
Scientific rationale for immunotherapy in lymphoma and predictors of response
Introduction
Lymphomas represent the first malignancy ever to be targeted with immunotherapy—in the 1990’s rituximab drastically changed treatment paradigms across all B-cell non-Hodgkin lymphomas (B-NHLs). However, a significant number of B-cell NHL patients will still relapse after first-line chemo-immunotherapy. In the relapsed setting for such patients, immunomodulatory agents like lenalidomide have been available. In relapsed/refractory Hodgkin lymphoma (HL), the Food and Drug Administration (FDA) approved the anti-CD30 antibody brentuximab vedotin, which has demonstrated some modulatory effects on the immune microenvironment. Unfortunately, despite the availability of such agents, the management of relapsed/refractory HL and NHL remains challenging.
Recently, a better understanding of the role of the immune system within the tumor microenvironment (TME) has advanced the field. Treatment strategies incorporating new immune therapies are emerging for both HL and NHL. Monoclonal antibodies that either block inhibitory signaling or co-stimulate anti-tumor immune effectors are being developed. Furthermore, combinatorial strategies to address these complementary mechanisms simultaneously for enhanced anti-tumor effect are being investigated with some success. There have also been exciting results with cellular therapies in B-NHL with consideration for expansion of this approach to T-cell NHL and HL.
We provide a comprehensive overview of passive immuno-oncologic strategies including monoclonal antibodies and cellular therapies, and active immunotherapeutic agents like vaccines, immune checkpoint molecules (inhibitors and stimulators), that are currently available or being developed for the treatment of lymphomas. We review possible potential predictors of response, and present the challenges associated with the utilization of immunotherapy agents including the ambiguity of predictive biomarkers, heterogeneity in responses seen with certain agents and limitations of available tools for the assessment of response to therapy.
Passive immunotherapy
Monoclonal antibodies
Rituximab
Rituximab, a chimeric IgG1 anti-CD 20 monoclonal antibody (mAb) functions to trigger antibody-dependent cell-mediated cytotoxicity (ADCC) by effector cells. It was the first immunotherapy agent to be utilized in cancer, receiving approval in the late 1990s based on efficacy in patients with relapsed or refractory low-grade or follicular lymphoma (FL) in non-comparative trials (1,2). Rituximab has since shown efficacy in patients with untreated advanced FL in combination with chemotherapy in several multicenter randomized trials (3-5). Similarly, in several randomized, open-label, multicenter trials in younger or elderly patients with previously untreated diffuse large B-cell lymphoma (DLBCL), clinical outcomes were significantly improved by the addition of rituximab to CHOP or CHOP-like chemotherapy (6-9). Rituximab serves as a prototype for the benefits of immunotherapy in cancer. The success of rituximab has driven the development of novel mAbs with the first approval of radio-immunotherapy agents in the 2000s and obinutuzumab most recently for the treatment of indolent lymphomas.
Obinutuzumab
Since the approval of rituximab, a new generation of anti-CD20 antibodies have been developed aimed at achieving improved efficacy. Obinutuzumab, a glyco-engineered type II anti-CD20 antibody, elicits enhanced ADCC and phagocytosis as compared with rituximab (10,11) with superiority demonstrated in pre-clinical studies. However, in a prospective, randomized study comparing safety and efficacy of obinutuzumab with rituximab in relapsed indolent lymphoma, a better overall response rate (ORR) with obinutuzumab did not translate into a progression-free survival (PFS) or overall survival (OS) advantage (12).
Despite these disappointing results in early clinical trials, more recent data suggest that there may be an advantage to using the antibody with chemotherapy. The phase III GALLIUM study reported results for 1,202 patients with previously untreated FL randomized to obinutuzumab versus rituximab with chemotherapy followed by maintenance (13). After a median follow-up of 34.5 months, there was a 34% reduction in the risk of progression or death in the obinutuzumab-chemotherapy arm relative to the rituximab-chemotherapy arm [hazard ratio (HR), 0.66; 95% CI, 0.51–0.85; P=0.001]. The obinutuzumab-chemotherapy arm induced rapid and more effective tumor cell clearance with increased depth of response in peripheral blood and bone marrow (14). Similarly, the obinutuzumab-bendamustine combination demonstrated an improvement in OS in a rituximab refractory population of iNHL (15,16), an approach however, made challenging by the increasing use of bendamustine in the front-line setting. It is noteworthy that obinutuzumab with chemotherapy in the upfront treatment of aggressive lymphoma has not demonstrated such success (17). By contrast, the phase III, randomized, open-label CLL 11 trial established obinutuzumab with or without chlorambucil as an appropriate treatment for chronic lymphocytic leukemia (CLL) in both the elderly and those with comorbidities lacking deletion 17p by demonstrating measurable impact of the mAb on OS. Obinutuzumab plus chlorambucil increased the ORR, prolonged PFS and OS (HR 0.41; 95% CI, 0.23–0.74; P=0.002) when compared with chlorambucil alone. The combination also resulted in a higher rate of negative testing for minimal residual disease in peripheral blood (37.7% versus 3.3%) when compared with rituximab plus chlorambucil (18).
Ofatumumab and other anti-CD20 monoclonal antibodies
The safety and efficacy of other anti-CD20 mAbs such as veltuzumab (19), ocrelizumab (20), and ocaratumumab (21), have been investigated in NHL without clear benefit over rituximab. Results for ofatumumab in DLBCL have been conflicting, perhaps dependent on when in the disease course it is being utilized (22,23). Ublituximab, a unique type I, chimeric, glycol-engineered anti-CD20 mAb, however has shown efficacy in rituximab-relapsed or -refractory patients with B-NHL. In a phase I/II trial, induction therapy (doses of 450–1,200 mg) consisting of 4 weekly infusions in cycle 1 for NHL and 3 weekly infusions in cycles 1 and 2 for CLL was administered. Patients then received ublituximab maintenance for up to 2 years. Enrolled patients with B-NHL (n=27) and CLL (n=8) had a median of three prior therapies. Median duration of response (DOR) and PFS were 9.2 and 7.7 months, respectively. Ublituximab was well-tolerated with no unanticipated adverse effects (24). This agent is now being explored in combinatorial strategies with ibrutinib (NCT02013128), and/or bendamustine (NCT02006485) and with the immunomodulatory agent lenalidomide (NCT01744912) in NHL.
Predictors of response to monoclonal antibodies
A number of predictive factors have been proposed for rituximab activity, including tumor burden and achievable serum levels of rituximab (Table 1). The latter appears to be gender and age-dependent, with a poorer quality of response achieved in men attributed to a higher body mass contributing to faster rituximab clearance, and altered pharmacokinetics and clearance noted in elderly males (25,26). Additionally, 25-OH-Vitamin D deficiency has been suggested as an alternative mechanism in the aging population that may impair rituximab mediated cellular toxicity with negative effects on response rates (27).
Table 1
| Drug class | Agents | Proposed predictors of response |
|---|---|---|
| Monoclonal anti-CD 20 antibodies | Rituximab, obinutuzumab, ofatumumab, veltuzumab, ocrelizumab, ocaratumumab, ublituximab | Extrapolated from rituximab data: tumor burden; age; sex; FCγRIIIA and FCγRIIB; 25-OH-Vitamin D deficiency is a predictor |
| Checkpoint inhibitors | ||
| PD-1/PD-L1 inhibitors | Nivolumab, pembrolizumab, pidilizumab, atezolizumab, avelumab, durvalumab | Higher expression of PD-L1 either at baseline or with treatment on peripheral blood T cells and monocytes |
| Anti-CTLA-4 antibody | Ipilimumab, tremelimumab | CTLA-4 single nucleotide variants |
| Anti-LAG3 antibody | BMS-986016 | – |
| Co-stimulatory agents | ||
| Anti-CD137/4-1BB antibody | Urelumab | – |
| OX-40 agonist | MEDI6469 | – |
| BMS-663513 | – | |
| Immunomodulatory agents | Lenalidomide | Cell of origin |
| Cellular therapies | ||
| CAR-T | CD19 CAR-T | Interleukin-15; single-cell CAR-T poly-functional strength index; amplitude of CAR-T cell expansion; presence of the cytokine release storm; early PET/CT negativity |
| BiTEs | Blinatumomab | – |
CAR-T, chimeric antigen receptor T-cell; CTLA-4, cytotoxic T-lymphocyte antigen 4; BiTE, bi-specific T cell engager molecules; PET, positron emission tomography; CT, computed tomography.
