strategy omitting autoSCT. In low intermediate risk patients, post-induction treatment could be individualized on the basis of the MRD levels. For intermediate high- and high-risk patients we propose a consolidating autoSCT.
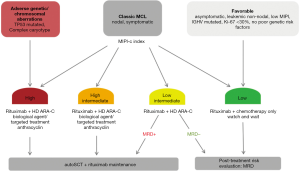
High-risk patients frequently harbor adverse chromosomal or genetic aberrations (e.g., complex karyotype, TP53 mutation). Especially in TP53 mutated patients, conventional chemotherapy might be less active. For these high-risk patients, we therefore propose to complement induction treatment by biological and targeted treatment approaches, which work independently of TP53 (46). Further intensification of induction treatment with doxorubicin might be useful for selected very high-risk patients.
For older and transplant-ineligible patients, individualized therapeutic approaches which consider performance status and co-morbidities in addition to the disease risk are needed. Bendamustine plus rituximab and R-CHOP followed by rituximab maintenance or replacing the vincristine with bortezomib are potential options for transplant-ineligible patients.
For relapsed MCL patients, risk adopted treatment strategies are even more difficult to establish. For young and fit patients who received a frontline autoSCT, we propose a risk adopted treatment strategy as outlined in Figure 2. Patients with early relapse or primary refractory disease should be considered for clinical studies [e.g., chimeric antigen receptor (CAR) T-cell therapy therapies]. We suggest to consider alloSCT or haplo-SCT if a remission with e.g. novel agents can be obtained in this high-risk group of patients. For patients with a late relapse, the percentage of Ki-67 positive cells might be informative. A low Ki-67 index (<10%) may indicate a more indolent disease course and could justify a watch and wait approach after completed reinduction in individual cases. For patients with relapse later than 12 months after the first autoSCT, high Ki-67 levels and/or other adverse parameters consolidation with an alloSCT should be considered. For relapsed patients who had not been eligible for frontline transplantation, data regarding prognostic parameters is even scarcer.
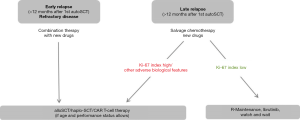
In conclusion, treatment of MCL remains challenging and further prospective trials for risk stratification are warranted. Whenever possible, both clinical and biological risk parameters should be assessed in the therapeutic management of MCL.
Acknowledgments
S Dietrich was supported by a grant of the Heidelberg Research Centre for Molecular Medicine (HRCMM), a BMBF junior group grant by the BMBF within the e:Med initiative and the Hairy Cell Leukemia Foundation.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Lymphoma for the series “Future Directions for Mantle Cell Lymphoma”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol.2018.12.03). The series “Future Directions for Mantle Cell Lymphoma” was commissioned by the editorial office without any funding or sponsorship. Martin Dreyling served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Hermine O, Hoster E, Walewski J, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet 2016;388:565-75. [Crossref] [PubMed]
- Dietrich S, Boumendil A, Finel H, et al. Outcome and prognostic factors in patients with mantle-cell lymphoma relapsing after autologous stem-cell transplantation: a retrospective study of the European Group for Blood and Marrow Transplantation (EBMT). Ann Oncol 2014;25:1053-8. [Crossref] [PubMed]
- Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol 2009;27:1209-13. [Crossref] [PubMed]
- Orchard J, Garand R, Davis Z, et al. A subset of t(11;14) lymphoma with mantle cell features displays mutated IgVH genes and includes patients with good prognosis, nonnodal disease. Blood 2003;101:4975-81. [Crossref] [PubMed]
- Fernàndez V , Salamero O, Espinet B, et al. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer Res 2010;70:1408-18. [Crossref] [PubMed]
- Royo C, Navarro A, Clot G, et al. Non-nodal type of mantle cell lymphoma is a specific biological and clinical subgroup of the disease. Leukemia 2012;26:1895-8. [Crossref] [PubMed]
- Aukema SM, Hoster E, Rosenwald A, et al. Expression of TP53 is associated with the outcome of MCL independent of MIPI and Ki-67 in trials of the European MCL Network. Blood 2018;131:417-20. [Crossref] [PubMed]