There is discordance in data regarding the impact of FcγRIIIA polymorphisms expressed on myeloid effectors on rituximab efficacy. Although earlier trials suggested correlations of improved clinical and molecular response in FL patients homozygous for FcγRIIIA-158VV (28,29), more recently, the larger PRIMA (30) and RESORT (31) trials were unable to confirm this association using genotyping analyses. Similarly, data are conflicting about the predictive role of FcγRIIB expression intensity (32,33). Finally, germline BCL2 single-nucleotide polymorphisms (SNPs) have also been investigated for their predictive value with SNP, rs7226979, found to be associated with survival in patients with DLBCL treated with R-CHOP vs. chemotherapy alone (34). Less is known about predictors of response for next generation anti-CD20 antibodies and certainly represents a field for future research.
Radioimmunotherapy (RIT)
RIT combines a radiation-emitting radionuclide with an anti-CD20 mAb to treat B-NHL. Currently, the only available RIT agent is 90Y-ibritumomab tiuxetan (Zevalin®, Spectrum Pharmaceuticals, Inc.), first approved for relapsed or refractory FL (35) with retrospective analyses confirming better responses when 90Y-ibritumomab tiuxetan is used earlier in the disease course (36). More recently, the phase III FIT trial established 90Y-ibritumomab consolidation after chemotherapy induction as an effective option for frontline treatment of FL (37). With long term follow-up of 409 patients, the estimated 8-year overall PFS was 41% with 90Y-ibritumomab versus 22% for the control arm (HR, 0.47; P<0.001). RIT has also demonstrated activity in aggressive lymphomas, utilized as consolidation after front-line therapy in both DLBCL (38,39) and MCL with durable remissions (40). Unfortunately, owing to the logistical limitations associated with the administration of this agent, this treatment approach has not been widely adopted.
Adoptive T-cell transfer
Chimeric antigen receptor T-cells (CAR-T cells) serve as a form of adoptive T-cell immunotherapy and has recently emerged as a feasible and effective passive immunotherapeutic modality. Autologous T-cells are genetically modified to express chimeric antigen receptors that include an external anti-CD19 single chain antibody domain and internal signaling domains. Anti-CD19 CAR-T cells recognize and kill CD19+ malignant B-cells and are being explored in refractory lymphomas.
In the 1990’s, it was first demonstrated that a single-chain Fv of an antibody molecule fused to the ζ chain of the CD3 complex could be expressed in T cells as an antigen-specific receptor. The chimeric scFvRζ endowed T cells with antibody-type specificity, transmitted a signal for IL-2 production, and mediated target cell lysis suggesting that this chimeric chain could not only direct specificity but also dictate selective reactivity of lymphocytes (41). Almost 20 years later, the first patient with refractory FL treated with CAR-T cells was reported—the patient achieved a partial response (PR) lasting 32 weeks but also experienced an ‘on-target, off-tumor’ effect of B-cell aplasia (42). Subsequent studies reported on the feasibility of this approach in a larger number of patients with B-cell malignancies providing first insights in to the inflammatory cytokine signature, (i.e., elevations in IFN-γ and/or IL-6 identified around the time of peak toxicity), associated with significant toxicities such as cytokine release syndrome (CRS) and neurotoxicity (43,44). These studies better characterized the pharmacokinetics of CAR-T cells with persistence measured in peripheral blood for up to 6 months in some after infusion, and demonstrated response rates of up to ~80% with potential durability in patients with aggressive chemo-refractory lymphoma (43,44). Early studies also reported on cellular anti-transgene immune rejection responses and contributed to the refinement of 2nd generation CAR-T cell products which utilize co-stimulatory domains (45,46).
There have since been a number of phase II studies evaluating various 2nd generation CAR-T cell products in B-NHL. At the NCI, 22 patients with advanced stage lymphoma, the majority with DLBCL, were treated with CAR-T cells after administration of lymphodepleting chemotherapy with fludarabine/cyclophosphamide. The overall remission rate was 73% with 55% CRs. Eleven of 12 complete remissions were ongoing at time of publication with the longest reported duration of 24 months. Neurologic toxicity was most prominent (55%) while CRS occurred in 18% of patients; only 3 patients required immunosuppressive therapy to abort toxicities while the rest had resolution with supportive measures only. Preliminary findings from an ongoing phase IIb study evaluating the safety and efficacy of anti-CD19 CAR-T cells (CTL019) in patients with heavily pre-treated, relapsed/refractory DLBCL or FL have been reported in abstract form (NCT02030834). In this group, no standard lymphodepleting chemotherapy was used prior to CAR-T cell administration. For 18 evaluable patients (12 DLBCL; 6 FL), at 3 months, the ORR was 67% (DLBCL 50%; FL 100%). At median follow-up of 6 months, PFS was 59% (DLBCL 37%; FL 100%) (47). This led to the JULIET trial evaluating the safety and efficacy of CTL019 in relapsed, refractory DLBCL (NCT02445248). In a planned interim analyses of the JULIET trial with 141 patients enrolled, CR and PR rates at 3 months were 37% and 8%, respectively with efficacy observed across prognostic subgroups. Median DOR was not reached (NR). CTL019 was detectable in peripheral blood by quantitative PCR for up to 355 days in responders. CRS, graded using the UPenn scale, occurred in 57% of infused pts (17% grade 3; 9% grade 4) and no CRS-associated deaths occurred (48).
Updated results for the ZUMA-1 trial reported on significant clinical benefit, and an acceptable safety profile in patients with refractory aggressive NHL treated with a different anti-CD19 CAR-T cell product. The phase II trial demonstrated an ORR of 82% (P<0.0001) and a CR of 54% in 101 patients treated, with consistent results across disease subtypes, refractory status, stage, and International Prognostic Index (IPI) score. With a median follow-up of 8.7 months, 44% of patients had an ongoing response and 39% were in CR. The median DOR was 8.2 months overall (49,50). Grade ≥3 CRS and neurologic events occurred in 13% and 28% of patients, respectively with one event of cerebral edema and subsequent death reported very early on in the trial. A summary of early pivotal clinical trials for CD19 CAR-T in B-NHL with rates of toxicity and predictors of response has been provided in Table 2 (47,49,51-53). CAR-T cells targeting CD30 are currently being developed in HL and T-NHL, with early results reported (54).
Table 2
| Description/endpoint | Kochenderfer et al. (51) | Locke et al. (49); Neelapu et al. (50) | Schuster et al. (47) | Turtle et al. (52) | Abramson et al. (53) |
|---|---|---|---|---|---|
| Institution | NCI | Moffitt Cancer Center | UPenn | FHCRC | Multicenter |
| Manufacturer/product | Kite | Kite/Axicabtagene Ciloleucel | Novartis/CTL019 | Juno/JCAR014 | Juno/JCAR017 |
| Co-stimulatory domain | CD28 | CD28 | 4-1BB | 4-1BB | 4-1BB |
| Disease | DLBCL/FL/PMBCL/MCL | DLBCL/FL/PMBCL | DLBCL/FL/MCL | DLBCL/transformed DLBCL/FL/MCL; |
DLBCL/FL3B/PMBCL |
| Patient number | 22 | 101 | 20 (18 evaluable for response) | 30 | 55 |
| ORR (%) | 73 | 82 | 67%; |
63 (82% if treated with Cy/Flu) | Best ORR is 76% (41/54) |
| CR rates (%) | 55 | 54 | – | 33 (64% if treated with Cy/Flu) | CR is 52% (28/54) |
| Median follow-up (months) | – | 8.7 | 6 | Cy ± E cohort: 25; |
3.2 months |
| DOR | 11/12 CRs ongoing; median duration of CRs 12.5 months | 44% ongoing responses | PFS for evaluable patients at 6 months, 59% | 8/9 CRs in Cy/Flu cohort ongoing (follow-up 2.3–11.2 months) | Three-month CR is 39% (16/41); |
| Predictor of response | Serum IL-15 ± IL-10; |
Peak and cumulative CAR levels | – | CAR peak expansion/AUC and persistence; |
CAR expansion |
| Neurotoxicity | 55% grade ≥3; |
28% (Grade ≥3); |
1% (Grade ≥3) | 28% (Grade ≥3); |
16% (Grade ≥3) |
| CRS | 18% | 13% (Grade ≥3) | 1% (Grade ≥3) | 13% (Grade ≥3); |
2% (Grade ≥3) |
| Median OS (months) | NR | NR | NR | NR | NR |
CR, complete response; CRP, C reactive protein; CRS, cytokine release syndrome; Cy, Cyclophosphamide; DLBCL, diffuse large B-cell lymphoma; E, etoposide; FHCRC, Fred Hutchinson Cancer Research Center; FL, follicular lymphoma; Flu, fludarabine; MCL, mantle cell lymphoma; NCI, National Cancer Institute; UPenn, University of Pennsylvania; ORR, overall response rate, PMBCL, primary mediastinal B-cell lymphoma.
In one study, seven patients with HL and two patients with T-cell anaplastic large cell lymphoma who relapsed or progressed after treatment with brentuximab vedotin were treated with CD30 CAR-T. Six weeks after treatment, one patient had a CR, one patient had a very good PR, and four patients had stable disease (SD) persisting for up to 8 months. In a second phase 1 study that included 18 patients with relapsed/refractory HL, seven achieved PR and six achieved SD with inconsistent responses dependent on site of involvement; better responses were observed in lymph nodes as compared to extra-nodal lesions including lung lesions. Interestingly, analysis of biopsied tissues by qPCR and immunohistochemistry revealed the trafficking of CAR-T cells into the targeted sites and reduction of the expression of CD30 in tumors (55). These findings are very preliminary but clearly warrant further evaluation.
Predictors of response to CAR-T cell therapy
Predictors of response to CAR-T cell therapy are being explored widely and are a subject of debate. In the study conducted by Kochenderfer et al., patients with advanced-stage lymphoma received a single dose of CD19 CAR-T cells, constructed with a CD28 co-stimulatory domain, 2 days after a low-dose chemotherapy conditioning with cyclophosphamide plus fludarabine (51). In this cohort, higher serum interleukin-15 levels were associated with higher median peak CAR-T cell levels (P=0.001) and remission rates (P<0.001). With a different CAR-T cell product comprised of a 4-1BB co-stimulatory domain and a 1:1 CD4+:CD8+ ratio, the group at the Fred Hutchinson Cancer Center demonstrated that intensification of lymphodepletion using fludarabine increased the peak of CAR-T cell expansion, AUC0-28 and long-term persistence of infused CAR-T cells, variables associated with improved CR rates, OS, and PFS. These associated changes in CAR-T cell expansion and AUC0-28 along with high IL-6, IL-8, IL-10, IL-15, and IFN-γ concentrations however also correlated with subsequent occurrences of severe CRS and neurotoxicity, suggesting that the presence of the CRS could represent a potential biomarker of response. It was also suggested that CAR-T cell dose reduction and/or manipulation of the cytokine microenvironment in an effort to minimize toxicity might result in loss of efficacy (56). In the ZUMA-1 trial, objective responses correlated best with a combined measure of single-cell CAR-T poly-functional strength index and amplitude of CAR-T cell expansion (49). By contrast, Schuster and colleagues found no correlation for CAR-T cell expansion and/or CRS with clinical outcomes but did suggest that early PET/CT may predict response to this novel immunotherapy (47,57). Of note, the Schuster protocol did not employ standardized lympho-depleting chemotherapy prior to CAR-T cell administration. With the TRANSCEND study, transformation from chronic lymphocytic leukemia or marginal zone lymphoma and an ECOG performance status of 2 were also found to be a negative predictor of response. Taken collectively, it appears that predictors of response may be dependent on patient variables such as performance status, the use of lympho-depleting protocols pre-CAR-T infusion, dose of cells and/or the actual product being used. Aligned with the task of patient selection and identification of predictive biomarkers of response is the challenge of developing more refined approaches to managing toxicities specific to each construct without compromising therapeutic effect. The two main toxicities of concern remain CRS and neurotoxicity with deaths attributed to both in early experience. The more expedient use of tocilizumab, an IL-6 receptor antagonist, and steroids have helped mitigate these toxicities with declining rates reported in clinical trials. However, the ideal approach to toxicity management remains controversial with questions raised regarding the impact of such an approach to the therapeutic benefit of CAR-T cell therapy. Nonetheless, in the ZUMA-1 trial, the use of tocilizumab or corticosteroids to manage CRS did not impact response rates despite the suggestion that CRS may serve as a predictive biomarker of response (49). Greater experience with CAR-T cell will clarify these issues (53).
Bi-specific B-cell Engagers (BiTE antibodies)
Also worthy of mention with respect to cellular therapies are the bi-specific T cell engager molecules (BiTEs). BiTEs are constructs that are composed of two single chain antibodies that bind not only the target antigen on malignant cells, but also stimulate cytotoxic T cells for directed tumor cell lysis (Figure 1). Blinatumomab, a bispecific T-cell engaging antibody construct, transiently links CD3-positive T cells to CD19-positive B cells has received approval for the treatment of relapsed/refractory ALL. However, in relapsed/refractory DLBCL, the agent demonstrated activity at the cost of significant toxicity making its future in the treatment of NHL uncertain (58).
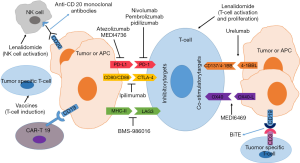
Active immunotherapy
Tumor vaccines and in situ vaccination
In addition to the passive immunotherapeutic strategies, investigators have continued to explore the utility of tumor vaccines in eliciting an anti-tumor effect via the engagement of the patient’s own adaptive immunity for decades. A variety of vaccines including protein- and cell-based vaccines are being developed with the functional goal of inducing long-lasting anti-tumor immune responses capable of tumor eradication (59,60). To date, the idiotype of the immunoglobulin protein expressed by B-cell lymphomas has been used to develop most of these vaccines with initial success in early phase trials but mixed results in phase III trials, perhaps dependent on tumor burden prior to vaccination (61-63). Nonetheless, a number of strategies including optimization of antigen presentation and T-cell function are being incorporated with the hopes of improving results.
Although in early stages of development, in situ vaccines are demonstrating promising anti-lymphoma activity. In a phase I/II trial, concurrent delivery of low dose radiation with the intratumoral injection of in situ vaccine CpG, a TLR9 agonist, elicited a significant abscopal effect with regression of distal lymphoma sites in a subset of patients. The was attributed to the priming of an adaptive immune response from immunogenic cell death and antigen release induced by the radiation, and the pro-inflammatory stimuli of the vaccine (64). A similar clinical effect has also been demonstrated with co-administration of intratumoral CpG and ibrutinib with concomitant stimulation of antigen-presenting cells in the TME inducing regression of local and distant tumors (65).
Checkpoint co-stimulatory molecules
Generating optimal cytotoxic CD8 T-cell responses requires T-cell receptor activation in addition to co-stimulation, which can be provided through ligation of tumor necrosis factor receptor family members, including 4-1BB (CD137) and OX40 (CD134; Figure 1). Co-stimulatory mAbs to 4-1BB and OX-40 are in early stages of development in lymphomas.
Anti-CD137 antibodies
A promising co-stimulatory immunologic target is 4-1BB, or CD137, a member of the tumor necrosis factor receptor superfamily. Ligation of 4-1BB activates CD8+ T-cells which then infiltrate and lyse tumors, promote natural killer cell activity with enhancement of ADCC, and induces pro-inflammatory type 1 cytokine secretion (66,67). Agonistic mAbs of 4-1BB exploit these functions with potent anti-tumor effects demonstrated in murine lymphoma tumor models in combination with rituximab (68). It is expected that optimal efficacy of 4-1BB-targeted agents, such as urelumab will continue to come from combinatorial strategies with checkpoint blockade offering a complementary yet distinct approach to harnessing immunity (69,70). But, early phase trials will need to focus on finding dosages in combination strategies that minimize toxicities.
OX40 agonists
Activating (agonistic) anti-OX40 mAb have been shown to augment anti-tumor immunity against a variety of tumors by enhancing T-cell differentiation and cytolytic function while turning off suppressive regulatory T-cells (Treg) (71). Data for this agent in lymphoma at present are limited but suggest that combinatorial strategies with other immunotherapy agents may be of greater benefit (72). A single phase 1b/2 trial has evaluated the safety and tolerability of MEDI6469 in combination with rituximab in relapsed DLBCL with outcomes yet to be reported (NCT02205333). In pre-clinical studies, investigators have demonstrated that potentially more potent systemic anti-tumor immune responses may be generated using sequential therapy with doxorubicin followed by an anti-OX40 agent and anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) blockade in mice (73). This approach has yet to be tested in humans.
Immune checkpoint inhibitors
The PD-1 receptor is an inhibitory transmembrane protein that is expressed on the surface of immune suppressor T-cells. The engagement of the PD-1 receptor to its ligand (PD-L1/PD-L2) on lymphoma cells blocks T-cell receptor signalling and T-cell response allowing for immune escape. PD-1/PD-L1 axis blockade with antibodies has been investigated as a therapeutic strategy. Similarly, CTLA-4 is a negative regulator of T-cell activation that serves to dampen antitumor immune responses (Figure 1). Unlike PD-1/PD-L1, which regulates later effector T-cell activity within tissue and tumors, CTLA-4 regulates T-cell activity at an earlier stage. Blocking anti-CTLA-4 mAbs have been shown to improve host resistance to immunogenic tumors leading to paradigm shifts in the management of solid tumors (74). Herein, we review the data that has led to the FDA approval of several anti-PD-1 agents in HL and discuss the promising efforts seen for anti-PD1/PD-L1 agents and other checkpoint inhibitors in lymphoid malignancies.