- Nygren L, Baumgartner Wennerholm S, Klimkowska M, et al. Prognostic role of SOX11 in a population-based cohort of mantle cell lymphoma. Blood 2012;119:4215-23. [Crossref] [PubMed]
- Clot G, Jares P, Gine E, et al. A gene signature that distinguishes conventional and leukemic nonnodal mantle cell lymphoma helps predict outcome. Blood 2018;132:413-22. [Crossref] [PubMed]
- Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 2008;111:558-65. [Crossref] [PubMed]
- Hoster E, Klapper W, Hermine O, et al. Confirmation of the mantle-cell lymphoma International Prognostic Index in randomized trials of the European Mantle-Cell Lymphoma Network. J Clin Oncol 2014;32:1338-46. [Crossref] [PubMed]
- Hoster E, Rosenwald A, Berger F, et al. Prognostic Value of Ki-67 Index, Cytology, and Growth Pattern in Mantle-Cell Lymphoma: Results From Randomized Trials of the European Mantle Cell Lymphoma Network. J Clin Oncol 2016;34:1386-94. [Crossref] [PubMed]
- Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nat Rev Cancer 2007;7:750-62. [Crossref] [PubMed]
- Sarkozy C, Terre C, Jardin F, et al. Complex karyotype in mantle cell lymphoma is a strong prognostic factor for the time to treatment and overall survival, independent of the MCL international prognostic index. Genes Chromosomes Cancer 2014;53:106-16. [Crossref] [PubMed]
- Greenwell IB, Staton AD, Lee MJ, et al. Complex karyotype in patients with mantle cell lymphoma predicts inferior survival and poor response to intensive induction therapy. Cancer 2018;124:2306-15. [Crossref] [PubMed]
- Hartmann EM, Campo E, Wright G, et al. Pathway discovery in mantle cell lymphoma by integrated analysis of high-resolution gene expression and copy number profiling. Blood 2010;116:953-61. [Crossref] [PubMed]
- Delfau-Larue MH, Klapper W, Berger F, et al. High-dose cytarabine does not overcome the adverse prognostic value of CDKN2A and TP53 deletions in mantle cell lymphoma. Blood 2015;126:604-11. [Crossref] [PubMed]
- Espinet B, Salaverria I, Bea S, et al. Incidence and prognostic impact of secondary cytogenetic aberrations in a series of 145 patients with mantle cell lymphoma. Genes Chromosomes Cancer 2010;49:439-51. [PubMed]
- Halldórsdóttir AM , Lundin A, Murray F, et al. Impact of TP53 mutation and 17p deletion in mantle cell lymphoma. Leukemia 2011;25:1904-8. [Crossref] [PubMed]
- Rosenwald A, Wright G, Wiestner A, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell 2003;3:185-97. [Crossref] [PubMed]
- Rubio-Moscardo F, Climent J, Siebert R, et al. Mantle-cell lymphoma genotypes identified with CGH to BAC microarrays define a leukemic subgroup of disease and predict patient outcome. Blood 2005;105:4445-54. [Crossref] [PubMed]
- Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood 2017;130:1903-10. [Crossref] [PubMed]
- Beà S , Valdes-Mas R, Navarro A, et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci U S A 2013;110:18250-5. [Crossref] [PubMed]
- Zhang J, Jima D, Moffitt AB, et al. The genomic landscape of mantle cell lymphoma is related to the epigenetically determined chromatin state of normal B cells. Blood 2014;123:2988-96. [Crossref] [PubMed]
- Cheung KJ, Horsman DE, Gascoyne RD. The significance of TP53 in lymphoid malignancies: mutation prevalence, regulation, prognostic impact and potential as a therapeutic target. Br J Haematol 2009;146:257-69. [Crossref] [PubMed]
- Hollstein M, Sidransky D, Vogelstein B, et al. p53 mutations in human cancers. Science 1991;253:49-53. [Crossref] [PubMed]
- Vogelstein B. Cancer. A deadly inheritance. Nature 1990;348:681-2. [Crossref] [PubMed]
- Stefancikova L, Moulis M, Fabian P, et al. Loss of the p53 tumor suppressor activity is associated with negative prognosis of mantle cell lymphoma. Int J Oncol 2010;36:699-706. [PubMed]
- Jethwa A, Slabicki M, Hullein J, et al. TRRAP is essential for regulating the accumulation of mutant and wild-type p53 in lymphoma. Blood 2018;131:2789-802. [Crossref] [PubMed]
- Kridel R, Meissner B, Rogic S, et al. Whole transcriptome sequencing reveals recurrent NOTCH1 mutations in mantle cell lymphoma. Blood 2012;119:1963-71. [Crossref] [PubMed]
- Bodet-Milin C, Bailly C, Meignan M, et al. Predictive Power of FDG-PET Parameters at Diagnosis and after Induction in Patients with Mantle Cell Lymphoma, Interim Results from the LyMa-PET Project, Conducted on Behalf of the Lysa Group. Blood 2015;126:335.