PD-1/PD-L1 inhibitors in HL
Reed-Sternberg (RS) cells of classical HL are characterized by genetic copy number alterations of chromosome 9p24.1 (75-77). This directly leads to increased expression of PD-L1 and PD-L2 on RS cells. Additionally, 9p24.1 amplification activates JAK2 signaling which also upregulates PD-L1 expression. This PD-L1 overexpression limits T-cell-mediated immune response and results in evasion of immune surveillance but also explains HL’s unique vulnerability to PD-1/PD-L1 blockade.
Nivolumab, a fully human immunoglobulin G4 (IgG4) immune checkpoint inhibitor antibody that targets PD-1, was recently approved for the treatment of relapsed or refractory classical HL (cHL) after failure of autologous hematopoietic stem cell transplantation (auto-HSCT) and brentuximab vedotin. This agent achieved accelerated approval based on combined results of two clinical trials (78,79). Efficacy was evaluated in 95 patients previously treated with auto-HSCT and post-transplantation brentuximab vedotin. Patients had a median of 5 prior systemic regimens (range, 3–15) and received a median of 17 doses of nivolumab (range, 3–48). Single-agent nivolumab produced a 65% ORR, with 58% PR and 7% complete response (CR) rates. The estimated median DOR was 8.7 months (80).
Pembrolizumab is a humanized IgG4 antagonistic anti-PD-1 antibody that is currently being studied in the multi-cohort open-label phase Ib KEYNOTE-013 clinical trial (NCT01953692), in patients with hematologic malignancies. In the HL cohort failing brentuximab vedotin, results were impressive: for patients who received pembrolizumab (10 mg/kg intravenously every 2 weeks) until tumor progression, excessive toxicity, or completion of 2 years of therapy, the ORR was 65% (CR, 16%; PR, 48%); 23% of patients achieved SD, with 90% exhibiting a reduction in target lesions. Responses were sustained for more than 24 weeks in 71% of patients and with a median follow-up of 18 months for survivors, the PFS at 24 weeks was 69% (81).
The non-randomized, open-label KEYNOTE-087 trial similarly enrolled 210 patients with relapsed or refractory cHL. Sixty-one percent of patients had undergone prior auto-HSCT and the majority had been exposed to brentuximab vedotin. With a median follow-up of 10.1 months, the ORR with pembrolizumab was 69.0% (95% CI, 62.3–75.2%) with CR in 22.4%. The median DOR was NR in all cohorts and 31 patients (75.6%) maintained a response of ≥6 months (82). Based on these data, the FDA approved this agent in March 2017 for patients with cHL who have relapsed after three or more prior lines of therapy at a dose of 200 mg every 3 weeks in adults and a dose of 2 mg/kg (up to 200 mg) every 3 weeks in pediatric patients.
Of note, although PD-1 agents have been well tolerated after auto-HSCT, caution must be exercised with the use of such agents after allogeneic HSCT—although they can achieve high and durable responses in this setting, they can also induce rapid and treatment-refractory GVHD (83,84).
PD-1/PD-L1 inhibitors in NHL
Subsets of NHL have also been shown to express PD-L1 (85). In fact, genetic/cytogenetic aberrations involving the PD-L1/PD-L2 locus can be identified in approximately 20% of DLBCLs with these alterations occurring mostly in the non-GCB subtype of the disease (86). These findings justify ongoing efforts to explore the effects of anti-PD-1/PD-L1 antibodies in NHL.
In a phase 1b study of nivolumab in relapsed/refractory hematologic malignancies, nivolumab therapy resulted in ORRs of 36% and 40% among patients with DLBCL and FL, respectively (87). With continued nivolumab therapy, the depth of objective responses improved as demonstrated by one patient with DLBCL with an initial PR (at 16 weeks) that converted to a CR (at 72 weeks) with extended treatment. Pembrolizumab as mentioned has demonstrated clinical activity in HL and is currently being investigated in NHL per the KEYNOTE-013 study (NCT01953692), as one of many clinical trials with this agent ± other targeted therapies (NCT02332980, NCT02446457, NCT02178722).
Pidilizumab, a humanized IgG4 antibody, has been evaluated in the relapsed/refractory DLBCL post-autologous stem cell transplant (ASCT) (88) with the rationale that this approach might enhance the anti-lymphoma immune response during the period of immune reconstitution after transplant. This trial included patients with DLBCL, primary mediastinal large B-cell lymphoma (PMBL), or transformed indolent B-cell lymphoma after ASCT. Among 66 evaluable patients, the 16-month PFS and OS were 72% and 85%, respectively. Pidilizumab treatment resulted in a significant increase in the absolute number of PD-L1-bearing activated helper T cells (CD4+/CD25+/PD-L1+), an effect that was sustained for at least 16 weeks after treatment.
The effects of checkpoint inhibitors have been scrutinized in other aggressive B-NHL histological subtypes including PMBL. PMBL accounts for up to 10% of DLBCL and is clinically and biologically distinct from the other subtypes of DLBCL. Among the most common genetic alterations in PMBL are amplifications of a region of chromosome 9p leading to rearrangements of the PD ligand locus at a frequency of approximately 20% (89), and much higher frequencies of amplification and overexpression of PD-L1 (75). More recently, mediastinal grey zone lymphomas (MGZL) have been recognized as a separate entity with clinical and pathologic features intermediate between PMBL and classical HL though with less known about their molecular characteristics (90). One study did report alterations of the JAK2/PDL2 locus in 9p24.1 in 55% of cases for this histology (91). Given these findings, it is not surprising that studies with checkpoint inhibitors have been more encouraging in patients with these unique histologic subtypes as compared to results in DLBCL. For example, among 18 patients with relapsed/refractory PMBCL enrolled as part of the KEYNOTE-013 multi-cohort phase 1b trial, ORR was 41% with 35% of additional patients acquiring SD with 81% demonstrating a decrease in target lesions. With a median follow-up of 11.3 months, median DOR was NR (92). A global, multicenter, phase 2 trial (KEYNOTE-170) is currently evaluating single-agent pembrolizumab in this setting (NCT02576990) while another trial is evaluating the same in MGZL (NCT03255018). Additionally, a cooperative group effort evaluating DA-EPOCH-R + PD-1 blockade in PMBCL is being designed for the frontline setting.
Checkpoint blockade has also been explored in indolent NHL. A single-institution phase II study evaluated pidilizumab in combination with rituximab in patients with relapsed, rituximab-sensitive FL (93). In this study, pidilizumab in combination with rituximab resulted in increases in absolute lymphocyte counts following therapy consistent with an immune response. Since these studies, it is noteworthy that the precise mechanism of pidilizumab is not entirely clear and its impact on the PD-1/L1 axis has been called into question.
Atezolizumab (MPDL3280A) and MEDI4736 are IgG1 mAbs directed against PD-L1, and are currently undergoing evaluation in NHL in combination with agents such as ibrutinib (NCT02205333), obinutuzumab (NCT02220842) or chemotherapy (NCT02596971).
Targeting CTLA-4
Data for anti-CTLA antibodies for lymphomas have not been as encouraging. In a phase I trial with ipilimumab though well tolerated, only 2 of 18 treated lymphoma patients demonstrated a response (94). Combinatorial strategies have since been evaluated.
As an example, the CheckMate 039 Trial first reported data of combination checkpoint blockade therapy with ipilimumab and nivolumab at 3 mg/kg IV and 1 mg/kg IV respectively, every 3 weeks for 4 doses, followed by nivolumab maintenance (3 mg/kg) every 2 weeks for up to 2 years in a cohort of relapsed/refractory hematologic malignancies (95), with preceding preclinical and solid tumor data showing superior efficacy of the combination as compared with nivolumab or ipilimumab alone (96,97). In total, 65 heavily pre-treated but predominantly transplant naïve patients (31 HL, 15 B-NHL, and 11 T-NHL), were enrolled. Combination therapy demonstrated a safety and efficacy profile similar to that previously reported for nivolumab monotherapy in HL and B-cell NHL. ORRs were 74% and 20% (CR of 19% and 0%), for each respective cohort. With median follow-up of 11.4 months, median PFS was NR vs. 1.5 months and median OS was NR vs. 2.9 months in the HL and B-NHL groups respectively.
E4412 is a Phase 1 ECOG-ACRIN sponsored study that has since taken a further step to explore triplet therapy with brentuximab vedotin, nivolumab and ipilimumab in relapsed/refractory HL with results on the triplet strategy awaited (NCT01896999).
Anti-LAG3 antibodies
Other immune checkpoint proteins that can be expressed on T cells include BTLA, LAG3, and TIM3. LAG 3 is a member of the immunoglobulin superfamily and binds to major histocompatibility complex (MHC) class II. Anti-LAG 3 mAbs bind to LAG 3 on tumor infiltrating lymphocytes with resultant enhancement of cytotoxic T-cell mediated tumor cell lysis (Figure 1) (98,99). These antibodies have been shown to be of clinical and therapeutic relevance in CLL (100) and are in early stages of exploration in lymphoma. Specifically, a phase 1 clinical trial is underway with the objective of characterizing safety, tolerability and maximum tolerated dose of BMS-986016 administered alone or in combination with nivolumab in patients with relapsed hematologic malignancies including HL and DLBCL (NCT02061761).
Predictors of response to checkpoint blockade
Immune checkpoint blockade can generate anti-tumor immune responses translating into clinical benefit in cHL though DOR is variable. Results thus far have indicated only modest effects of anti-PD1/PD-L1 and anti-CTLA-4 therapy in NHL. For patients with primary resistance or short durations of response to checkpoint inhibitors, the question is whether predictive biomarkers can be identified to better select patients more likely to derive benefit from these agents.
For ipilimumab, CTLA-4 single nucleotide variants, 1577G>A and CT60G>A, were found to be significantly associated with clinical outcomes in metastatic melanoma (101). To our knowledge, similar studies have not been conducted in lymphoma.
The overexpression of PD-L1 is an important and widely-explored predictive biomarker for the response to PD-1/PD-L1 antibodies in solid and hematologic malignancies. In cHL, biomarker studies were conducted in patients treated with pembrolizumab after brentuximab vedotin failure and demonstrated a high prevalence of PD-L1 and PD-L2 expression and treatment-induced expansion of T-cells and natural killer cells though not predictive of response. RNA analysis was performed on a NanoString platform at baseline and after 7 cycles of treatment in 19 patients. Although gene signatures involving expanded immune-related signaling pathways, T cell receptor signaling, and activation of IFN-γ, were significantly upregulated after treatment, no specific gene expression profile reliably predicted for response to treatment (81).
In NHL, studies with pidilizumab suggest that higher expression of PD-L1 either at baseline or with treatment on peripheral blood T-cells and monocytes may predict for improved response to the agent (88,93). In indolent B-cell NHL, a unique genetic signature predicting response to pidilizumab was also identified but needs to be validated in a larger cohort (93).
These variable results reflect the limitations associated with PD-L1 as a biomarker, including the low prediction accuracy and dynamic changes of the marker. Given the complexity of the immune microenvironment, it is only practical to consider that PD-L1’s predictive value is co-dependent on tumor-infiltrating immune cells and molecules in the TME, a concept that needs to be explored in lymphoma (102).
Immunomodulatory agents
Lenalidomide
Lenalidomide is an immunomodulatory agent with activity in lymphoid malignancies. It exerts anti-lymphoma effects through T-cell immune synapse enhancement while maintaining anti-proliferative effects in a cereblon-dependent manner (103,104). Lenalidomide also enhances natural killer cell and monocyte-mediated ADCC (Figure 1). The latter explains lenalidomide’s ability to enhance the activity of rituximab against CD20+ tumor cells in vitro and in animal models (105,106).
This expected synergy was confirmed in vivo in a phase-2 study for patients with untreated indolent lymphomas—lenalidomide (20 mg/day on days 1–21 of each 28-day cycle), and rituximab (375 mg/m2 on day 1 of each cycle), were given for up to 12 cycles. Of 46 evaluable patients with FL, 40 (87%) had a CR/unconfirmed CR (CRu) with 79% of FL patients remaining in CR with 3 years follow-up (107). Similarly, the SAKK 35/10 phase-2-study compared the activity of rituximab plus lenalidomide versus single-agent rituximab as first-line therapy for FL and demonstrated significantly higher CR/CRu rates in the combination arm (108). The response improvement translated into significant prolonged time to next treatment (P=0.01) and superior PFS rates (P=0.03) (108). The RELEVANCE Trial (NCT01476787), an international phase III study comparing the combination of rituximab and lenalidomide with rituximab plus chemotherapy in a larger cohort of patients with untreated FL has since begun accrual.
In aggressive lymphomas, lenalidomide has similar results of efficacy with some specificity to DLBCL of non-germinal center (non-GC) or activated B-cell (ABC) origin. In a heavily pretreated population with DLBCL, a randomized phase II/III study of lenalidomide as a single agent vs. single-agent investigator’s choice (IC) in patients ineligible for stem cell transplantation or further combination chemotherapy was conducted (109). Lenalidomide monotherapy improved ORR, PFS, and OS in the non-GCB population (defined by IHC) over IC, with more pronounced benefits in the ABC population as defined by GEP. In the frontline setting, lenalidomide as an adjunct to R-CHOP (R2-CHOP) in a phase II trial resulted in improved survival in the non-GCB subset, as compared to historical control rates (110). For patients with non-GCB phenotype treated with R-CHOP vs. R2-CHOP, the 2-year OS was 46% vs. 78%, respectively. We will wait to see if these results will be confirmed by the ROBUST trial, a randomized, double-blind, global, phase III study of R-CHOP-21 ×6 cycles versus R2-CHOP with lenalidomide days 1–14, in patients with treatment naive ABC-type DLBCL determined by GEP (NCT 02285062). Of note, maintenance with lenalidomide in aggressive lymphomas does not improve survival as demonstrated by the REMARC study (111).
Predictors of response to lenalidomide
The preferential activity for lenalidomide is not entirely reliant on cell of origin as evidenced by its activity in FL and conflicting data in DLBCL. In the front-line and relapsed/refractory settings, several investigators have concluded that lenalidomide has more pronounced benefits in the non-GC population by IHC or the ABC population as defined by GEP (109,110). By contrast, in a recent report of lenalidomide maintenance in relapsed DLBCL, identical PFS in GC and non-GC cases and in small subgroups of GCB-DLBCL and ABC-DLBCL was shown (112). It is anticipated that the REMARC and RELEVANCE trials will help to identify reliable biomarkers that predict lenalidomide responses for the treatment of indolent and aggressive lymphomas.
Special considerations in immuno-oncology
Measuring responses to immunotherapy
To date, the traditional RECIST (113) and/or the Lugano Criteria (114) have been reliable tools used to stage and define response to standard therapy in solid tumors and lymphoma respectively. However, immunotherapy including immunomodulatory agents and cellular therapy with CAR-T cells can be associated with “pseudo-progression” whereby inflammation and recruitment of immune cells to disease sites may transiently cause tumor flare and/or increase in burden of measurable disease. This phenomenon limits the applicability of these traditional tools in measuring response, which define PD as tumor burden increase above a specified threshold (20% for RECIST and 50% for Lugano Criteria), or any new lesions. Similarly, other agents can alter tumor metabolism or glucose uptake with potential for either false-positive or false-negative FDG-PET results.
Misinterpretation of these findings can lead to premature termination of such therapies. To avoid this error in solid tumors, immune-related response criteria (irRC) were developed which required confirmation of PD on two consecutive scans at least 4 weeks apart, and inclusion of new lesion measurements to the total tumor burden (115). More recently, for lymphoma response in patients receiving targeted agents and immune therapy, two response evaluation tools were outlined including the RECIL 2017, a modified version of RECIST criteria for lymphoma, and LRYIC, a refinement of the Lugano Classification (116,117).
The RECIL 2017 criteria suggests that tumor burden in lymphoma can be assessed using the unidimensional measure of the sum of the longest diameters (SLD) of a maximum of three target lesions and introduces a provisional category of a minor response defined as a reduction in SLD of ≥10% but <30%. In those receiving immunotherapy, confirmation of progressive disease on 2 consecutive scans 4 weeks apart are recommended to evaluate for true progression versus pseudo-progression (116).
The LYRIC is a comprehensive refinement of the Lugano Classification which now includes the term “indeterminate response (IR)” in the context of immunomodulatory therapy (117). This is defined as either an (I) increase in overall tumor burden within the first 12 weeks of therapy without clinical deterioration (IR1); (II) the appearance of new lesions or growth of 1 or more existing lesions ≥50% but in the context of lack of progression (<50% increase) of overall tumor burden (IR2); and/or (III) increase in PET uptake in one or more lesion(s) without a concomitant increase in lesion number or size (IR3). For IR1 and IR2, biopsies are encouraged if feasible. Serial imaging studies within 12 weeks for any IR category would then be necessary to confirm that changes are related to an early manifestation of disease progression rather than a tumor flare.
As response for lymphoma is currently assessed by the Lugano Classification, by natural extension, it would be appropriate to consider the incorporation of LYRIC into clinical trials for novel agents as a standardized assessment of response. With this, it is expected that insight about unique patterns of response would be gained for single and/or combination therapies and anticipate ongoing modifications to LYRIC to match evolving treatment strategies. That being said, future directions should also be aimed at evaluating a combination of radiologic and biologic data to eliminate the ambiguity of CTs and or FDG-PETs as tools of response assessment and to achieve a more accurate measure of depth of response.