- Cohen JB, Hall NC, Ruppert AS, et al. Association of pre-transplantation positron emission tomography/computed tomography and outcome in mantle cell lymphoma. Bone Marrow Transplant 2013;48:1212-7. [Crossref] [PubMed]
- Mato AR, Svoboda J, Feldman T, et al. Post-treatment (not interim) positron emission tomography-computed tomography scan status is highly predictive of outcome in mantle cell lymphoma patients treated with R-HyperCVAD. Cancer 2012;118:3565-70. [Crossref] [PubMed]
- Kedmi M, Avivi I, Ribakovsky E, et al. Is there a role for therapy response assessment with 2-[fluorine-18] fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in mantle cell lymphoma? Leuk Lymphoma 2014;55:2484-9. [Crossref] [PubMed]
- Pott C, Schrader C, Gesk S, et al. Quantitative assessment of molecular remission after high-dose therapy with autologous stem cell transplantation predicts long-term remission in mantle cell lymphoma. Blood 2006;107:2271-8. [Crossref] [PubMed]
- Pott C, Hoster E, Delfau-Larue MH, et al. Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: a European MCL intergroup study. Blood 2010;115:3215-23. [Crossref] [PubMed]
- Dietrich S, Tielesch B, Rieger M, et al. Patterns and outcome of relapse after autologous stem cell transplantation for mantle cell lymphoma. Cancer 2011;117:1901-10. [Crossref] [PubMed]
- Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med 2014;370:2286-94. [Crossref] [PubMed]
- Chiron D, Di Liberto M, Martin P, et al. Cell-cycle reprogramming for PI3K inhibition overrides a relapse-specific C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Discov 2014;4:1022-35. [Crossref] [PubMed]
- Rahal R, Frick M, Romero R, et al. Pharmacological and genomic profiling identifies NF-kappaB-targeted treatment strategies for mantle cell lymphoma. Nat Med 2014;20:87-92. [Crossref] [PubMed]
- Wu C, de Miranda NF, Chen L, et al. Genetic heterogeneity in primary and relapsed mantle cell lymphomas: Impact of recurrent CARD11 mutations. Oncotarget 2016;7:38180-90. [PubMed]
- Herling CD, Abedpour N, Weiss J, et al. Clonal dynamics towards the development of venetoclax resistance in chronic lymphocytic leukemia. Nat Commun 2018;9:727. [Crossref] [PubMed]
- Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol 2016;17:48-56. [Crossref] [PubMed]
- Dreyling M, Ferrero SEuropean Mantle Cell Lymphoma Network. The role of targeted treatment in mantle cell lymphoma: is transplant dead or alive?. Haematologica 2016;101:104-14. [Crossref] [PubMed]
- Jerkeman M, Eskelund CW, Hutchings M, et al. Ibrutinib, lenalidomide, and rituximab in relapsed or refractory mantle cell lymphoma (PHILEMON): a multicentre, open-label, single-arm, phase 2 trial. Lancet Haematol 2018;5:e109-16. [Crossref] [PubMed]
Cite this article as: Liebers N, Dreger P, Dreyling M, Dietrich S. Risk stratification of mantle cell lymphoma (MCL). Ann Lymphoma 2018;2:10.