Novel surveillance techniques
Imaging modalities remain poor surrogates for overall tumor burden and measure of response to therapy, particularly in an era of novel immunotherapies. Recently, novel sequencing-based methods to detect circulating tumor DNA (ctDNA) have emerged. Although in early stages of use, assays using immunoglobulin high-throughput sequencing are being developed to detect cell-free circulating ctDNA during the course of therapy for response assessment. Though not ready for use in lymphoma, investigators have demonstrated that ctDNA in metastatic melanoma patients receiving treatment with PD-1 inhibitors is an accurate predictor of tumor response, PFS and OS (118). Building on this technology, cancer personalized profiling by deep sequencing (CAPP-seq) promises the added benefit of identifying clonal evolution with therapy that may help predict for relapse (119,120). It is expected that these techniques may eventually lead to more accurate measures of disease burden, depth of response, and improved disease surveillance but will require validation against established, albeit sub-optimal tools currently used for these purposes.
Response to immunotherapy—interplay of tumor and host factors
Immunotherapies have shown significant activity in lymphoma. However, there remains a heterogeneity in depth and DOR with growing efforts to identify mechanisms of resistance. It is clear that a number of factors affect responses to immunotherapy from the tumor micro-environment, to cancer-cell autonomous cues, to host-related factors.
The TME is comprised of the extracellular matrix, stromal cells and immune cells, all contributing to a state of chronic inflammation and immunosuppression that favors tumor cell evasion from the immune system. The TME’s suppression of tumor infiltrating lymphocyte recruitment along with epigenetic silencing of chemokines and Th1 immunity have been suggested to contribute to resistance to immune checkpoint blockade (121,122).
As for host-factors, age, diet, hormones, human leukocyte antigen (HLA) type, genetic polymorphisms as outlined for predictors of response to rituximab, and the gut microbiota have all been implicated in successful response to immunotherapy (123). For example, with aging, lymphocyte number with refractoriness to activation, up-regulation of suppressive immune cells including Tregs, and chronic inflammation all lead to immune senescence. It has been suggested that these changes may mute responses to immunotherapy (124). With respect to intestinal microflora, there is a mutualistic symbiosis linking intestinal flora and the host—alterations in this microbiome can result in pathologies of immune dysregulation including cancer (125). In fact, it has been shown that immune checkpoint blockade can mobilize gut microbiota to promote anti-tumor response by enhancing DC antigen-processing and -presentation functions with recruitment of intratumoral T cells in solid tumors (126). By extrapolation, alterations in the composition of the gut microbiota would be expected to affect host anti-cancer immunity and response to immunotherapies in lymphoma, but this has yet to be explored.
Conclusions
Passive and active immune therapies are being aggressively evaluated in HL and NHL with promising results for checkpoint inhibitors, agonistic co-stimulatory antibodies, cellular therapies and immunomodulatory agents. But, there is clearly a differential effect of these passive and active immune-therapeutic strategies in HL and NHL. Furthermore, the reason for variable depth and DOR among patients with similar disease histology is unclear, underscoring the heterogeneity in tumor biology and a clear need for predictive biomarkers of response. Though not the focus of this summary, immune related toxicities are also quite unpredictable and a subject of growing interest. As our experience matures with these agents, it is expected that these gaps will be addressed. With the hope of achieving improved anti-lymphoma effect, combinations of immunotherapies to further enhance our ability to harness the immune system and address heterogeneous tumor biology are already underway. Ongoing studies will ideally provide us with direction on effective combinations and predictive biomarkers to guide patient selection for optimal response as well as how best to monitor for response. With time, such questions as whether immunotherapy can effectively replace cytotoxics, how best to sequence agents, and timing and safety in the context of stem cell transplantation will be answered and reflect the future of the field.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Bruce D. Cheson) for the series “Inaugural Issue” published in Annals of Lymphoma. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aol.2017.11.02). The series "Inaugural Issue" was commissioned by the editorial office without any funding or sponsorship. R Karmali received speakers’ bureau from Celgene; JA Sosman is one of advisory board of BMS, Incyte and Merck; LI Gordon is Chair of Data Monitoring Committees for clinical trials for Jansen. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 1998;16:2825-33. [Crossref] [PubMed]
- Ghielmini M, Schmitz SF, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood 2004;103:4416-23. [Crossref] [PubMed]
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203-10. [Crossref] [PubMed]
- Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 2014;123:2944-52. [Crossref] [PubMed]
- Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood 2005;105:1417-23. [Crossref] [PubMed]
- Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood 2010;116:2040-5. [Crossref] [PubMed]
- Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 2005;23:4117-26. [Crossref] [PubMed]
- Molina TJ, Canioni D, Copie-Bergman C, et al. Young patients with non-germinal center B-cell-like diffuse large B-cell lymphoma benefit from intensified chemotherapy with ACVBP plus rituximab compared with CHOP plus rituximab: analysis of data from the Groupe d'Etudes des Lymphomes de l'Adulte/lymphoma study association phase III trial LNH 03-2B. J Clin Oncol 2014;32:3996-4003. [Crossref] [PubMed]
- Pfreundschuh M, Kuhnt E, Trumper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011;12:1013-22. [Crossref] [PubMed]
- Herter S, Herting F, Mundigl O, et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther 2013;12:2031-42. [Crossref] [PubMed]
- Mössner E, Brunker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 2010;115:4393-402. [Crossref] [PubMed]
- Sehn LH, Goy A, Offner FC, et al. Randomized Phase II Trial Comparing Obinutuzumab (GA101) With Rituximab in Patients With Relapsed CD20+ Indolent B-Cell Non-Hodgkin Lymphoma: Final Analysis of the GAUSS Study. J Clin Oncol 2015;33:3467-74. [Crossref] [PubMed]
- Marcus R, Davies A, Ando K, et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med 2017;377:1331-44. [Crossref] [PubMed]
- Pott C, Hoster E, Kehden B, et al. Minimal Residual Disease in Patients with Follicular Lymphoma Treated with Obinutuzumab or Rituximab As First-Line Induction Immunochemotherapy and Maintenance in the Phase 3 GALLIUM Study. Blood 2016;128:abstr 613.
- Sehn LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol 2016;17:1081-93. [Crossref] [PubMed]
- Cheson B, Trněný M, Bouabdallah K, et al. Obinutuzumab plus Bendamustine Followed by Obinutuzumab Maintenance Prolongs Overall Survival Compared with Bendamustine Alone in Patients with Rituximab-Refractory Indolent Non-Hodgkin Lymphoma: Updated Results of the GADOLIN Study. Blood 2016;128:abstr 615.
- Vitolo U, Treny M, Belada D, et al. Obinutuzumab or rituximab plus CHOP in patients with previously untreated diffuse large B-cell lymphoma: Final results from an open-label, randomized phase 3 study (GOYA). Blood 2016;128:abstr 470.
- Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014;370:1101-10. [Crossref] [PubMed]
- Morschhauser F, Leonard JP, Fayad L, et al. Humanized anti-CD20 antibody, veltuzumab, in refractory/recurrent non-Hodgkin's lymphoma: phase I/II results. J Clin Oncol 2009;27:3346-53. [Crossref] [PubMed]
- Morschhauser F, Marlton P, Vitolo U, et al. Results of a phase I/II study of ocrelizumab, a fully humanized anti-CD20 mAb, in patients with relapsed/refractory follicular lymphoma. Ann Oncol 2010;21:1870-6. [Crossref] [PubMed]
- Forero-Torres A, de Vos S, Pohlman BL, et al. Results of a phase 1 study of AME-133v (LY2469298), an Fc-engineered humanized monoclonal anti-CD20 antibody, in FcgammaRIIIa-genotyped patients with previously treated follicular lymphoma. Clin Cancer Res 2012;18:1395-403. [Crossref] [PubMed]
- van Imhoff GW, McMillan A, Matasar MJ, et al. Ofatumumab Versus Rituximab Salvage Chemoimmunotherapy in Relapsed or Refractory Diffuse Large B-Cell Lymphoma: The ORCHARRD Study. J Clin Oncol 2016;Jco2016690198 [PubMed]
- Peyrade F, Bologna S, Delwail V, et al. Combination of ofatumumab and reduced-dose CHOP for diffuse large B-cell lymphomas in patients aged 80 years or older: an open-label, multicentre, single-arm, phase 2 trial from the LYSA group. Lancet Haematol 2017;4:e46-e55. [Crossref] [PubMed]
- Sawas A, Farber CM, Schreeder MT, et al. A phase 1/2 trial of ublituximab, a novel anti-CD20 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma or chronic lymphocytic leukaemia previously exposed to rituximab. Br J Haematol 2017;177:243-53. [Crossref] [PubMed]
- Pfreundschuh M, Muller C, Zeynalova S, et al. Suboptimal dosing of rituximab in male and female patients with DLBCL. Blood 2014;123:640-6. [Crossref] [PubMed]
- Müller C, Murawski N, Wiesen MH, et al. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood 2012;119:3276-84. [Crossref] [PubMed]
- Bittenbring JT, Neumann F, Altmann B, et al. Vitamin D deficiency impairs rituximab-mediated cellular cytotoxicity and outcome of patients with diffuse large B-cell lymphoma treated with but not without rituximab. J Clin Oncol 2014;32:3242-8. [Crossref] [PubMed]
- Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002;99:754-8. [Crossref] [PubMed]
- Ghielmini M, Rufibach K, Salles G, et al. Single agent rituximab in patients with follicular or mantle cell lymphoma: clinical and biological factors that are predictive of response and event-free survival as well as the effect of rituximab on the immune system: a study of the Swiss Group for Clinical Cancer Research (SAKK). Ann Oncol 2005;16:1675-82. [Crossref] [PubMed]
- Ghesquières H, Cartron G, Seymour JF, et al. Clinical outcome of patients with follicular lymphoma receiving chemoimmunotherapy in the PRIMA study is not affected by FCGR3A and FCGR2A polymorphisms. Blood 2012;120:2650-7. [Crossref] [PubMed]
- Kenkre VP, Hong F, Cerhan JR, et al. Fc Gamma Receptor 3A and 2A Polymorphisms Do Not Predict Response to Rituximab in Follicular Lymphoma. Clin Cancer Res 2016;22:821-6. [Crossref] [PubMed]
- Camilleri-Broët S, Mounier N, Delmer A, et al. FcgammaRIIB expression in diffuse large B-cell lymphomas does not alter the response to CHOP+rituximab (R-CHOP). Leukemia 2004;18:2038-40. [Crossref] [PubMed]
- Lee CS, Ashton-Key M, Cogliatti S, et al. Expression of the inhibitory Fc gamma receptor IIB (FCGR2B, CD32B) on follicular lymphoma cells lowers the response rate to rituximab monotherapy (SAKK 35/98). Br J Haematol 2015;168:145-8. [Crossref] [PubMed]
- Bashash M, Connors JM, Gascoyne RD, et al. Genetic polymorphism at BCL2 as a predictor for rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone efficacy in patients with diffuse large B-cell lymphoma. Haematologica 2017;102:e199-e202. [Crossref] [PubMed]
- Highlights of prescribing information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125019s0156.pdf
- Emmanouilides C, Witzig TE, Gordon LI, et al. Treatment with yttrium 90 ibritumomab tiuxetan at early relapse is safe and effective in patients with previously treated B-cell non-Hodgkin's lymphoma. Leuk Lymphoma 2006;47:629-36. [Crossref] [PubMed]
- Morschhauser F, Radford J, Van Hoof A, et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol 2008;26:5156-64. [Crossref] [PubMed]
- Karmali R, Larson ML, Shammo JM, et al. Phase 2 study of CHOP-R-14 followed by 90Y-ibritumomab tiuxetan in patients with previously untreated diffuse large B-cell lymphoma. Mol Clin Oncol 2017;6:627-33. [Crossref] [PubMed]
- Stefoni V, Casadei B, Bottelli C, et al. Short-course R-CHOP followed by (90)Y-Ibritumomab tiuxetan in previously untreated high-risk elderly diffuse large B-cell lymphoma patients: 7-year long-term results. Blood Cancer J 2016;6:e425 [Crossref] [PubMed]
- Smith MR, Hong F, Li H, et al. Mantle cell lymphoma initial therapy with abbreviated R-CHOP followed by 90Y-ibritumomab tiuxetan: 10-year follow-up of the phase 2 ECOG-ACRIN study E1499. Leukemia 2017;31:517-9. [Crossref] [PubMed]
- Eshhar Z, Waks T, Gross G, et al. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A 1993;90:720-4. [Crossref] [PubMed]
- Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010;116:4099-102. [Crossref] [PubMed]
- Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor–transduced T cells. Blood 2012;119:2709-20. [Crossref] [PubMed]
- Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-Refractory Diffuse Large B-Cell Lymphoma and Indolent B-Cell Malignancies Can Be Effectively Treated With Autologous T Cells Expressing an Anti-CD19 Chimeric Antigen Receptor. J Clin Oncol 2015;33:540-9. [Crossref] [PubMed]
- Jensen MC, Popplewell L, Cooper LJ, et al. Anti-Transgene Rejection Responses Contribute to Attenuated Persistence of Adoptively Transferred CD20/CD19-Specific Chimeric Antigen Receptor Re-directed T Cells in Humans. Biol Blood Marrow Transplant 2010;16:1245-56. [Crossref] [PubMed]
- Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011;121:1822-6. [Crossref] [PubMed]
- Schuster S, Svoboda J, Nasta S, et al. Phase IIa trial of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed or refractory CD19+ lymphomas. J Clin Oncol 2015;33: abstr 8516.
- Schuster SJ, Bishop MR, Tam C, et al. Global pivotal phase 2 trial of the CD19-targeted therapy CTL019 in adult patients with relapsed or refractory (R/R) diffuse large b-cell lymphoma (DLBCL)—an interim analysis. Hematol Oncol 2017;35:27. [Crossref]
- Locke FL, Neelapu SS, Bartlett NL, et al. Abstract CT019: Primary results from ZUMA-1: a pivotal trial of axicabtagene ciloleucel (axicel; KTE-C19) in patients with refractory aggressive non-Hodgkin lymphoma (NHL). Annual Meeting of the American Association for Cancer Research. Washington DC, April 1-5, 2017.
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel (axi-cel; kte-c19) in patients with refractory aggressive non-hodgkin lymphomas (nhl): Primary results of the pivotal trial zuma-1. Hematol Oncol 2017;35:28. [Crossref]
- Kochenderfer JN, Somerville RP, Lu T, et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels. J Clin Oncol 2017;35:1803-13. [Crossref] [PubMed]
- Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 2016;8:355ra116 [Crossref] [PubMed]
- Abramson JS, Palomba ML, Gordon LI, et al. CR rates in relapsed/refractory (R/R) aggressive B-NHL treated with the CD19-directed CAR T cell product JCAR017 (TRANSCEND NHL 001). J Clin Oncol 2017;35:7513.
- Ramos CA, Ballard B, Liu E, et al. Chimeric T cells for therapy of CD30+ Hodgkin and non-Hodgkin lymphomas. Blood 2015;126:185. [PubMed]
- Wang CM, Wu ZQ, Wang Y, et al. Autologous T Cells Expressing CD30 Chimeric Antigen Receptors for Relapsed or Refractory Hodgkin Lymphoma: An Open-Label Phase I Trial. Clin Cancer Res 2017;23:1156-66. [Crossref] [PubMed]
- Hay KA, Turtle CJ. Chimeric Antigen Receptor (CAR) T Cells: Lessons Learned from Targeting of CD19 in B-Cell Malignancies. Drugs 2017;77:237-45. [Crossref] [PubMed]
- Shah N, Torigian DA, Farwell M, et al. Utility of FDG-PET/CT in lymphoma patients undergoing immunotherapy with autologous CTL019 T-cells. J Clin Oncol 2015;33:abstr 3022.
- Viardot A, Goebeler ME, Hess G, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 2016;127:1410-6. [Crossref] [PubMed]
- Brody J, Kohrt H, Marabelle A, et al. Active and passive immunotherapy for lymphoma: proving principles and improving results. J Clin Oncol 2011;29:1864-75. [Crossref] [PubMed]
- Lee ST, Neelapu SS, Kwak LW. Therapeutic Vaccine for Lymphoma. Yonsei Med J 2007;48:1-10. [Crossref] [PubMed]
- Brody J, Levy R. Lymphoma immunotherapy: vaccines, adoptive cell transfer and immunotransplant. Immunotherapy 2009;1:809-24. [Crossref] [PubMed]
- Kwak LW, Campbell MJ, Czerwinski DK, et al. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med 1992;327:1209-15. [Crossref] [PubMed]
- Levy R, Ganjoo KN, Leonard JP, et al. Active idiotypic vaccination versus control immunotherapy for follicular lymphoma. J Clin Oncol 2014;32:1797-803. [Crossref] [PubMed]
- Marron TU, Kalac M, Brody J. An Update on the Use of Immunotherapy in the Treatment of Lymphoma. Curr Hematol Malig Rep 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Sagiv-Barfi I, Kohrt HE, Burckhardt L, et al. Ibrutinib enhances the antitumor immune response induced by intratumoral injection of a TLR9 ligand in mouse lymphoma. Blood 2015;125:2079-86. [Crossref] [PubMed]
- Curran MA, Geiger TL, Montalvo W, et al. Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J Exp Med 2013;210:743-55. [Crossref] [PubMed]
- Li Q, Carr A, Ito F, et al. Polarization effects of 4-1BB during CD28 costimulation in generating tumor-reactive T cells for cancer immunotherapy. Cancer Res 2003;63:2546-52. [PubMed]
- Kohrt HE, Houot R, Goldstein MJ, et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood 2011;117:2423-32. [Crossref] [PubMed]
- Sánchez-Paulete AR, Cueto FJ, Martinez-Lopez M, et al. Cancer Immunotherapy with Immunomodulatory Anti-CD137 and Anti-PD-1 Monoclonal Antibodies Requires BATF3-Dependent Dendritic Cells. Cancer Discov 2016;6:71-9. [Crossref] [PubMed]
- Kohrt HE, Houot R, Marabelle A, et al. Combination strategies to enhance antitumor ADCC. Immunotherapy 2012;4:511-27. [Crossref] [PubMed]
- Aspeslagh S, Postel-Vinay S, Rusakiewicz S, et al. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer 2016;52:50-66. [Crossref] [PubMed]
- Redmond WL, Linch SN, Kasiewicz MJ. Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Cancer Immunol Res 2014;2:142-53. [Crossref] [PubMed]
- Makkouk A, Joshi VB, Lemke CD, et al. Three steps to breaking immune tolerance to lymphoma: a microparticle approach. Cancer Immunol Res 2015;3:389-98. [Crossref] [PubMed]
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med 2016;375:1845-55. [Crossref] [PubMed]
- Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 2010;116:3268-77. [Crossref] [PubMed]
- Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J Clin Oncol 2016;34:2690-7. [Crossref] [PubMed]
- Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 2013;19:3462-73. [Crossref] [PubMed]
- Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311-9. [Crossref] [PubMed]
- Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 2016;17:1283-94. [Crossref] [PubMed]
-
Nivolumab (Opdivo) for Hodgkin Lymphoma - Armand P, Shipp MA, Ribrag V, et al. Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure. J Clin Oncol 2016;34:3733-9. [Crossref] [PubMed]
- Chen R, Zinzani PL, Fanale MA, et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J Clin Oncol 2017;35:2125-32. [Crossref] [PubMed]
- Herbaux C, Gauthier J, Brice P, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood 2017;129:2471-8. [Crossref] [PubMed]
- Haverkos BM, Abbott D, Hamadani M, et al. PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood 2017;130:221-8. [Crossref] [PubMed]
- Rossille D, Gressier M, Damotte D, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia 2014;28:2367-75. [Crossref] [PubMed]
- Georgiou K, Chen L, Berglund M, et al. Genetic basis of PD-L1 overexpression in diffuse large B-cell lymphomas. Blood 2016;127:3026-34. [Crossref] [PubMed]
- Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J Clin Oncol 2016;34:2698-704. [Crossref] [PubMed]
- Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol 2013;31:4199-206. [Crossref] [PubMed]
- Twa DDW, Chan FC, Ben-Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood 2014;123:2062-5. [Crossref] [PubMed]
- Dunleavy K, Wilson WH. Primary mediastinal B-cell lymphoma and mediastinal gray zone lymphoma: do they require a unique therapeutic approach? Blood 2015;125:33-9. [Crossref] [PubMed]
- Eberle FC, Salaverria I, Steidl C, et al. Gray zone lymphoma: chromosomal aberrations with immunophenotypic and clinical correlations. Mod Pathol 2011;24:1586-97. [Crossref] [PubMed]
- Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood 2017;130:267-70. [Crossref] [PubMed]
- Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol 2014;15:69-77. [Crossref] [PubMed]
- Ansell SM, Hurvitz SA, Koenig PA, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res 2009;15:6446-53. [Crossref] [PubMed]
- Ansell S, Gutierrez ME, Shipp MA, et al. A Phase 1 Study of Nivolumab in Combination with Ipilimumab for Relapsed or Refractory Hematologic Malignancies (CheckMate 039). Blood 2016;128:abstr 183.
- Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:1270-1. [Crossref] [PubMed]
- Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Grosso JF, Kelleher CC, Harris TJ, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest 2007;117:3383-92. [Crossref] [PubMed]
- Woo SR, Turnis ME, Goldberg MV, et al. Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-cell Function to Promote Tumoral Immune Escape. Cancer Research 2012;72:917-27. [Crossref] [PubMed]
- Shapiro M, Herishanu Y, Katz BZ, et al. Lymphocyte activation gene 3: a novel therapeutic target in chronic lymphocytic leukemia. Haematologica 2017;102:874-82. [Crossref] [PubMed]
- Queirolo P, Dozin B, Morabito A, et al. Association of CTLA-4 Gene Variants with Response to Therapy and Long-term Survival in Metastatic Melanoma Patients Treated with Ipilimumab: An Italian Melanoma Intergroup Study. Front Immunol 2017;8:386. [Crossref] [PubMed]
- Meng X, Huang Z, Teng F, et al. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev 2015;41:868-76. [Crossref] [PubMed]
- Kater AP, Tonino SH, Egle A, et al. How does lenalidomide target the chronic lymphocytic leukemia microenvironment? Blood 2014;124:2184-9. [Crossref] [PubMed]
- Lopez-Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012;26:2326-35. [Crossref] [PubMed]
- Wu L, Adams M, Carter T, et al. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res 2008;14:4650-7. [Crossref] [PubMed]
- Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol 2008;140:36-45. [PubMed]
- Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol 2014;15:1311-8. [Crossref] [PubMed]
- Kimby E, Rondeau S, Vanazzi A, et al. Rituximab Plus Lenalidomide Versus Rituximab Monotherapy in Untreated Follicular Lymphoma Patients in Need of Therapy First Analysis of Survival Endpoints of the Randomized Phase-2 Trial SAKK 35/10. Blood 2016;128:abstr 1099.
- Czuczman MS, Trneny M, Davies A, et al. A Phase 2/3 Multicenter, Randomized, Open-Label Study of Lenalidomide vs. Investigator's Choice in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Clin Cancer Res 2017;23:4127-37. [Crossref] [PubMed]
- Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol 2015;33:251-7. [Crossref] [PubMed]
- Thieblemont C, Tilly H, Gomes da Silva M, et al. Lenalidomide Maintenance Compared With Placebo in Responding Elderly Patients With Diffuse Large B-Cell Lymphoma Treated With First-Line Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone. J Clin Oncol 2017;JCO2017726984 [PubMed]
- Ferreri AJ, Sassone M, Zaja F, et al. Lenalidomide maintenance in patients with relapsed diffuse large B-cell lymphoma who are not eligible for autologous stem cell transplantation: an open label, single-arm, multicentre phase 2 trial. Lancet Haematol 2017;4:e137-e46. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059-68. [Crossref] [PubMed]
- Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20. [Crossref] [PubMed]
- Younes A, Hilden P, Coiffier B, et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol 2017;28:1436-47. [Crossref] [PubMed]
- Cheson BD, Ansell S, Schwartz L, et al. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood 2016;128:2489-96. [Crossref] [PubMed]
- Lee JH, Long GV, Boyd S, et al. Circulating tumour DNA predicts response to anti-PD1 antibodies in metastatic melanoma. Ann Oncol 2017;28:1130-6. [Crossref] [PubMed]
- Scherer F, Kurtz DM, Newman AM, et al. Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA. Sci Transl Med 2016;8:364ra155 [Crossref] [PubMed]
- Kurtz DM, Green MR, Bratman SV, et al. Noninvasive monitoring of diffuse large B-cell lymphoma by immunoglobulin high-throughput sequencing. Blood 2015;125:3679-87. [Crossref] [PubMed]
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing Tumor-Promoting Chronic Inflammation: A Magic Bullet? Science 2013;339:286-91. [Crossref] [PubMed]
- Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015;348:74-80. [Crossref] [PubMed]
- Pitt Jonathan M, Vétizou M, Daillère R, et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity ;44:1255-69. [Crossref] [PubMed]
- Nishijima TF, Muss HB, Shachar SS, et al. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: A systematic review and meta-analysis. Cancer Treat Rev 2016;45:30-7. [Crossref] [PubMed]
- Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 2014;12:661-72. [Crossref] [PubMed]
- Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015;350:1079-84. [Crossref] [PubMed]
Cite this article as: Karmali R, Sosman JA, Gordon LI. Scientific rationale for immunotherapy in lymphoma and predictors of response. Ann Lymphoma 2017;1:10.


